Abstract
The functional roles of the orphan nuclear receptor, Nurr1, have been extensively studied and well established in the development and survival of midbrain dopamine neurons. Since Nurr1 and other NR4A members are widely expressed in the brain in overlapping and distinct manners, it has been an open question whether Nurr1 has important function(s) in other brain areas. Recent studies suggest that up-regulation of Nurr1 expression is critical for cognitive functions and/or long-term memory in forebrain areas including hippocampal formation. Questions remain about the association between Nurr1 expression and Alzheimer’s disease (AD) brain pathology. Here, using our newly developed Nurr1-selective antibody, we report that Nurr1 protein is prominently expressed in brain areas with Aβ accumulation, i.e., the subiculum and the frontal cortex, in the 5XFAD mouse and that Nurr1 is highly co-expressed with Aβ at early stages. Furthermore, the number of Nurr1-expressing cells significantly declines in the 5XFAD mouse in an age-dependent manner, accompanied by increased plaque deposition. Thus, our findings suggest that altered expression of Nurr1 is associated with AD progression.
Keywords: Alzheimer’s disease, Nurr1, NR4A2, 5XFAD mice, amyloid beta, plaque deposition
Introduction
The orphan nuclear receptor Nurr1 belongs to the nuclear receptor subfamily 4A (NR4A) comprising NR4A1, NR4A2, and NR4A3 (also known as Nur77, Nurr1, and Nor1) (Hawk & Abel 2011). Nurr1 was identified as a novel transcription factor in 1992 (Law et al. 1992), and its function in the central nervous system was elucidated through knockout mouse studies (Zetterstrom et al. 1997). These initial studies established that Nurr1 is essential for generation of midbrain dopamine (mDA) neurons in the substantia nigra and the ventral tegmental area. In addition, a conditional knockout study showed that inactivation of Nurr1 in adult midbrain area leads to loss of mDA neuron-specific gene expression and neuron degeneration (Kadkhodaei et al. 2009). Thus, based on these seminal reports, Nurr1’s function has been mostly studied in the context of the mDA neuronal system and DA-related brain disorders such as Parkinson’s disease (PD) (Decressac et al. 2013).
Since Nurr1 is expressed in diverse brain areas beyond the mDA neuronal area (Law et al. 1992), it is possible that Nurr1 has important functional roles in non-DA areas. Indeed, multiple lines of evidence suggest that Nurr1 plays important roles in various brain functions. For instance, Nurr1 expression is up-regulated in the hippocampus following memory-inducing activities such as learning, or other hippocampus-dependent tasks (Pena de Ortiz et al. 2000, Vecsey et al. 2007), suggesting Nurr1’s involvement in learning and memory. Indeed, several laboratories showed that lowering Nurr1’s level in the hippocampus impairs long-term memory and/or synaptic plasticity (Colon-Cesario et al. 2006, McQuown et al. 2011, Hawk et al. 2012, Bridi & Abel 2013). These interesting studies indicating Nurr1’s prominent role in learning and memory prompted us to hypothesize that Nurr1 is directly or indirectly involved in the pathogenic mechanisms of cognition-related diseases such as Alzheimer’s disease (AD).
Toward this long-term goal, it is critical to identify Nurr1 expression at cellular level and distinguish it from those of other NR4A members. Thus, we developed a highly selective antibody against Nurr1 with undetectable cross-reactivity with other NR4A members (see below) and investigated the expression pattern of Nurr1 in wild-type and an animal model of AD, 5XFAD mice, of different ages and correlated them to amyloid deposition. Our study, for the first time to our knowledge, establishes that (1) Nurr1 protein is highly expressed in the subiculum and cortical layer 5/6, two regions showing most prominent amyloid deposits in the 5XFAD animal model of AD, and (2) the number of Nurr1-expressing cells is significantly reduced during disease progression in 5XFAD mice. Therefore, this study reveals a highly specific co-expression of Nurr1 and Aβ in pathological areas in an AD model, suggesting that Nurr1 is implicated in AD pathogenesis.
Materials and methods
Development and characterization of Nurr1-selective antibody without cross-reactivity to Nor-1 or Nur77
Rabbit anti-Nurr1, anti-Nor1, and anti-Nur77 antibodies were generated against recombinant mouse Nurr1-LBD, Nor1-LBD, and Nur77-LBD proteins, respectively. Nurr1-LBD, Nor1-LBD, and Nur77-LBD were amplified by PCR using 5′-AAA AAA CAT ATG GTT AAA GAA GTG GTT CG-3′ and 5′-GTC AAT CTC GAG TTA GAA AGG TAA GGT GTC CAG GAA AAG- 3′ for Nurr1 LBD, 5′-AAA CCA CAT ATG AAG AGC CCA TTA CAA CAG GAA CC-3′ and 5′-AAA AAA CTC GAG TTA GAA AGG TAG GGT GTC CAG-3′ for Nor1 LBD, and 5′-AAA CCC CAT ATG AAG CAG CCC CCA GAT GCC-3′ and 5′-AAA AAA CTC GAG TCA GAA GGG CAG CGT -3′ for Nur77 LBD (underlined letters indicate the restriction enzyme sites, NdeI and XhoI) and then subcloned into vector pET15b to express the His-tagged fusion protein. The integrity of all sequences was verified by DNA sequence analyses. The recombinant proteins were purified by Ni-NTA column chromatography (Qiagen) according to the manufacturer’s instruction. Immunizations were carried out by Covance Inc. (Denver, PA). Rabbits were immunized with 250 μg of purified Nurr1 LBD in complete Freund’s adjuvant (CFA) followed by boosts with 150 μg of proteins in incomplete Freund’s adjuvant (IFA) according to Covance’s standard immunization protocol. Anti-Nurr1 LBD titers were assessed by ELISA. Briefly, 100 μl of Nurr1 LBD (2.5 μg/ml) were used to coat the wells of a 96-well plate (Immulon® 2HB; Thermo 3455) overnight at 4°C. Plates were washed with PBS Tween 20 (0.05%) and wells filled (300 μl) with Blocking buffer consisting of 1% Normal Horse serum (NHS), 1% Normal Goat Serum (NGS) in 1X KPL milk diluent (KPL# 50-82-01). Plates were blocked for 2 hours at room temperature. Serial dilutions of immune and non-immune sera (in blocking buffer) were applied to the wells and incubated at room temperature for 1 hour. Wells were washed 3 times with PBS Tween 20 (0.05%) followed by addition of horseradish peroxidase (HRP) conjugated Goat anti-rabbit IgG (H+L, KPL; 1:8000). After 1-hour incubation at room temperature, plates were washed 3 times with PBS Tween 20 (0.05%) and rinsed with water. 100 μl of tetramethylbenzidine (TMB; Thermo 34022) was added and plates incubated in the dark for 20 minutes. The reaction was stopped by addition of 50 μl 2N H2SO4 and the optical density at 450 nm minus background at 650 nm determined. Sera cross-reactivities were assessed by coating the wells with Nor-1 or Nur77 LBDs (2.5 μg/ml) and carrying the ELISA as described. The anti-Nurr1 specific antibodies were obtained by passing rabbit sera through a Protein A Plus spin column (Thermo, 89978) followed by passage through an AminoLink column (Pierce cat# 20381) coupled with Nor1 LBD and then through an AminoLink column coupled with Nur77 LBD. The recovered antibodies were free of cross-reactivity to Nur77 or Nor-1 LBDs. Purified antibodies were stored in phosphate-buffered saline (PBS) containing 0.02% sodium azide at −20 °C.
Animals
Wild-type and transgenic mice with five familial AD mutations (5XFAD) used in this study were purchased from Jackson Laboratory (Bar Harbor, ME). 5XFAD mice express transgenes for both mutant human presenilin 1, harboring two FAD mutations (M146L and L286V), and mutant human APP (695) with the Swedish mutation (K670N, M671L), Florida mutation (I716V), and London mutation (V717I). Animal use was in accordance with McLean Hospital’s Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines.
Immunoperoxidase staining
For immunohistochemical study of Nurr1 expression in the hippocampus of C57BL/6 mice (9 weeks old), free floating sections on cover slips were briefly rinsed in PBS and treated with 1% hydrogen peroxide for 15 min to remove endogenous peroxidase activity. They were incubated overnight at 4°C with rabbit anti-Nurr1 (1:1,000 dilution) in the presence of 0.3% triton X-100 and normal goat serum. Then, brain sections were incubated with biotinylated anti-rabbit IgG (1:200 dilution; Vector Laboratories, Burlingame, CA, USA) for 90 min, followed by incubation with an avidin-biotin complex (1:100 dilution; Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. Peroxidase activity was visualized by 3,3-diaminobenzidine (DAB) in 0.05 M Tris–buffered saline (pH 7.6). They were washed three times with PBS after every incubation step. Finally, the sections were mounted on gelatin-coated slices, dehydrated, and cover-slipped using histomount medium. The cover slips were mounted on glass slides and air-dried. The images were photographed at 200x magnification using an optical light microscope (Carl Zeiss, Jena, Germany).
Immunofluorescence staining
Eight brain sections at 210–240 μm intervals were taken from each mouse from the region between −2.6 mm and −4.3 mm relative to the bregma using a mouse brain atlas (2001). To detect the immunofluorescence of Aβ and Nurr1, free-floating sections were incubated with mouse anti-Aβ antibody 4G8 (1:2,000; Covance, Princeton, NJ), rabbit anti-Nurr1 antibody (1:1,000), and mouse anti-NeuN antibody (1:1,000; EMD Millipore, Billerica, MA) overnight at 4°C. Following extensive washes in PBS, the sections were incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG (1:500; Invitrogen, Carlsbad, CA) and Alexa Fluor 594-conjugated goat anti-rabbit IgG (1:500; Invitrogen, Carlsbad, CA) for 1 h at room temperature (RT). All sections were counterstained with DAPI before mounting. The immunoreactivity was observed and analyzed with ImageJ software (Bethesda, MD, USA).
Statistical analyses
All data are shown as mean ± SEM. Statistical differences between groups were defined by one-way analysis of variance (ANOVA), followed by Fisher’s LSD post hoc test using SigmaStat for Windows Version 3.10 (Systat Software, Inc., Point Richmond, CA). A p-value < 0.05 was considered statistically significant.
Results
Generation of Nurr1-specific antibodies without cross-reactivity to Nor1 or Nur77
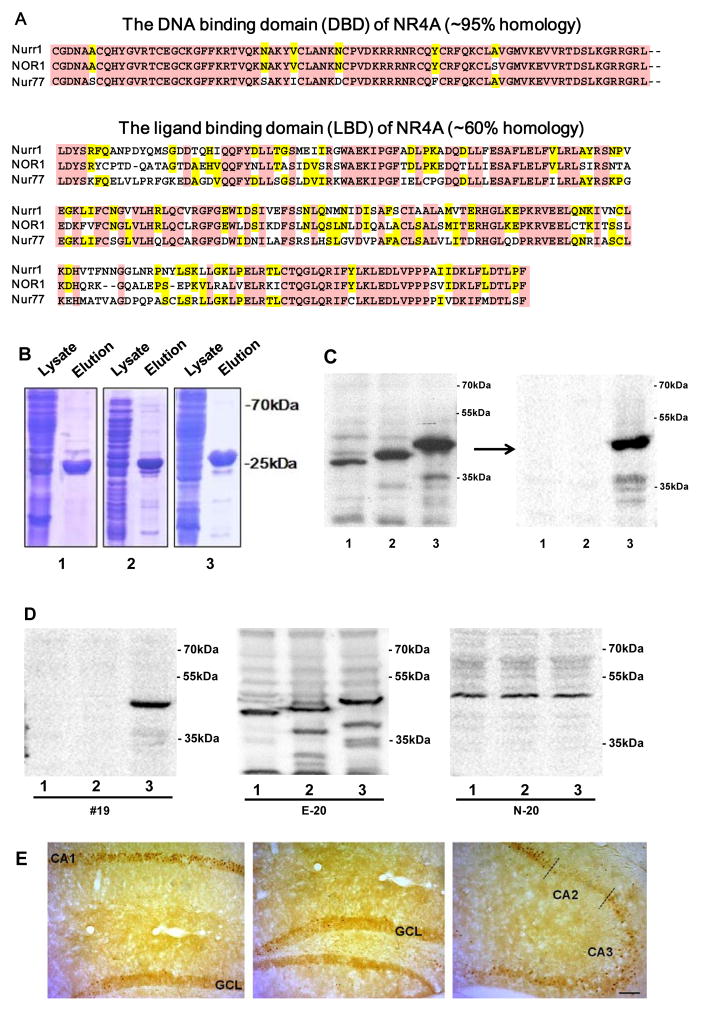
Three members of the NR4A subfamily (Nurr1, Nor1 and Nur77) are expressed in an overlapping but distinct pattern in the brain (Saucedo-Cardenas & Conneely 1996). Furthermore, they are known to play distinct and overlapping roles in diverse brain functions such as learning, survival, and stress (Fernandez et al. 2000, McNulty et al. 2012, Hawk & Abel 2011). Thus, to clearly understand the expression and the regulation of Nurr1 protein in AD, it is of paramount significance to specifically detect Nurr1 using specific antibodies against Nurr1 without cross-reactivity to Nor1 or Nur77. Toward this goal, we sought to generate highly specific antibodies against Nurr1, using a polypeptide encompassing Nurr1’s LBD (amino acid 328–598). We speculated that it is a better strategy because the DNA binding domain shares a higher homology between all NR4A members (Fig. 1A). We successfully generated a high-titer Nurr1-specific antibody using the purified Nurr1’s LBD (endpoint titers ≥ 108; Fig. 1B). However, it also showed minor cross-reactivity to Nor1 and Nur77. Thus, we attempted to further purify it by sequential pre-adsorption to affinity columns bound with the LBDs of Nor1 and Nur77, respectively. As shown in Fig. 1C, this purified antibody demonstrated that it can efficiently detect Nurr1’s LBD, with no detectable cross-reactivity to Nor1 or Nur77. In contrast, commercially available Nurr1 antibodies showed prominent cross reactivities to Nor1 and Nur77 as well as non-specific binding (Fig. 1D). To further characterize our Nurr1 antibody, we conducted immunohistochemistry with mouse brain tissues and observed clear and specific immunoreactivity against Nurr1 in the hippocampus (Fig. 1E).
Fig. 1.
(A) Murine sequence comparison and homology of the DBD and LBD domains of three NR4A members. (B) Production and isolation of Nurr1, Nor1, and Nur77 LBDs for antibody production and purification. Lane 1, Nur77-LBD; lane 2, Nor1-LBD; lane 3, Nurr1-LBD. Each LBD with histidine tag (molecular weight of 26, 27, and 30 kDa for Nur77, Nor1, Nurr1, respectively) was expressed in E. coli and purified to >90% purity by histidine tag purification. (C) Step-wise purification of Nurr1-specific antibody: Although our Nurr1 antibody exhibited strong binding to Nurr1 protein, it also showed cross-reactivity to Nor1 and Nur77 proteins. Following two consecutive pre-adsorptions against Nor1 and Nur77 LBDs, the final Nurr1 antibody did not show any detectable cross-reactivity to Nor1 and Nur77. Each lane contained 20 μg of CHO cell extracts expressing Nur77-LBD (lane 1), Nor1-LBD (lane 2), and Nurr1-LBD (lane 3). (D) Comparison of different Nurr1 antibodies cross-reactivities: While our purified #19 antibody was highly specific for Nurr1 LBD, two commercially available antibodies showed cross-reactivities to Nor1 and Nur77 LBDs. Each lane contained 20 μg of CHO cell extracts expressing Nur77-LBD (lane 1), Nor1-LBD (lane 2), and Nurr1-LBD (lane 3). (E) Immunoreactivity of Nurr1 in the hippocampus of C57BL/6 mice. Nurr1-positive cells are abundantly expressed in CA1 of the hippocampus. Hippocampal CA3 exhibited moderate numbers of Nurr1-immunoreactive cells. The granule cell layer (GCL) of the dentate gyrus and the CA2 region showed the weak Nurr1immunoreactivity. Scale bar=100 μm.
Nurr1 is highly expressed in the subiculum and deep cortical layers, two regions showing most prominent amyloid deposits in 5XFAD mice
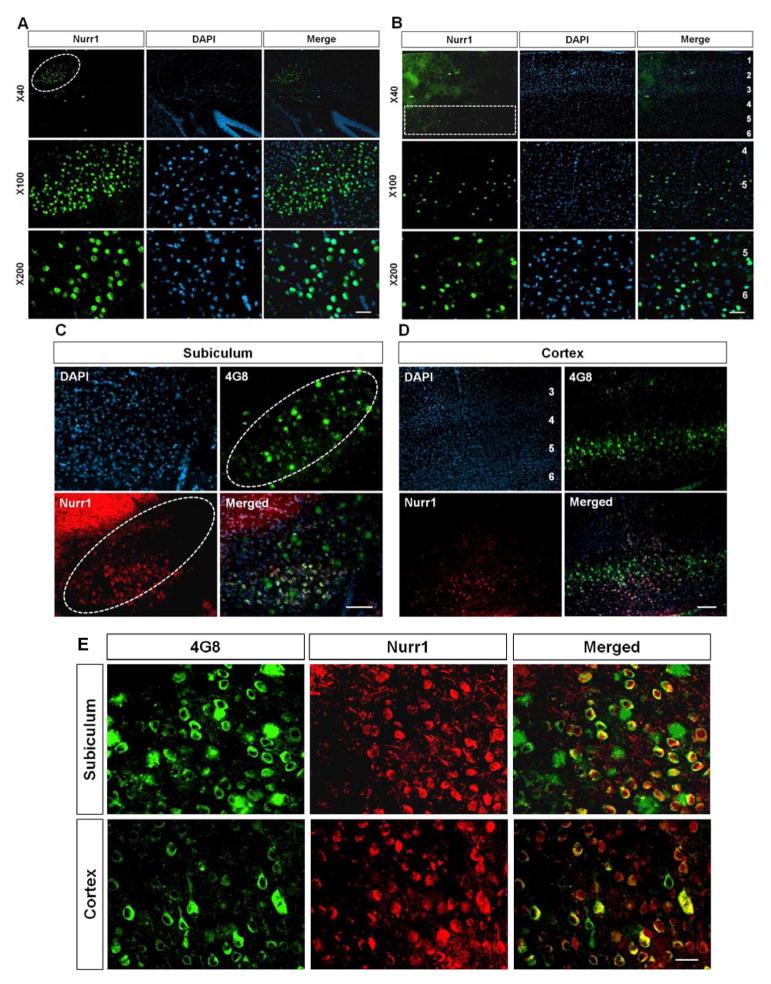
Using our specific antibody, we next examined the expression of Nurr1 protein in brain areas relevant to AD pathology. Since the subiculum and deep cortical layers prominently express Nurr1 mRNA in rodent brains (Hirokawa et al. 2008) and exhibit the highest levels of amyloid deposition in 5XFAD mice (Oakley et al. 2006, Moon et al. 2012), we tested if Nurr1 protein is highly expressed in these brain areas by performing immunohistochemistry. Remarkably, we found that Nurr1 is robustly expressed both in the subiculum (Fig. 2A and 2C) and in the deep cortical layers (Fig. 2B and 2D) of both wild-type and the early-stage brains of 5XFAD, indicating that Nurr1-expressing patterns in these areas are strikingly similar to those of amyloid deposition in 5XFAD mice. Co-localization of Nurr1 and Aβ was further validated in higher magnification images (Fig. 2E). These findings suggest the possibility that Nurr1 is directly or indirectly involved in the Aβ pathology of 5XFAD mice. Notably, it was previously reported that Nurr1 mRNA is restrictively expressed in the cerebral cortex layers 5/6 (Watakabe et al. 2007). Indeed, our immunostaining results of deep cortical layers demonstrated that Nurr1 protein is specifically detected in cortical layers 5/6 but not in other layers with almost no background staining (Fig. 2B), further validating the specificity of our Nurr1 antibody. In contrast, we could not observe this layer-specific expression of Nurr1 protein in cerebral cortex by using commercially available Nurr1 antibodies (data not shown).
Fig. 2.
Immunoreactivity of Nurr1 antibody in the subiculum (A) and the frontal cortex (B) of wild-type mice. Nurr1-expressing cells were restricted to specific cortical layers and subiculum (indicated by white circle and box). (C and D) A comparison of Nurr1-expressed areas with amyloid-deposited regions in the brain of 5XFAD mice using 4G8 antibody and Nurr1 antibody. Nurr1-positive cells are detected in subiculum (C, indicated by dotted white circle) and specific cortical layers 5/6 (D) of 2 month-old 5XFAD mice. Remarkably, Nurr1 is highly expressed in two amyloid-deposited regions, deep cortical layers and subiculum of 5XFAD mice. (E) Representative high magnification images of Nurr1 and 4G8 double labeling in the subiculum and cerebral cortex of 2 months old 5XFAD mice. Scale bar=20 μm (A–C and E) and 100 μm (D).
Nurr1-containing cells are degenerated during AD progression in a region-specific manner
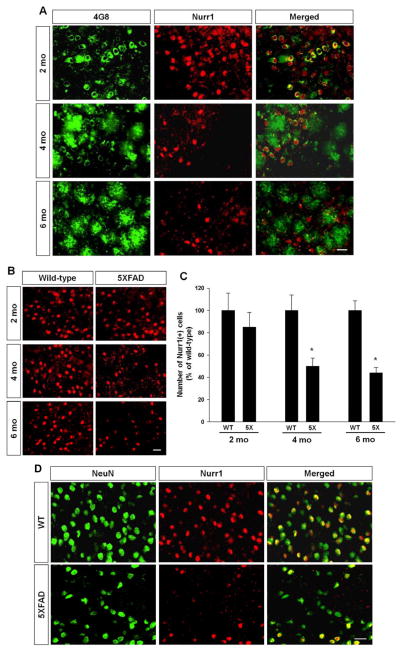
We next addressed whether Nurr1 expression is altered in the subiculum during disease progression. We stained the brains of 2, 4, and 6 month-old 5XFAD mice with antibodies against Nurr1 and Aβ and found that the number of Nurr1-postive cells robustly decreased in the subiculum with age (Fig. 3A and 3B), and concomitantly, the number of 4G8-stained cells and plaques increased in the same regions (Fig. 3A). Next, we counted the number of Nurr1-immunoreactive cells in the subiculum of wild-type and 5XFAD mice at 2, 4 and 6 months of ages (Fig. 3C). Our quantification revealed that there is no significant difference in the numbers of Nurr1-expressing cells in the subiculum of 2 month-old wild-type and 5XFAD mice although there was a declining trend in the subiculum of 5XFAD mice (Fig. 3C). However, there was a prominent decrease of Nurr1-expressing cells in the subiculum of 4 and 6 month-old 5XFAD mice, compared to those of wild-type littermates (Fig. 3B). Together, the decrease of Nurr1-expressing cells appears to be age-dependent with approximately 51% and 57% Nurr1-expressing cells in 4-month and 6-month old 5XFAD mice, respectively, compared to wild-type mice of the same age (Fig. 3C). When we performed double labeling of the neuronal marker NeuN (neuronal nuclear antigen) and Nurr1, we found there is a significant neuronal loss in the subiculum of 6 month-old 5XFAD mice, paralleled with the loss of Nurr1-positive cells (Fig. 3D). We next examined whether Nurr1-positive neurons are similarly degenerated in the ventral midbrain areas of 5XFAD mice. As expected, this ventral midbrain region of 5XFAD mice was devoid of amyloid deposits (Fig. 4A). In addition, there was no reduction of Nurr1-positive cells in ventral midbrain of 5XFAD mice, compared with wild-type littermates (Fig. 4B).
Fig. 3.
Temporal changes of Aβ deposits and Nurr1-positive cell density in the subiculum area during disease progression. (A) Representative pictures of Nurr1 and 4G8 double-labeling in the subiculum of 5XFAD mice at 2, 4 and 6 months of ages. Scale bar=20 μm. (B) Representative figures of Nurr1-positive cells in the subiculum of 5XFAD mice. Scale bar=20 μm. (C) Quantification of Nurr1-positive cells in the subiculum of wild-type littermates and 5XFAD mice (n=4~5). * < 0.05 indicates that mean value was significantly different from wild-type mice. WT: wild-type littermates of 5XFAD mice, 5X: 5XFAD mice. (D) Nurr1 and NeuN double labeling in the subiculum of 6 months-old 5XFAD and wild-type (WT) mice. The number of NeuN-positive cells decreased concomitantly with reduction of Nurr1-positive cells in the subiculum of 5XFAD mice. Scale bar=25 μm.
Fig. 4.
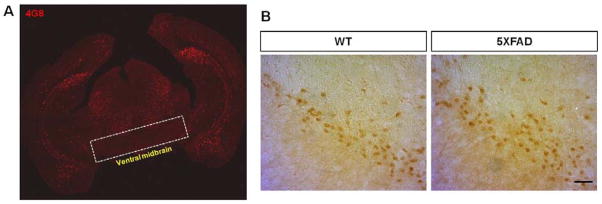
Nurr1 expression in the ventral midbrain of 5XFAD mice. (A) The distribution pattern of 4G8-stained amyloid plaques in the coronal brain section of 4 month-old 5XFAD mice. The ventral midbrain, in which dopaminergic substantia nigra neurons and ventral tegmental area neurons are located, remains devoid of amyloid deposits in 5XFAD mice. (B) Nurr1-immunoreactive cells in substantia nigra of wild-type (WT) and 5XFAD mice. There was no reduction of Nurr1-expressing cells in ventral midbrain of 5XFAD mice at 4 months of age, compared with wild-type littermates. Scale bar=50 μm.
Discussion
Despite emerging evidence of Nurr1’s functional roles in learning and memory, there has been no study examining Nurr1’s expression and regulation in brain areas with direct relevance to AD during disease progression. Using a highly specific antibody against Nurr1 without detectable cross reactivity to Nor1 or Nur77, we here show that Nurr1 is highly expressed in the subiculum and the cortical layers 5/6, two brain regions that show most prominent amyloid deposits and degeneration in 5XFAD mouse model of AD. Furthermore, we demonstrated that Nurr1 is highly co-expressed with Aβ and that there is significant decrease in Nurr1 protein expression in AD brains, compared with control brain.
Although the exact mechanisms of AD pathogenesis are not fully understood, Aβ is considered to have a critical role in AD-related pathologies, such as neurodegeneration and cognitive decline (LaFerla et al. 2007). The selective neurodegeneration in AD brain might result from the selective accumulation of Aβ. Among numerous AD models, the 5XFAD mouse is very useful due to its selective plaques deposition and intracellular Aβ accumulation, showing the highest levels of Aβ in the subiculum and the cortical layers 5/6 (Oakley et al. 2006). Strikingly, our results demonstrate that Nurr1 is also highly expressed in the subiculum and the deep cortical layers of the 5XFAD mouse brain. Additionally, at early stages of Aβ deposition, the majority of Nurr1-expressing cells are highly co-localized with the APP or Aβ, as examined by 4G8 staining of the brain of 5XFAD mice (Fig. 2E and Fig. 3A). These similar expression patterns and colocalization of Nurr1 and Aβ imply that Nurr1 may be directly or indirectly involved in the APP processing or Aβ generation in the brain of 5XFAD mice.
At present, it is not clear whether Nurr1 plays a beneficial/protective role, a harmful/pathological role, or no role in AD-related brain areas for Aβ accumulation. One scenario is that Nurr1 contributes to Aβ synthesis in the subiculum and the cortical layer 5/6, leading to selective degeneration of these Nurr1+ and Aβ+ cells. Alternatively, another possibility is that Nurr1 plays a protective role in Aβ+ cells at early stages but these neurons eventually degenerate in aging brains of AD. While this question awaits further investigation, it is worthwhile to note that Nurr1 has been known to be specifically down regulated in substantia nigra (SN) DA neurons with α-synuclein inclusions in human brain with PD (Chu et al. 2006). Since the loss of Nurr1 is restricted to DA neurons with signs of pathology, it has been suggested that Nurr1 may contribute to the disease process (Decressac et al. 2013). It was also reported that Nurr1 expression is compromised in SN DA neurons of AD patient brain (Chu et al. 2006). However, it is unknown if Nurr1 is selectively expressed or regulated in neurons with signs of pathology, non-DA neurons, or extra-mesencephalic areas of AD brain during disease progression. We found that neuronal loss concomitantly occurs with down-regulation of Nurr1 in the hippocampal formation of brains with AD (Fig. 3D). In contrast, there is no reduction of Nurr1-expressing cells in ventral midbrain of 5XFAD mice, compared with wild-type littermates (Fig. 4), strongly suggesting that degeneration of Nurr1-positive cells occurs in a region-specific manner in AD brain.
This study, for the first time to our knowledge, reports that Nurr1 expression is significantly compromised in brain areas that are highly relevant to AD pathogenesis in 5XFAD mice. In particular, it has been suggested that reduced expression or knockdown of Nurr1 results in deficits of cognitive function (Colon-Cesario et al. 2006, McQuown et al. 2011, Hawk et al. 2012). Moreover, it has been demonstrated that neuronal loss can be caused by decreased expression of Nurr1 and prevented by up-regulation of Nurr1 (Barneda-Zahonero et al., Volakakis et al., Zhang et al. 2009, Le et al. 1999, Lin et al., Sousa et al. 2007, Pan et al. 2008, Decressac et al. 2012). Taken together, these observations indicate that reduced Nurr1 expression in hippocampal formation is an important cause of memory and neuronal loss in the AD brain. Further investigation is warranted to investigate if there is significant difference in Nurr1 protein expression between normal and AD human brains, as found in AD animal model. In these future studies, our Nurr1-specific antibody will be a useful tool to address behavioral, pathological or physiological effects of altered Nurr1 expression or activation in normal or AD brains.
Acknowledgments
This work was supported by NIH grants (NS070577 and NS084869) and a grant from Michael J. Fox Foundation.
Footnotes
The authors declare that they have no conflicts of interest.
References
- Barneda-Zahonero B, Servitja JM, Badiola N, Minano-Molina AJ, Fado R, Saura CA, Rodriguez-Alvarez J. Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA) receptor-mediated neuronal survival. J Biol Chem. 287:11351–11362. doi: 10.1074/jbc.M111.272427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridi MS, Abel T. The NR4A orphan nuclear receptors mediate transcription-dependent hippocampal synaptic plasticity. Neurobiol Learn Mem. 2013;105:151–158. doi: 10.1016/j.nlm.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Le W, Kompoliti K, Jankovic J, Mufson EJ, Kordower JH. Nurr1 in Parkinson’s disease and related disorders. J Comp Neurol. 2006;494:495–514. doi: 10.1002/cne.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Cesario WI, Martinez-Montemayor MM, Morales S, et al. Knockdown of Nurr1 in the rat hippocampus: implications to spatial discrimination learning and memory. Learn Mem. 2006;13:734–744. doi: 10.1101/lm.407706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Bjorklund A. alpha-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med. 2012;4:163ra156. doi: 10.1126/scitranslmed.3004676. [DOI] [PubMed] [Google Scholar]
- Decressac M, Volakakis N, Bjorklund A, Perlmann T. NURR1 in Parkinson disease--from pathogenesis to therapeutic potential. Nat Rev Neurol. 2013;9:629–636. doi: 10.1038/nrneurol.2013.209. [DOI] [PubMed] [Google Scholar]
- Fernandez PM, Brunel F, Jimenez MA, Saez JM, Cereghini S, Zakin MM. Nuclear receptors Nor1 and NGFI-B/Nur77 play similar, albeit distinct, roles in the hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141:2392–2400. doi: 10.1210/endo.141.7.7562. [DOI] [PubMed] [Google Scholar]
- Hawk JD, Abel T. The role of NR4A transcription factors in memory formation. Brain Res Bull. 2011;85:21–29. doi: 10.1016/j.brainresbull.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk JD, Bookout AL, Poplawski SG, Bridi M, Rao AJ, Sulewski ME, Kroener BT, Manglesdorf DJ, Abel T. NR4A nuclear receptors support memory enhancement by histone deacetylase inhibitors. J Clin Invest. 2012;122:3593–3602. doi: 10.1172/JCI64145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa J, Watakabe A, Ohsawa S, Yamamori T. Analysis of area-specific expression patterns of RORbeta, ER81 and Nurr1 mRNAs in rat neocortex by double in situ hybridization and cortical box method. PLoS One. 2008;3:e3266. doi: 10.1371/journal.pone.0003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadkhodaei B, Ito T, Joodmardi E, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Law SW, Conneely OM, DeMayo FJ, O’Malley BW. Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol. 1992;6:2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- Le W, Conneely OM, He Y, Jankovic J, Appel SH. Reduced Nurr1 expression increases the vulnerability of mesencephalic dopamine neurons to MPTP-induced injury. J Neurochem. 1999;73:2218–2221. [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Sgobio C, et al. Conditional expression of Parkinson’s disease-related mutant alpha-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 32:9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty SE, Barrett RM, Vogel-Ciernia A, Malvaez M, Hernandez N, Davatolhagh MF, Matheos DP, Schiffman A, Wood MA. Differential roles for Nr4a1 and Nr4a2 in object location vs. object recognition long-term memory. Learn Mem. 2012;19:588–592. doi: 10.1101/lm.026385.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, et al. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon M, Hong HS, Nam DW, Baik SH, Song H, Kook SY, Kim YS, Lee J, Mook-Jung I. Intracellular amyloid-beta accumulation in calcium-binding protein-deficient neurons leads to amyloid-beta plaque formation in animal model of Alzheimer’s disease. J Alzheimers Dis. 2012;29:615–628. doi: 10.3233/JAD-2011-111778. [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Zhu W, Zhao H, Deng H, Xie W, Jankovic J, Le W. Nurr1 deficiency predisposes to lactacystin-induced dopaminergic neuron injury in vitro and in vivo. Brain Res. 2008;1222:222–229. doi: 10.1016/j.brainres.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Pena de Ortiz S, Maldonado-Vlaar CS, Carrasquillo Y. Hippocampal expression of the orphan nuclear receptor gene hzf-3/nurr1 during spatial discrimination learning. Neurobiol Learn Mem. 2000;74:161–178. doi: 10.1006/nlme.1999.3952. [DOI] [PubMed] [Google Scholar]
- Saucedo-Cardenas O, Conneely OM. Comparative distribution of NURR1 and NUR77 nuclear receptors in the mouse central nervous system. J Mol Neurosci. 1996;7:51–63. doi: 10.1007/BF02736848. [DOI] [PubMed] [Google Scholar]
- Sousa KM, Mira H, Hall AC, Jansson-Sjostrand L, Kusakabe M, Arenas E. Microarray analyses support a role for Nurr1 in resistance to oxidative stress and neuronal differentiation in neural stem cells. Stem Cells. 2007;25:511–519. doi: 10.1634/stemcells.2006-0238. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volakakis N, Kadkhodaei B, Joodmardi E, Wallis K, Panman L, Silvaggi J, Spiegelman BM, Perlmann T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc Natl Acad Sci U S A. 107:12317–12322. doi: 10.1073/pnas.1007088107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A, Ichinohe N, Ohsawa S, Hashikawa T, Komatsu Y, Rockland KS, Yamamori T. Comparative analysis of layer-specific genes in Mammalian neocortex. Cereb Cortex. 2007;17:1918–1933. doi: 10.1093/cercor/bhl102. [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wang P, Ren H, Fan J, Wang G. NGFI-B nuclear orphan receptor Nurr1 interacts with p53 and suppresses its transcriptional activity. Mol Cancer Res. 2009;7:1408–1415. doi: 10.1158/1541-7786.MCR-08-0533. [DOI] [PubMed] [Google Scholar]