Abstract
We are developing a novel treatment for heart failure by increasing myocardial 2 deoxy-ATP (dATP). Our studies in rodent models have shown that substitution of dATP for adenosine triphosphate (ATP) as the energy substrate in vitro or elevation of dATP in vivo increases myocardial contraction and that small increases in the native dATP pool of heart muscle are sufficient to improve cardiac function. Here we report, for the first time, the effect of dATP on human adult cardiac muscle contraction. We measured the contractile properties of chemically-demembranated multicellular ventricular wall preparations and isolated myofibrils from human subjects with end-stage heart failure. Isometric force was increased at both saturating and physiologic Ca2+ concentrations with dATP compared to ATP. This resulted in an increase in the Ca2+ sensitivity of force (pCa50) by 0.06 pCa units. The rate of force redevelopment (kTR) in demembranated wall muscle was also increased, as was the rate of contractile activation (kACT) in isolated myofibrils, indicating increased cross-bridge binding and cycling compared with ATP in failing human myocardium. These data suggest dATP could increase dP/dT and end systolic pressure in failing human myocardium. Importantly, even though the magnitude and rate of force development was increased, there was no increase in the time to 50% and 90% myofibril relaxation. These data, along with our previous studies in rodent models shows the promise of elevating myocardial dATP to enhance contraction and restore cardiac pump function. These data also support further pre-clinical evaluation of this new approach for treating heart failure.
Keywords: Heart failure, contraction, myofibrils, Ca2+ sensitivity, treatment
1. Introduction
Heart failure is a growing epidemic in developed countries, with the incidence and prevalence rising each year [1]. Despite advancements in treatment, the five year mortality approaches fifty percent [2]. At least half of the patients suffering from heart failure have low systolic function [3]. However, currently available inotropic agents which increase contractility via altering intracellular Ca2+ do not improve survival in patients with heart failure [4, 5]. Some of the reasons for failure of inotropic agents include tachyarrhythmias, increased myocardial oxygen consumption, decreased coronary perfusion and alteration of intracellular Ca2+ [5]. Hence, there is an urgent need for development of novel agents that improve contractility and systolic function. One way to avoid unwanted side effects may be to directly target myofilaments to alter contractility at level of the sarcomere and motor proteins.
In previous animal studies we found that use of 2-deoxy adenosine triphosphate (dATP) instead of adenosine triphosphate (ATP) as the energy source improves contractility in striated muscle by enhancing crossbridge binding and cycling kinetics and improving allosteric activation [6–9]. In fact, increasing the dATP level from the typical ≤0.1% of the adenosine nucleotide pool to 1% is enough to significantly increase contraction [10]. We have developed a novel approach to elevate dATP in vivo by increasing the expression of the enzyme ribonucleotide reductase (R1R2), the rate-limiting step in de novo dNTP biosynthesis. This results in increased levels of 2-deoxy ATP (dATP). We have shown that increasing dATP in intact cardiomyocytes via adenovirus mediated transfection increased contractile magnitude and kinetics [10]. In addition, transgenic mice that overexpress R1R2 have increased left ventricular systolic function compared to control animals [11]. Based on these results, overexpression of R1R2 and increased cardiomyocytes dATP constitutes an exciting and novel therapy with potential to treat heart failure. However, before clinical studies in humans are convened, a critical step is to test the efficacy of elevated dATP levels on human cardiac myocardium to ensure that effect of dATP is consistent across species.
Here we report for the first time that dATP improves contraction in myocardial samples isolated from human subjects with end-stage heart failure. By measuring isometric force of demembranated multicellular samples we show that dATP enhances force development at both maximal and submaximal Ca2+ concentrations and increases Ca2+ sensitivity of force. We also show that for isolated myofibrils there is an increase in activated force and rate of activation without alteration of relaxation. This study represents an important next logical step in the progression toward using dATP therapy in a clinical setting. We conclude that elevation of myocardial dATP has merit as an approach worth further investigation for the treatment of heart failure and in particular patients with low systolic function.
2. Methods
2.1. Human Left Ventricular Tissue collection
Adult heart tissue was obtained following written informed consent from subjects who were undergoing cardiac placement of left ventricular-assist device or cardiac transplantation for end stage heart failure under a study protocol approved by the University of Washington Institutional Review Board. For samples from transplanted patients, a piece of the left ventricular free wall was obtained. Samples were transported to the laboratory in cold phosphate buffered saline solution and immediately used with preparations as detailed in sections below. The time from harvest in the operating room to arrival to the laboratory was less than one hour.
2.2. NTPase and In vitro motility assays
Myosin was purified on the day of acquisition from human left ventricular samples as previously described [12]. Purified myosin was stored at 4°C and used for up to three days. ATPase and dATPase (NTPase) activities of human cardiac myosin were measured in presence of actin at 23°C using a colorimetric method to detect the nucleotide hydrolysis rate as described [7]. Myosin, actin and NTP (ATP or dATP) concentrations were 0.2 μM, 10 μM and 1 mM respectively. Heavy meromyosin (HMM) was prepared by digestion of myosin with 0.05 mg/ml chymotrypsin as previously described [7, 12, 13] and stored for up to three days at 4°C. In vitro motility assays were performed at 30°C using unregulated Rhodamine Phalloidin-labeled F-actin in presence of 2 mM ATP or dATP as previously described [7, 12, 13]. Images of filaments were recorded and digitally analyzed using custom-built software as previously described. Previously described filtering methods were used to define non-erratically moving filaments based on the ratio of standard deviation to mean of the velocity of the filament (0.75 cutoff). Weighted mean and standard deviation for each condition was calculated using the deviation of each filament’s speed, the number of filaments on each slide and in each condition, as described in our previous studies [13]. Comparison between the two groups was done on the weighted average and standard deviation using a Two-sample T-test with equal variance.
2.3. Multicellular left ventricular force measurements
Left ventricular wall tissue was demembranated in relaxing solution (in mM: 100 KCl, 10 imidazole, 2 EGTA, 5 MgCl2, and 4 ATP) containing 50% glycerol (vol:vol) and 1% Triton X-100 overnight at 4°C then stored in glycerinated relaxing solution at −20°C and used within one week. Demembranating and storage solutions contained protease inhibitor cocktail (P8340; Sigma-Aldrich). Thin left ventricular strips (189±9 μm wide and 0.79±0.05 mm long) were dissected out from the trabeculated layer with fibers going in a single direction. Ends of the strips were wrapped in aluminum T-clips and mounted between a motor (Aurora Scientific, Model 312B) and force transducer (Aurora Scientific, Model 403A). Sarcomere length (SL) was set to 2.3 μm either by direct measuring using fast Fourier transform analysis of the preparation image obtained via MyoCam (IonOptix Corp.) or by stretching the sample to 15% over non strained length. Steady-state force was measured using a custom built mechanical apparatus at 15°C during Ca2+ activation at various concentrations in presence of 5 mM ATP or dATP [14] with half of the preparations measured first in ATP and half in dATP. Experimental solutions were maintained pH 7.0 at 15°C and contained (in mM): 15 phosphocreatine, 15 EGTA, 80 MOPS, 1 free Mg2+, 1 DTT, and 5 Mg2ATP or 5 Mg2dATP. Ca2+ concentration (reported as pCa = −log[Ca2+]) was adjusted by varying amounts of CaCl2. Ionic strength was set to 0.17 M with KCl [14]. Relaxing and activating solutions were prepared using a custom software package as described previously [15]. Passive force (pCa 9.0) was subtracted from force at other pCa concentration to calculate Ca activated force. Force-pCa curves were fit using the Hill Equation to calculate pCa at half maximum force (pCa50) and slope (nH).
| (Eq. 1) |
Reported pCa50 and nH values for force–pCa relationships are the average of individual ts for each experimental curve ±S.E.M.
The rate of isometric tension redevelopment (ktr) was calculated in each activation solution following a rapid release-restretch protocol[16] and fitting the resulting tension trace with mono exponential equation [8].
| (Eq.2) |
For additional demonstration of the ability of dATP to increase contraction, force was measured at submaximal (pCa=5.6) and maximal (pCa=4.5) Ca2+ activation first in ATP, then dATP and back to ATP. In order to eliminate the effect of the order of activation, for one half of the samples the first ATP activation force was used and for the other half the force of the second ATP activation was used.
2.4. Myofibril Mechanical Measurements
Small myofibril bundles were prepared from demembranated left ventricular wall tissue as previously described [13]. Briefly, bundles were rinsed twice in Rigor solution containing 2 mM DTT and 1:200 dilution of protease inhibitor (Sigma-Aldrich, St. Louis, MO) before being homogenized for 1 or 2 pulses of 30s at high speed, stored at 4°C, and used for up to three days. Experiments were performed on a custom set up as previously described [13, 17]. In brief, myofibrils were mounted between two needles; one acted as a cantilever force transducer and the other as an inflexible mount attached to a piezo-electric computer controlled motor. A duel diode system was used to measure needle displacement and developed force was measured based on this displacement and the known stiffness of the needle. Needle stiffness was 2–7 nN/μm for this study. Relaxing (pCa=9.0) and activating (pCa=5.6) solutions were delivered to the mounted myofibril using a double-barreled glass pipette. Activation and relaxation data were collected at 15°C and fit with either single-exponential curves, linear coefficients, or 50% times as previously described [13, 17, 18].
2.5. Statistics
Comparison between groups of data was performed using paired or unpaired Student’s t test as appropriate. All the data passed the normality test (Shapiro-Wilk method). Data are expressed as mean ± standard error of mean and “n” represents the number of experimental samples in each group, and “N” represents the number of patients in each group. Statistical significance was accepted as p<0.05 and when p> 0.05 the result was designated as not statistically significant. In case of outliers in the data set, the interquartile range was calculated and the outliers identified using Tukey’s method.
3. Results
3.1. Patient Characteristics
Sixteen samples were collected from fifteen patients that were enrolled in the study. For one patient, there was sample collected both at time of left ventricular assist device (LVAD) and transplant and used for different assays at the two time points. Thirteen out of fifteen patients (87%) were males and the average age of the cohort was 49±13 years old (26–67 years). Twelve of the sixteen samples were collected from patients undergoing LVAD implantation and four samples were collected at the time of cardiac transplantation. Samples used for isometric contraction were exclusively from patients undergoing LVAD implantation. For the myofibril measurements, NTPase and in vitro motility assays, there was a mixture of samples at time of LVAD surgery and transplantation. The etiology of heart failure in 87% (13 out of 15) of the patients was non-ischemic and 13% (2 out of 15) was ischemic.
3.2. NTPase and In vitro motility assays
The Vmax (actin activated) of isolated human myosin at 23° calculated by NTPase assay was increased by sixty percent from 1.00 ± 0.20 s−1/head to 1.60 ± 0.32 s−1/head (N=5, p=0.042) when dATP was substituted for ATP (Figure 1A). Our in vitro motility measurements were performed at 30°, which is closer to the physiologic temperature, and these measurements also provide an indication of NTPase. Maximum sliding velocity (Vf) for isolated human cardiac HMM was increased by 47%, from 1.78 ± 0.53 μm/s to 2.62 ± 0.79 μm/s (N=3), when dATP was substituted for ATP (Figure 1B). This suggests a higher NTPase rate when dATP is used by failing human adult myosin.
Figure 1.
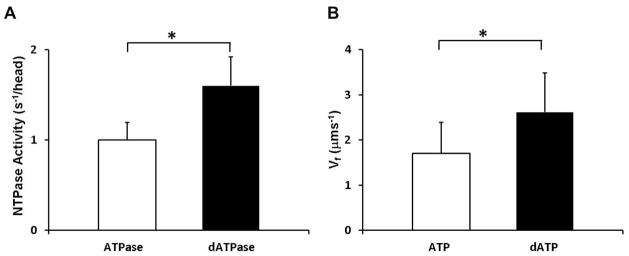
ATP increases NTPase activity. A) NTPase activity of myosin from failing human myocardium in presence of actin and 1 mM ATP or dATP (N=5). Data are reported as mean with standard error of the mean. *p <0.05 by paired student’s t-test B) Speed of smoothly moving actin filaments (Vf) over failing human cardiac heavy meromyosin in presence of 2 mM ATP (n=576 filaments) or dATP (n=1493 filaments). Data are reported as weighted mean with standard deviation (N=3) * p< 0.05 by non-paired student’s t-test
3.3. Isometric contraction of multicellular preparations
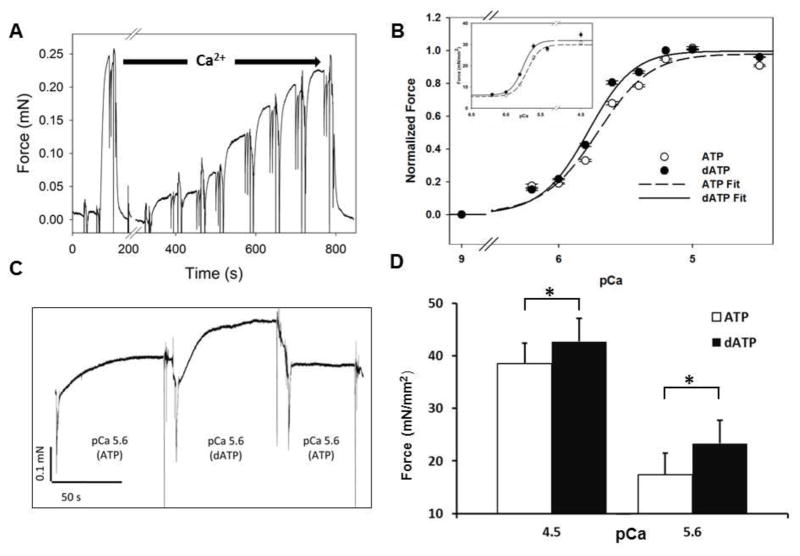
An example multicellular preparation of ventricular wall tissue from human failing myocardium is shown in Figure 2. For each multicellular preparation, we first determined the effect of dATP on the Ca2+ sensitivity of force by activating the preparations in a series of solutions with increasing Ca2+ concentrations in the presence of either ATP or dATP (Figure 3A). The order of nucleotide used in the first curve was reversed for half of the samples to avoid bias due to decreased force after multiple activations (run down). To determine the Ca2+ sensitivity of force, values were normalized to Fmax with either ATP or dATP for each preparation and fitted the Hill equation (Figure 3A, Methods). Substitution of 5 mM dATP for 5 mM ATP resulted in increased Ca2+ sensitivity, seen as a left-shift of the isometric force-pCa curve (Figure 3B). The [Ca2+] required to elicit half maximum force (pCa50) decreased from 5.68±0.03 for ATP to 5.76±0.03 (n=26, N=5, p=2.0 × 10−4). There was no difference between the Hill coefficients (2.54 ± 0.04 vs. 2.88 ± 0.05, n=21, N=5, p=0.21) or passive force (20.51 ± 0.49 vs. 20.85 ± 0.49 mN/mm2, n=26, N=5, p=0.47) between the two groups.
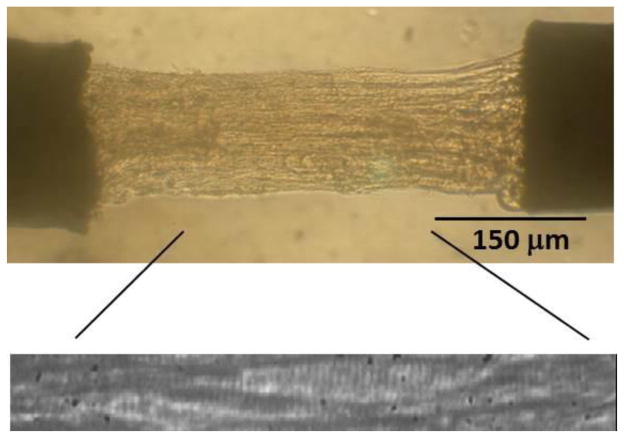
Figure 2.
Human end-stage heart failure left ventricular multicellular preparation. Representative demembranated multicellular preparation under microscope. The zoomed image demonstrates sarcomeres and it was used for measuring sarcomere length to ensure that experiments were under isometric conditions.
Figure 3.
dATP increases force and Ca2+ sensitivity. A) Example of an experiment on human left ventricular multicellular preparation. An initial activation at saturating Ca2+ level (pCa=4.5) is followed by activation at increasing amounts of Ca2+ at pCa 6.4, 6.2, 6.0, 5.8, 5.6, 5.2 and 4.5. Each activation is followed by a rapid release-restrech protocol to determine the rate of isometric tension redevelopment (ktr). B) Normalized and non-normalized (inset) force vs. pCa in presence of ATP (Open Circle) vs. dATP (Closed Circle). Force is normalized to pCa 4.5 for each preparation. Data are reported as the mean and standard error for each pCa (n=25, N= 5). Data were fit to the Hill Equation and values of pCa50 and ηH are reported in the text. C) Representative tracing of isometric force measurement on demembranated multicellular preparation at pCa=5.6 in presence of 5 mM ATP or dATP. D) Force measured at saturating (pCa=4.5) and working Ca2+ concentrations (pCa=5.6) in presence of 5 mM ATP (white) or 5 mM ATP (Black). Measurements represent average and standard error of mean. * p<0.05 by paired student’s t-test
Increase in active force by dATP was also determined by side-by-side measurement in ATP and dATP containing solutions during maximal (pCa 4.5) and sub-maximal (pCa 5.6) activations (Figure 3C, D). Maximal isometric force increased from 38.5±3.9 mN/mm2 with ATP to 42.8±4.4 mN/mm2 with dATP (p=3.89 × 10−6, n =25, N=5), which corresponds to an 11% increase. The effect was greater at submaximal Ca2+ concentration where the heart operates. At pCa=5.6, when substituting dATP for ATP, isometric force increased by 35% from 17.3 ± 4.2 mN to 23.3 ± 4.4 mN (p=1.04 ×10−5, n=18, N=6).
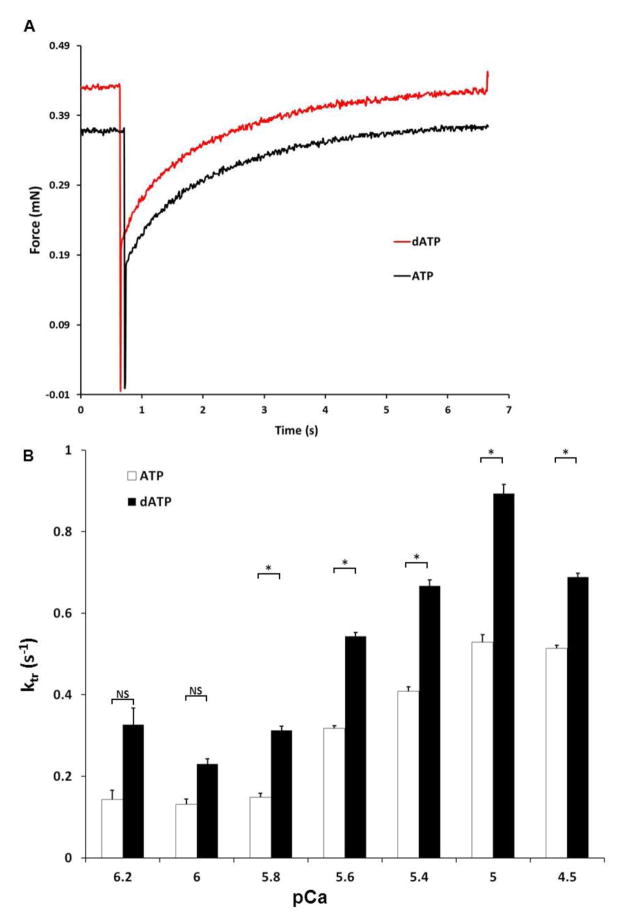
In addition to the magnitude of force, the rate of force development in muscle preparations was also significantly increased at most levels of Ca2+ activation (Figure 4). Example tracings at pCa=5.6 are shown for ATP and dATP, indicating the faster rate of force redevelopment. For each sample, using a rapid release and restrech protocol (Methods), dATP increased the maximum rate of force redevelopment (ktr) by <30%, from 0.62±0.01 to 0.82±0.01 s−1 (p=3.8 × 10−4, n=25, N=5). Thus, dATP results in greater force development at a faster rate in failing human myocardium.
Figure 4.
dATP increases rate of isometric tension redevelopment following a rapid release-restretch protocol. A) Example tracings of isometric tension redevelopment (ktr) at pCa=5.6 in presence of 5 mM ATP (Black) or dATP (Red). B) Rate of isometric tension redevelopment (ktr) in demembranated human failing multicellular preparations at different pCa in presence of 5 mM ATP or dATP (n= 5–21, N=5).* p< 0.05 by paired student’s t-test
To determine the nucleotide concentration dependence of changes in force caused by dATP, we substituted 10%, 25%, 50% and 100% dATP, keeping the total concentration of ATP and dATP at 5 mM, and measured isometric contraction in a subset of demembranated preparations at pCa=5.6. Within individual preparations, a nucleotide mixture containing only 10% dATP was sufficient to significantly increase isometric force by 14%, from 26.3 ± 5.1 mN/mm2 to 29.9 ± 5.5 mN/mm2 (p=0.0091, n=11, N=4) as shown in Figure 5. The augmentation of force by dATP appeared to be linear, from 10 to 100% dATP, with each 10% increase in dATP increasing the force by approximately 1.68 mN/mm2 (R2=0.985).
Figure 5.
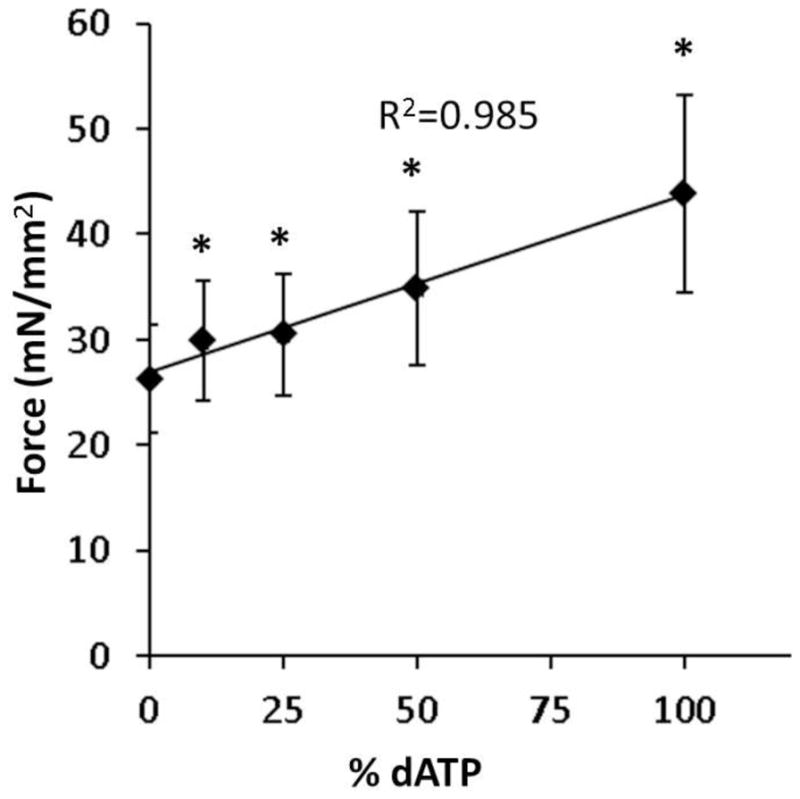
A small amount of dATP increases isometric force. Isometric force measured at pCa=5.6 in multicellular demembranated preparations at 10%, 25%, 50% and 100% dATP compared to 100% ATP (n=11, N=4 at each dATP concentration). Measurements represent mean and standard error of the mean. Linear regression performed on the data. * p < 0.05 compared to 100% ATP (0% dATP) by paired student’s t-test
3.4. Myofibrillar contraction measurements
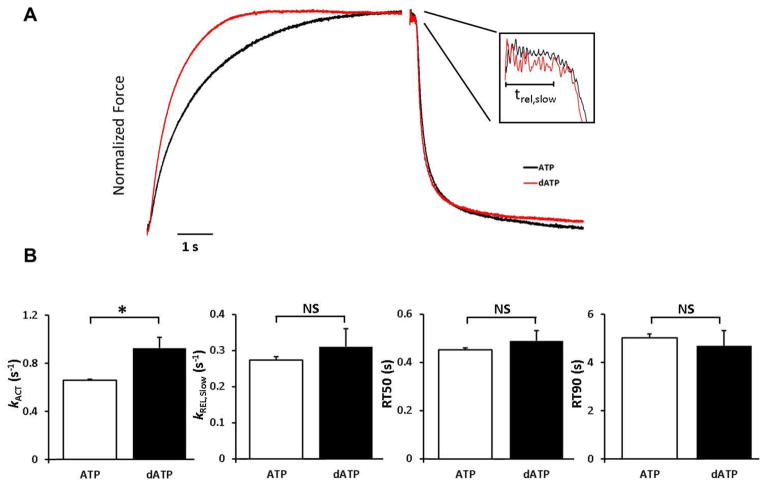
Small myofibril bundles were used to assess the kinetics of activation and relaxation. To control for the effects of repeated activations, separate myofibril bundles from each patient were activated and relaxed at pCa 5.6 in the presence of 2 mM ATP and 2 mM dATP. Figure 6A shows an example of activation and relaxation of 2 myofibril bundles, one in presence of 2 mM ATP and the other in the presence of 2 mM dATP. The force for both traces is normalized to highlight the rates of activation and relaxation. This example demonstrates the increase in the rate of activation in the presence of dATP. The data for all measurements is summarized in Figure 6B. The relaxation trace shows that the time course of relaxation to baseline was similar. dATP significantly increased both force production (76.1 ± 1.0 vs. 85.9 ± 6.9 mN/mm2, p=0.039, n=27, N=7) and rate of activation (0.64 ± 0.01 vs. 0.92 ± 0.08 s−1, p=0.003, n=27, N=7) at pCa=5.6. Myofibril relaxation measurements did not show a decrease in time to 50% (0.38 ± 0.03 vs. 0.47 ± 0.05 s, p=0.064, n=16, N=7) or 90% (4.44 ± 0.75 vs.5.11 ± 1.01 s, p=0.31, n=16, N=7) relaxation. Myofibril tension relaxation consists of an initial slow (kREL,slow) phase, associated with crossbridge detachment rates, and a secondary, large amplitude fast (kREL,fast) phase that dominates overall relaxation time [19]. Neither the duration (0.182 ± 0.002 msec (ATP) vs. 0.194 ± 0.009 s (dATP), p=0.17, n=25, N=7), nor rate of the slow phase of relaxation (kREL,slow) (0.273 ± 0.009 vs. 0.310 ± 0.05 s−1, p=0.32, n=25, N=7) are significantly increased when dATP was substituted for ATP. Similarly, there was no significant increase in the rate of the fast phase of relaxation (kREL,fast) (1.81 ± 0.03 vs. 1.70 ± 0.11 s−1, p=0.29, n=27, N=7).
Figure 6.
dATP increases myofibril activation rate without altering relaxation. A) Typical normalized myofibril force trace from a patient with end-stage congestive heart failure in presence of ATP (Black) or dATP (Red). The left trace is the activation trace at pCa=5.6 and the right trace is relaxation when switched to pCa=9.0 solution. The inset is close up of the slow phase of relaxation. B) Rate of activation (kac), rate of the slow phase of relaxation (kREL,slow), time to 50% (RT50) and 90% (RT90) relaxation of human myofibrils in presence of 2 mM ATP (white) and dATP (black). Data are reported as mean±SE (n=25–27, N=7). * P<0.05 compared to ATP by non-paired student’s t-test
4. Discussion
Here we have reported the first observations of the effect of dATP on the contractile properties of cardiac muscle from human heart failure patients. This cohort of patients have severe systolic function (Table 1), indicating severe contractile dysfunction in the cardiac muscle. The main findings from our studies are that dATP 1) increases human cardiac myosin binding (to actin) and cycling kinetics and 2) the magnitude and rate of contraction at all levels of Ca2+ activation. Importantly, this occurs without altering the relaxation properties of cardiac muscle, which can occur with other cardiac inotropes (discussed below). Our pCa50 values (5.44 to 5.87 for ATP group) are within the range reported in the literature (5.6–5.8 in non-failing hearts and 5.8–6.0 in failing heart) [20, 21]. These previous studies were performed on skinned isolated cells rather than multicellular preparations, as in our study.
Table 1.
Demographic, clinical and echocardiographic characteristics of the patients including in this study. Data represents mean ± SD.
| Demographic Data | ||
| Age (years) | 48.9 ± 13.6 | |
| Male/Female | 13(87%)/2(13%) | |
|
| ||
| Clinical Data | ||
| Etiology | ||
| Ischemic | 2 (13%) | |
| Non-ischemic | 13 (87%) | |
| Medications | ||
| Inotropic agents | 6 (40%) | |
| Beta blockers | 8 (53%) | |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 12 (80%) | |
| Diuretics | 14 (93%) | |
| Digoxin | 11 (73%) | |
|
| ||
| Echocardiographic Data | ||
| Ejection Fraction (%) | 16 ± 5 | |
| Left ventricular end-diastolic dimension (cm) | 7.4 ± 1.4 | |
| Left ventricular end-diastolic dimension (cm) | 6.7 ± 1.4 | |
Our previous studies in rodent models demonstrated that the magnitude and rate of force development was increased at all Ca2+ activation levels in cardiac muscle preparations when dATP is substituted for ATP [6, 7, 10, 11]. However, there are known differences between contractile proteins in humans and rodents. The predominant myosin subtype in human is β-MHC, compared to the faster α-MHC subtype that is predominant in rodents [22]. While the two isoforms show significant homology, β-MHC exhibits a slower ATPase rate, slower sliding velocity and maximal shortening velocity, and significantly lower power generation capabilities [23]. At the same time, β-MHC is more energy efficient and produces the same amount of force per crossbridge [24].
On the molecular level, differences between the two cardiac myosin isoforms are thought to be primarily in loop 1 and loop 2 of the myosin head [25]. Our molecular dynamic simulations predict that dATP changes the charge exposure on loop 2[26], so it was possible that the effect of dATP could be MHC dependent. We have reported that dATP increases force and the rate of force development at all levels of Ca2+ activation in demembranated cardiac preparations from normal and PTU treated rats, the latter of which express primarily β-MHC respectively [8]. In a report by Schoffstall et al., porcine cardiac preparations had a similar dATP-induced increase in maximal force production and at sub-maximal Ca2+ levels > pCa 5.5, but did not show change in Ca2+ sensitivity of force, a result that was attributed to troponin and tropomyosin isoform differences by the authors [27]. It may also be that phosphorylation of myofilament proteins was significantly different in their cardiac muscle preparations, as phosphorylation of proteins such as troponin I, troponin T, myosin binding protein C and titin are known to affect the Ca2+ sensitivity of force and affect the myosin dependence of thin filament activation and force development. They did report a 47% increase in unregulated (no troponin or tropomyosin) actin filament sliding velocity over immobilized myosin heads via the in vitro motility assay, which is the same magnitude increase we report for actin motility on surface covered with myosin from failing human hearts (Figure 1B), supporting a myosin specific effect of dATP.
The interspecies difference in ATPase rates and actin sliding velocity within α and β MHC [22] and reported alterations in myosin subtype expression in human failing hearts [28–30] warranted an assessment of the effect of dATP on failing human myocardium. To our knowledge, this is the first study to evaluate the effect of dATP on the contraction of human samples from end-stage heart failure patients. Our data demonstrate that, in addition to augmenting cardiac muscle contraction at all levels of Ca2+ activation, dATP increases crossbridge cycling in purified human cardiac myosin and HMM. Coupled with our previous studies, this suggests that dATP enhances crossbridge activity independent of myosin isoform and Ca2+ levels present in cardiac muscle cells. Additionally, we show that even a minority concentration of dATP (10% dATP: 90% ATP in this study) can significantly increase the isometric force generated in failing human myocardium at physiologic Ca2+ levels. Our isometric force measurements from both isolated myofibrils and demembranated left ventricular samples demonstrate an increase in the magnitude (Figure 2C) and the rate of force development (Figure 4) at all levels of Ca2+ activation with dATP. Our current human data confirms the previous mechanism that has been shown in rodents, where we demonstrated an increase in myosin binding to actin with dATP, which leads to increased thin filament activation at all levels of Ca2+ activation, and that the level of thin filament activation is an important determinant in the rate that force develops [7, 8]. Our data can be interpreted using the 2-state model of crossbridge to understand how dATP improves contraction in human cardiac samples. In the 2-state crossbridge model, the rate constants for crossbridge attachement and force generation are summed together as fapp and rate of crossbridge detachment is gapp. Our current data in human samples shows that dATP increases rate of force redevelopment (ktr=fapp+gapp), Fmax (~ fapp/(fapp+gapp) and Vf, indicating an increase in fapp+gapp and fapp/gapp. This is also consistent with recent modeling results from our group showing dADP stabilizes myosin in a conformation that favors binding to actin. Thus dATP elevates contractile activation via increased myosin binding to actin, without extending the time of myosin-actin interaction, such that it should increase the amount and rate of force development, but not retard the rate of myosin detachment and muscle relaxation.
The increase in both magnitude and rate of force development at the sub-cellular and tissue levels correlates (respectively) to systolic ventricular function, particularly the isovolumic phase of pressure development (+dP/dT). Indeed, we have reported that +dP/dT is significantly increased in transgenic mice with elevated [dATP] [11], in normal and infarcted mice and infarcted pigs treated with a viral vector to overexpress R1R2 and elevate dATP in the heart [31–33]. As such, it suggests that +dP/dT could be increased by elevating myocardial dATP in failing human hearts. An aspect that may be unique to elevated dATP as a treatment for heart failure may be that, in addition to improving systolic pressure development, myocardial relaxation and the rate of ventricular pressure decline (−dP/dT) at beginning of diastole is not prolonged and may even be improved. This may be related to faster nucleotide hydrolysis product release by myosin with dATP as the substrate [6].
We found significant variability of pCa50 between patients. The etiology of heart failure, duration of LVAD treatment and medication profiles (Tables 1 and S1) may account for some of these differences. We evaluated phosphorylation profile of the left ventricular wall preparations (3–5 per patient) used in generating the force-pCa plot using We also performed western blot analysis of the phosphorylated S23/24 troponin I and total troponin I of the same samples. While there was slight differences in the total phosphorylation and tropoinin I phosphorylation between the samples, there was no relationship between pCa50 and amount of phosphorylation. However, the strength of our study is dATP had a dramatic effect in all the samples tested despite baseline differences. In order to eliminate even the small contribution of decreasing force with multiple activations (run down) in our samples, we performed sequential isometric activation with ATP and dATP. We found that the increase in force was much higher in the physiologic range of Ca2+ concentrations (about 30%) compared to saturating Ca2+ concentrations (about 10%).
One of the limitations of our study is that it was skewed towards non-ischemic cardiomyopathy. We would expect similar increase in contractility in other forms of systolic heart failure. We also need to evaluate our approach in isolated cardiomyocytes treated with R1R2 overexpression viral vector to ensure the effect is present event with the elevated diastolic Ca2+ levels seen in end-stage heart failure. ATP is an indispensable biological molecule and in our novel therapeutic approach, we anticipate replacing less than 10% of the ATP content by dATP. It is noted that dATP can be used instead of ATP by enzymes such as hexokinase, polynucleotide kinase, T4 DNA ligase, T4 RNA ligase and sarcoplasmic reticulum Ca2+ dependent ATPase (SERCA) [34, 35]. It has been shown that ATP and dATP are hydrolysed at similar rate by SERCA [34]. Whether these ATPases would have enhanced activity with a dATP pool that is less than 10% of ATP, as is the case for myofilament contractility, is unclear.
We have previously shown that overexpression of the enzyme ribonucleotide reductase in rodents, using transgene or gene therapy, can improve contraction via increased dATP [10, 11]. Now we show that dATP can increase contraction in failing human myocardial tissue. Our novel approach to treat heart failure focuses at the myofilament where the primary contractile deficit occurs. Levosimendan and Omecamtiv mercarbil are two medications in investigational trials that also focus on treating heart failure at the level of the myofilament. Levosimendan is a Ca2+ sensitizer [36] that binds to Ca2+-saturated cTnC. Levosimendan does have off-target effects, such as interaction with the vascular smooth muscle K+ channel, and has shown favorable hemodynamic effects without any clear clinical benefit [37]. Omecamtiv mercarbil enhances the ATPase activity of myosin [38], and also improves cardiac function in both healthy volunteers and those with stable heart failure. However, this medication increases the duration of cardiomyocyte twitches, increases systolic ejection time, and decreases time in diastole. Furthermore, there is concern that Omecamtiv marcarbil decreases coronary perfusion and coronary filling [39, 40]. Our approach to treating heart failure is myofilament specific, and we have previously shown the nucleotide dATP can transfer from one cell to another via gap junctions [41] so elevation of ribonucleotide reductase need not be accomplished throughout the myocardium. Additionally, cardiomyocyte contractions are stronger and faster, but not prolonged, and both +dP/dT and −dP/dT are increased with a resulting increase in ejection fraction [10, 11].
5. Conclusions
We have demonstrated for the first time that dATP can increase contraction and the rate of crossbridge cycling in cardiac muscle from patients with end-stage heart failure, without retarding relaxation. We have used isolated protein, myofibril and multicellular assays to show this occurs at the level of the contractile apparatus of the heart over the entire range of Ca2+ activation. Coupled with our previous and ongoing work in animal models, these data support a novel myofilament approach for treating heart failure that warrants further pre-clinical evaluation.
Supplementary Material
Highlights.
contraction in human heart failure sample was measured in presences of ATP or dATP
dATP increases isometric force and Ca2+ sensitivity in demembranated samples
dATP increases rate of force redevelopment in demembranated samples
10% dATP substitution significantly increases isometric force
dATP increases rate of myofibril activation without altering relaxation kinetics
Acknowledgments
We thank Galina Flint for assistance with in vitro motility assay, Stephen Farris for obtaining samples from the University of Washington Medical Center and Farshid Moussavi-Harami for help with data analysis.
Sources of Funding:
Research is supported by NIH R01 HL11119, awarded to MR and NIH R01HL094384 awarded to ASO. FMH is supported by Cardiovascular Training Program (NIH T32 HL07828) at University of Washington and an American Heart Association Postdoctoral Fellowship.
Nonstandard Abbreviations and Acronyms
- ATP
Adenosine triphosphate
- dATP
2-deoxy adenosine triphosphate
- HMM
Heavy meromyosin
- LVAD
Left ventricular assist device
- R1R2
Ribonucleotide reductase
- RT50
Time to 50% relaxation
- RT90
Time to 90% relaxation
- SL
Sarcomere length
Footnotes
Disclosures:
Dr. Regnier and the University of Washington (UW ref. 45511.01US1) have filed an international patent application (PCT/US12/39897) on R1R2 over-expression to improve cardiac contractile function. Dr. Regnier is a founder of BEATbio, Inc. that has licensed this technology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA : the journal of the American Medical Association. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ. Clinical practice. Systolic heart failure. The New England journal of medicine. 2010;362:228–38. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) Journal of the American College of Cardiology. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Teerlink JR, Metra M, Zaca V, Sabbah HN, Cotter G, Gheorghiade M, et al. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond. Heart failure reviews. 2009;14:243–53. doi: 10.1007/s10741-009-9153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regnier M, Lee DM, Homsher E. ATP analogs and muscle contraction: mechanics and kinetics of nucleoside triphosphate binding and hydrolysis. Biophysical journal. 1998;74:3044–58. doi: 10.1016/S0006-3495(98)78012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regnier M, Rivera AJ, Chen Y, Chase PB. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circulation research. 2000;86:1211–7. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- 8.Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophysical journal. 2004;87:1815–24. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regnier M, Homsher E. The effect of ATP analogs on posthydrolytic and force development steps in skinned skeletal muscle fibers. Biophysical journal. 1998;74:3059–71. doi: 10.1016/S0006-3495(98)78013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korte FS, Dai J, Buckley K, Feest ER, Adamek N, Geeves MA, et al. Upregulation of cardiomyocyte ribonucleotide reductase increases intracellular 2 deoxy-ATP, contractility, and relaxation. Journal of molecular and cellular cardiology. 2011;51:894–901. doi: 10.1016/j.yjmcc.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowakowski SG, Kolwicz SC, Korte FS, Luo Z, Robinson-Hamm JN, Page JL, et al. Transgenic overexpression of ribonucleotide reductase improves cardiac performance. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1220693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon AM, LaMadrid MA, Chen Y, Luo Z, Chase PB. Calcium regulation of skeletal muscle thin filament motility in vitro. Biophysical journal. 1997;72:1295–307. doi: 10.1016/S0006-3495(97)78776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racca AW, Beck AE, Rao VS, Flint GV, Lundy SD, Born DE, et al. Contractility and kinetics of human fetal and human adult skeletal muscle. The Journal of physiology. 2013;591:3049–61. doi: 10.1113/jphysiol.2013.252650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao VS, Korte FS, Razumova MV, Feest ER, Hsu H, Irving TC, et al. N-terminal phosphorylation of cardiac troponin-I reduces length-dependent calcium sensitivity of contraction in cardiac muscle. The Journal of physiology. 2013;591:475–90. doi: 10.1113/jphysiol.2012.241604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martyn DA, Chase PB, Hannon JD, Huntsman LL, Kushmerick MJ, Gordon AM. Unloaded shortening of skinned muscle fibers from rabbit activated with and without Ca2+ Biophysical journal. 1994;67:1984–93. doi: 10.1016/S0006-3495(94)80681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner B. The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin-ATPase in solution. Basic research in cardiology. 1986;81 (Suppl 1):1–15. doi: 10.1007/978-3-662-11374-5_1. [DOI] [PubMed] [Google Scholar]
- 17.Kreutziger KL, Piroddi N, Scellini B, Tesi C, Poggesi C, Regnier M. Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle. The Journal of physiology. 2008;586:3683–700. doi: 10.1113/jphysiol.2008.152181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colomo F, Piroddi N, Poggesi C, te Kronnie G, Tesi C. Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. The Journal of physiology. 1997;500 (Pt 2):535–48. doi: 10.1113/jphysiol.1997.sp022039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poggesi C, Tesi C, Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflugers Archiv : European journal of physiology. 2005;449:505–17. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- 20.van der Velden J, Boontje NM, Papp Z, Klein LJ, Visser FC, de Jong JW, et al. Calcium sensitivity of force in human ventricular cardiomyocytes from donor and failing hearts. Basic research in cardiology. 2002;97 (Suppl 1):I118–26. doi: 10.1007/s003950200040. [DOI] [PubMed] [Google Scholar]
- 21.Ambardekar AV, Walker JS, Walker LA, Cleveland JC, Jr, Lowes BD, Buttrick PM. Incomplete recovery of myocyte contractile function despite improvement of myocardial architecture with left ventricular assist device support. Circulation Heart failure. 2011;4:425–32. doi: 10.1161/CIRCHEARTFAILURE.111.961326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malmqvist UP, Aronshtam A, Lowey S. Cardiac myosin isoforms from different species have unique enzymatic and mechanical properties. Biochemistry. 2004;43:15058–65. doi: 10.1021/bi0495329. [DOI] [PubMed] [Google Scholar]
- 23.Herron TJ, Korte FS, McDonald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. American journal of physiology Heart and circulatory physiology. 2001;281:H1217–22. doi: 10.1152/ajpheart.2001.281.3.H1217. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura S, Kobayakawa N, Fujita H, Yamashita H, Momomura S, Chaen S, et al. Comparison of unitary displacements and forces between 2 cardiac myosin isoforms by the optical trap technique: molecular basis for cardiac adaptation. Circulation research. 1998;82:1029–34. doi: 10.1161/01.res.82.10.1029. [DOI] [PubMed] [Google Scholar]
- 25.Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, et al. Analysis of myosin heavy chain functionality in the heart. The Journal of biological chemistry. 2003;278:17466–74. doi: 10.1074/jbc.M210804200. [DOI] [PubMed] [Google Scholar]
- 26.Nowakowski SG, Adamek N, Geeves MA, Gay E, Kolwicz SC, Jr, Murry CE, et al. 2-deoxy-ATP Alters Myosin Structure to Enhance Cross-Bridge Cycling and Improve Cardiac Function. Biophysical journal. 2013:104. [Google Scholar]
- 27.Schoffstall B, Clark A, Chase PB. Positive inotropic effects of low dATP/ATP ratios on mechanics and kinetics of porcine cardiac muscle. Biophysical journal. 2006;91:2216–26. doi: 10.1529/biophysj.105.079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiser PJ, Portman MA, Ning XH, Schomisch Moravec C. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. American journal of physiology Heart and circulatory physiology. 2001;280:H1814–20. doi: 10.1152/ajpheart.2001.280.4.H1814. [DOI] [PubMed] [Google Scholar]
- 29.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circulation research. 2000;86:386–90. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi T, Camp P, Jr, Alix SL, Gorga JA, Begin KJ, Leavitt BJ, et al. Myosin from failing and non-failing human ventricles exhibit similar contractile properties. Journal of molecular and cellular cardiology. 2003;35:91–7. doi: 10.1016/s0022-2828(02)00282-1. [DOI] [PubMed] [Google Scholar]
- 31.Gay E, Nowakowski SG, Kolwicz SC, Odom GL, Flint GV, Stuppard R, et al. AAV6-Mediated Overexpression of Ribonucleotide Reductase (R1R2) Enhances 2-Deoxy-ATP Concentration in Vivo and Improves Cardiac Function. Biophysical journal. 2014:106. [Google Scholar]
- 32.Nowakowski SG, Kolwicz SC, Moussavi-Harami F, Odom GL, Flint GV, Gay E, et al. AAV6 -mediated overexpression of Ribonucleotide Reductase (R1R2) that increases myocardial dATP and improves function of normal and failing hearts. International Society for Heart Research; Miami Beach, FL: 2014. [Google Scholar]
- 33.Korte FS, Odom GL, Dai J, Kolwicz SC, Robinson-Hamm JN, Tian R, et al. Broad Transgenic, and Cardiac-Specific Viral Mediated, Over-Expression of Ribonucleotide Reductase Increases In Vivo Cardiac Contractility. Biophysical journal. 2012:102. [Google Scholar]
- 34.Trumble WR, Sutko JL, Reeves JP. Cardiac sarcolemmal and sarcoplasmic reticulum membrane vesicles exhibit distinctive (Ca-Mg)-ATPase substrate specificities. The Journal of biological chemistry. 1981;256:7101–4. [PubMed] [Google Scholar]
- 35.Kinoshita Y, Nishigaki K. Unexpectedly general replaceability of ATP in ATP-requiring enzymes. Journal of biochemistry. 1997;122:205–11. doi: 10.1093/oxfordjournals.jbchem.a021730. [DOI] [PubMed] [Google Scholar]
- 36.Papp Z, Edes I, Fruhwald S, De Hert SG, Salmenpera M, Leppikangas H, et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. International journal of cardiology. 2012;159:82–7. doi: 10.1016/j.ijcard.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart failure. 2013;1:103–11. doi: 10.1016/j.jchf.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Meijs MF, Asselbergs FW, Doevendans PA. Omecamtiv mecarbil: a promising new drug in systolic heart failure. European journal of heart failure. 2012;14:232–3. doi: 10.1093/eurjhf/hfr178. [DOI] [PubMed] [Google Scholar]
- 39.Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–83. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 40.Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–75. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- 41.Lundy SD, Murphy SA, Dupras SK, Dai J, Murry CE, Laflamme MA, et al. Cell-based delivery of dATP via gap junctions enhances cardiac contractility. Journal of molecular and cellular cardiology. 2014 doi: 10.1016/j.yjmcc.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.