Abstract
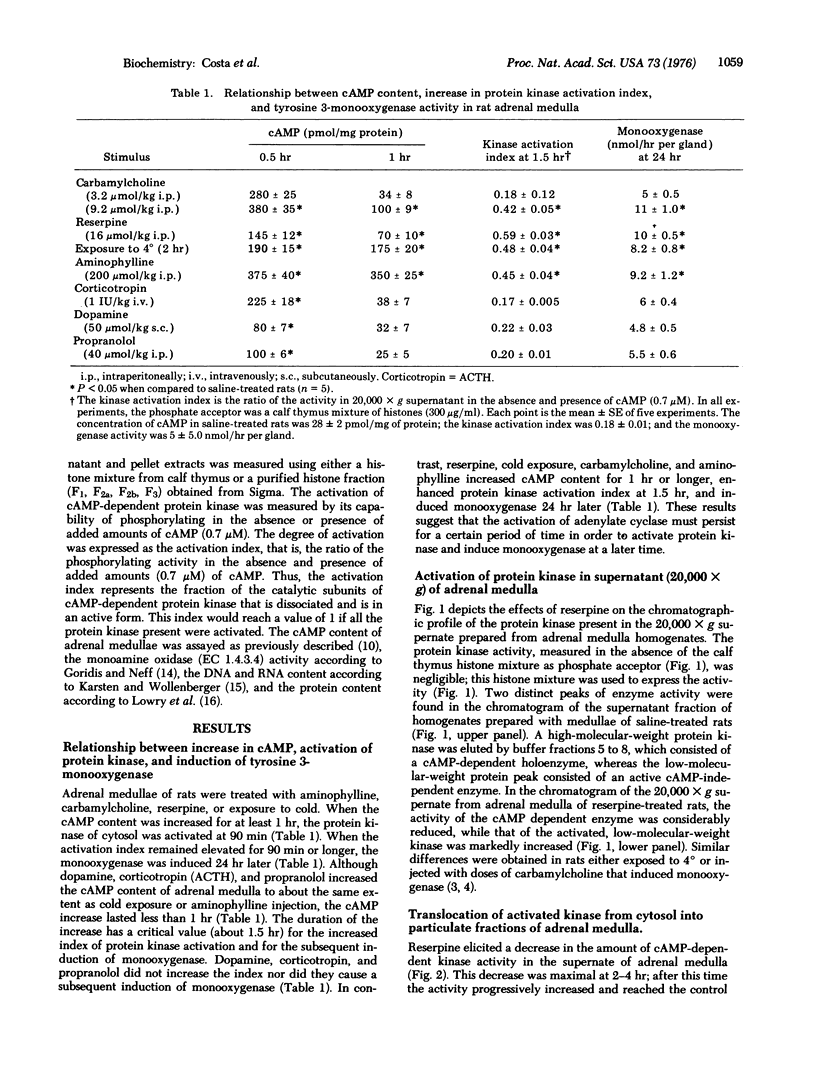
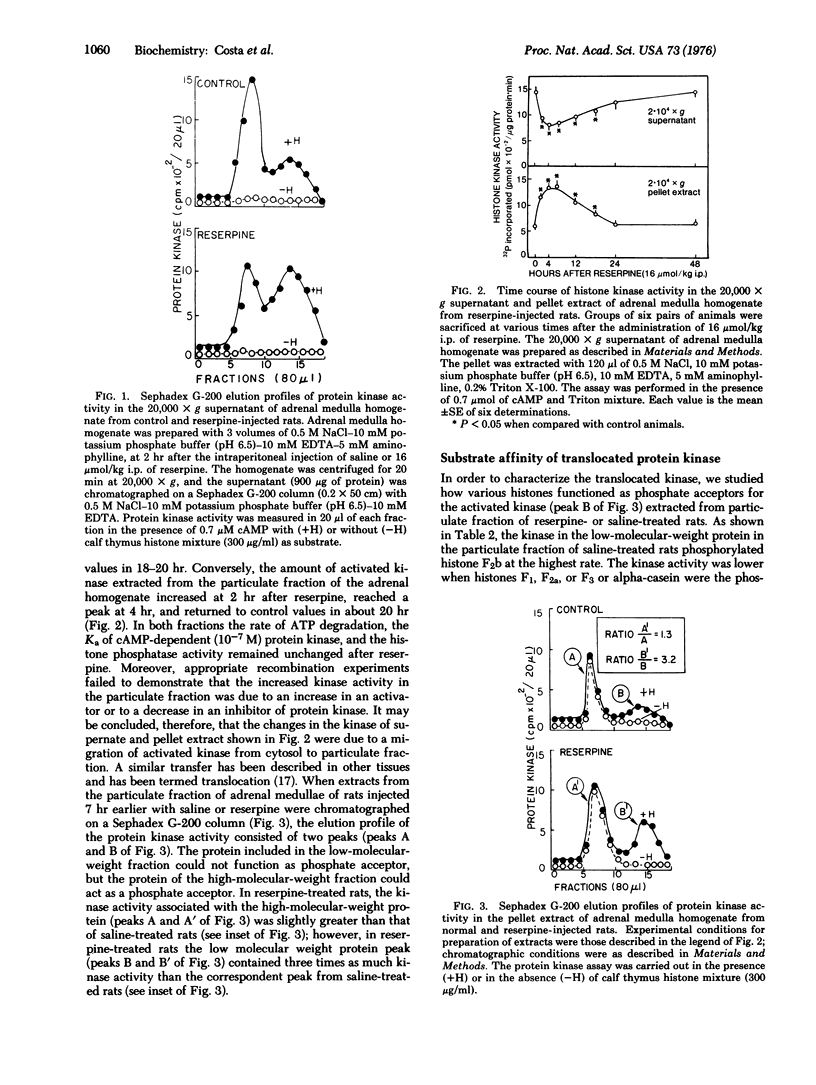
The tyrosine-3-monooxygenase activity [L-tyrosine, tetrahydropteridine: oxygen oxidoreductase (3-hydroxylating); EC 1.14.16.2] of rat adrenal medulla is induced 20-24 hr after the injection of reserpine (16 mumol/kg intraperitoneally). This and other inducing stimuli increase the 3': 5'-cyclic AMP (cAMP) content in the medulla for longer than 60 min and activate the cAMP-dependent protein kinase (ATP: protein phosphotransferase; EC 2.7.1.37) for several hours. Corticotropin (ACTH), dopamine, and propranolol do not induce the monooxygenase, but elicit an increase in the cAMP content of the medulla which fails to activate protein kinase and lasts less than 1 hr. A high- and low-molecular-weight protein kinase are separated by gel filtration from the 20,000 X g pellet extract of adrenal medulla homogenate. The activity of the low-molecular-weight enzyme is expressed as its ability to phosphorylate histone. The protein kinase activity of the pellet is increased between 3 and 17 hr after reserpine injection. Our evidence indicates that this increase is due to a translocation from cytosol to subcellular structures of a kinase that utilizes lysine-rich histone as phosphate acceptor. The protein kinase activity that is extracted from a purified nuclear fraction prepared from the adrenal medulla of rats injected 7 hr previously with reserpine is greater than that extracted from medulla of saline-treated rats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balhorn R., Bordwell J., Sellers L., Granner D., Chalkley R. Histone phosphorylation and DNA synthesis are linked in synchronous cultures of HTC cells. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1326–1333. doi: 10.1016/s0006-291x(72)80120-7. [DOI] [PubMed] [Google Scholar]
- Castagna M., Palmer W. K., Walsh D. A. Nuclear protein-kinase activity in perfused rat liver stimulated with dibutyryl-adenosine cyclic 3':5'-monophosphate. Eur J Biochem. 1975 Jun 16;55(1):193–199. doi: 10.1111/j.1432-1033.1975.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Smith D. L., Bruegger B. B., Halpern R. M., Smith R. A. Occurrence and distribution of acid-labile histone phosphates in regenerating rat liver. Biochemistry. 1974 Aug 27;13(18):3785–3789. doi: 10.1021/bi00715a026. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Soderling T. R., Park C. R. Regulation of adenosine 3',5'-monophosphate-dependent protein kinase. I. Preliminary characterization of the adipose tissue enzyme in crude extracts. J Biol Chem. 1973 Mar 10;248(5):1813–1821. [PubMed] [Google Scholar]
- Goodman R., Otten U., Thoenen H. Organ culture of the rat adrenal medulla: a model system for the study of trans-synaptic enzyme induction. J Neurochem. 1975 Oct;25(4):423–427. doi: 10.1111/j.1471-4159.1975.tb04340.x. [DOI] [PubMed] [Google Scholar]
- Goridis C., Neff N. H. Monoamine oxidase: an approximation of turnover rates. J Neurochem. 1971 Sep;18(9):1673–1682. doi: 10.1111/j.1471-4159.1971.tb03740.x. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Costa E. A role for nicotinic receptors in the regulation of the adenylate cyclase of adrenal medulla. J Pharmacol Exp Ther. 1974 Jun;189(3):665–675. [PubMed] [Google Scholar]
- Guidotti A., Costa E. Involvement of adenosine 3',5'-monophosphate in the activation of tyrosine hydroxylase elicited by drugs. Science. 1973 Mar 2;179(4076):902–904. doi: 10.1126/science.179.4076.902. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Hanbauer I., Costa E. Role of cyclic nucleotides in the induction of tyrosine hydroxylase. Adv Cyclic Nucleotide Res. 1975;5:619–639. [PubMed] [Google Scholar]
- Guidotti A., Kurosawa A., Chuang D. M., Costa E. Protein kinase activation as an early event in the trans-synaptic induction of tyrosine 3-monooxygenase in adrenal medulla. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1152–1156. doi: 10.1073/pnas.72.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann R. A., Hiestand P. C., Schweppe J. S. Mechanism of action of gonadotropin. IV. Cyclic adenosine monophosphate-dependent translocation of ovarian cytoplasmic cyclic adenosine monophosphate-binding protein and protein kinase to nuclear acceptor sites. Endocrinology. 1974 Jan;94(1):168–183. doi: 10.1210/endo-94-1-168. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Lee S., DeAngelo A. B. Translocation of cytoplasmic protein kinase and cyclic adenosine monophosphate-binding protein to intracellular acceptor sites. Adv Cyclic Nucleotide Res. 1975;5:281–306. [PubMed] [Google Scholar]
- Karsten U., Wollenberger A. Determination of DNA and RNA in homogenized cells and tissues by surface fluorometry. Anal Biochem. 1972 Mar;46(1):135–148. doi: 10.1016/0003-2697(72)90405-8. [DOI] [PubMed] [Google Scholar]
- Keely S. L., Jr, Corbin J. D., Park C. R. On the question of translocation of heart cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1501–1504. doi: 10.1073/pnas.72.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Cyclic AMP and histone phosphorylation. Ann N Y Acad Sci. 1971 Dec 30;185:166–180. doi: 10.1111/j.1749-6632.1971.tb45246.x. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Phosphorylation of liver histone following the administration of glucagon and insulin. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1276–1283. doi: 10.1073/pnas.64.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Adrenal tyrosine hydroxylase: compensatory increase in activity after chemical sympathectomy. Science. 1969 Jan 31;163(3866):468–469. doi: 10.1126/science.163.3866.468. [DOI] [PubMed] [Google Scholar]
- Otten U., Mueller R. A., Thoenen H. Effect of hypophysectomy on cAMP changes in rat adrenal medulla evoked by catecholamines and carbamylcholine. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(2):157–170. doi: 10.1007/BF00501303. [DOI] [PubMed] [Google Scholar]
- Palmer W. K., Castagna M., Walsh D. A. Nuclear protein kinase activity in glucagon-stimulated perfused rat livers. Biochem J. 1974 Nov;143(2):469–471. doi: 10.1042/bj1430469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawse A. R., Ord M. G., Stocken L. A. Histone kinase and cell division. Biochem J. 1971 May;122(5):713–719. doi: 10.1042/bj1220713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970 Nov 28;228(5274):861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Kettler R., Burkard W., Saner A. Neurally mediated control of enzymes involved in the synthesis of norepinephrine; are they regulated as an operational unit? Naunyn Schmiedebergs Arch Pharmakol. 1971;270(2):146–160. doi: 10.1007/BF00997085. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M. The metabolic code. Science. 1975 Sep 5;189(4205):760–763. doi: 10.1126/science.169570. [DOI] [PubMed] [Google Scholar]
- Wastila W. B., Stull J. T., Mayer S. E., Walsh D. A. Measurement of cyclic 3',5'-denosine monophosphate by the activation of skeletal muscle protein kinase. J Biol Chem. 1971 Apr 10;246(7):1996–2003. [PubMed] [Google Scholar]
- Yasmineh W. G., Yunis J. J. Isolation of mammalian heterochromatin and euchromatin. Methods Cell Biol. 1974;8(0):151–177. doi: 10.1016/s0091-679x(08)60450-1. [DOI] [PubMed] [Google Scholar]



