Abstract
Hypoxia in ischemic limbs typically initiates angiogenic and inflammatory factors to promote angiogenesis in attempt to restore perfusion. There is a gap in our knowledge concerning the role of anti-inflammatory interleukins in angiogenesis, macrophage polarization, and endothelial cell activation. Interleukin-19 is a uniquely anti-inflammatory Th2 cytokine that promotes angiogenic effects in cultured endothelial cells (EC); the purpose of this study was to characterize a role for IL-19 in restoration of blood flow in hind-limb ischemia, and define potential mechanisms. Hindlimb ischemia was induced by femoral artery ligation, and perfusion quantitated using Laser Doppler Perfusion Imaging (LDPI). Wild type mice which received i.p. injections of rIL-19 (10ng/g/day) showed significantly increased levels of perfusion compared to PBS controls. LDPI values were significantly decreased in IL-19−/− mice when compared to wild type mice. IL-19−/− mice injected with rIL-19 had significantly increased LDPI compared with PBS control mice. Significantly increased capillary density was quantitated in rIL-19 treated mice, and significantly less capillary density in IL-19−/−. Multiple cell types participate in IL-19 induced angiogenesis. IL-19 treatment of human microvascular EC induced expression of angiogenic cytokines. M2 macrophage marker and VEGF-A expression were significantly increased in macrophage and spleen from rIL-19 injected mice, and M1 marker expression was significantly increased in spleen from IL-19−/− compared with controls. Plasma VEGF-A levels are higher in rIL-19 injected mice. IL-19 decreased expression of anti-angiogenic IL-12 in spleen and macrophage. This study is the first to implicate IL-19 as a novel pro-angiogenic interleukin and suggests therapeutic potential for this cytokine.
Keywords: angiogenesis, interleukin-19, macrophage polarization, endothelial cell, hind limb ischemia
Introduction
Peripheral artery disease (PAD) is often associated with diabetes and coronary artery disease, leading to significant morbidity (amputation) and mortality (myocardial infarction) in patients. Identification and characterization of molecules which can not only limit tissue inflammation but also increase capillary density, collateral formation and perfusion have the potential to salvage ischemic tissue and can lead to new therapies for tissue repair and neovascularization. Hypoxia in ischemic limbs typically initiates angiogenic and inflammatory factors to promote angiogenesis in attempt to restore perfusion, and accordingly ischemic revascularization is a complex process involving multiple processes and cell types. While neovascularization and inflammation are independent biological processes, they are linked in response to injury and ischemia, and both inflammatory and anti-inflammatory cytokines participate in these processes. Endothelial cell (EC) paracrine and autocrine stimulation can result in migration and proliferation, and is an essential component of normal and pathophysiological processes including, wound healing, and angiogenesis1–3. In addition to well characterized angiogenic cytokines like VEGF, FGF, and CXCL1, it is accepted that many pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and IL-18 increase EC migration, proliferation, tube formation, and increased vascularity in vivo4–6. One exception is Interleukin-12, which is both pro-inflammatory and potently anti-angiogenic7. On the other hand, the role of and direct effects of anti-inflammatory interleukins on EC in initiation of angiogenesis are less clear. The prototypical anti-inflammatory cytokine, IL-10, has anti-angiogenic activity and is associated with VEGF down regulation, reduction of FGF and VEGF induced proliferation of microvascular EC8. Similarly, IL-4 can inhibit VEGF production and reduce vascularization, but can also induce migration and tube like structure formation in EC, activities consistent with angiogenesis9–11. IL-13 attenuates EC tube formation, and IL-20 has both pro and antiangiogenic effects12–15. Macrophage also participate in angiogenesis as the M2, or alternatively activated macrophage express several pro-angiogenic cytokines and thus must be included in any discussion of angiogenesis in vivo16,17. In summary, direct pro-angiogenic effects on EC, polarization of macrophage M2 phenotype, and inhibition of anti-angiogenic cytokines all are recognized pathways leading to angiogenesis; a modality which could reduce inflammation but not impair revascularization would have obvious clinical benefits.
Interleukin-19 (IL-19) was discovered in 200118, and is considered to be part of the IL-10 sub-family which includes IL-20, IL-22 and IL-24.19,20. IL-19 promotes an anti-inflammatory Th2 rather than the Th1 response in T-lymphocytes21,22. Unlike IL-10, IL-19 expression and activity is not restricted to leukocytes and is rather unique among interleukins. For example, neither IL-10, IL-4, nor IL-33 are expressed by EC or vascular smooth muscle cells (VSMC), precluding potential autocrine effects of these interleukins on the vasculature23. Little is reported regarding IL-19 effects on macrophage.
We recently reported that IL-19 was expressed in angiogenic tissue, and has potent pro-angiogenic effects on multiple human EC types, (umbilical vein, coronary artery, and microvascular)24. This manuscript reported that IL-19 is chemotactic and mitogenic for EC, promotes tube-like structure formation on Matrigel, and microvessel formation in the mouse aortic ring assay. These inaugural studies, though novel, were all cell culture or ex vivo based, and lacked validation in a relevant in vivo model of angiogenesis. Rodent hindlimb ligation and ischemia is a well-established model for induction of neovascularization in vivo25,26. In the present study, multiple but complementary approaches were used to determine if IL-19 regulated neovascularization in the hind-limb ischemia model. In this manuscript we determine that in contrast to IL-10, IL-19 can increase perfusion in ischemic hind limbs and exerts its angiogenic effects by at least three mechanisms: direct effects on EC gene expression; local and systemic M2 macrophage polarization and VEGF-A expression, and suppression of IL-12 expression in macrophage. Together, this implicates IL-19 as a link for two major processes; anti-inflammation and angiogenesis, and could identify IL-19 as a previously unrecognized proangiogenic modality in treatment of PAD.
Materials and methods
Animals
Wild type C57BL/6 mice were purchased from Jackson Labs. IL-19 knockout mice were generated using the VelociGene method and IL-19−/− mice identified by genotyping of tail DNA by PCR using specific primers as we described27. Age and sex-matched male and female littermates were used for these studies. The hind limb ischemia model was performed as described28. Briefly, mice were anesthetized by injection of ketamine and xylazine, the femoral artery is dissected from the femoral vein, the artery occluded at two points using double knots, and the femoral artery between these knots excised. Ten ng/g/day recombinant murine IL-19 was administered i.p. 24 hours post-surgery. Laser Doppler scanning was performed by an operator blinded to the identity of each mouse, immediately following surgery, and at days 3, 7, 10, and 15 days post-surgery using a Laser Doppler Imager (Moor Instruments, Cambridge, UK). After 15 days, mice were euthanized and gastrocnemius muscle prepared for immunohistochemistry. Some mice were injected i.p. with 10ng/g/day murine rIL-19 (eBioscience) or an equivalent volume of PBS five days per week for the duration of the study. All animal procedures followed Temple University-IACUC approved protocols.
Immunohistochemistry
Five-micrometer sections from paraffin embedded gastrocnemius muscle were blocked in 10% goat serum. Sections were incubated with primary antibody (anti-CD31 and anti-IL-19, from AbCam, Inc) at 1µg/ml in 1%BSA/PBS and were applied for 1 hr., followed by incubation with biotinylated secondary antibody (1:200), followed by avidin-biotin peroxidase complex each for 30 min as we have described24,27. Non-specific identical isotype control (Neomakers # NC-100-P, and Biolegend #400601) antibodies were used as negative controls. Macrophage were identified by immunostaining with anti-CD68 antibody (BioRad, Inc), and quantitated by manual counting of 9 randomly chosen HPFs from at least three different sections of ischemic hind limb of 6 animals per group 5 days post ligation surgery. For quantitation of capillary density, three transverse serial sections of gastrocnemius muscle spaced 100–200µm apart were immunostained from at least 6 mice in each group. Capillaries (CD31 positive structures surrounding a lumen) were counted from three per high powered fields per section and reported per mm2.
Human microvascular EC culture
Human microvascular endothelial cells (hmvEC) were obtained from Lonza, Inc. and cultured in growth media from the manufacturer as we described24. Cells from passage 2 to 4 were used. For gene expression studies, growth media was replaced with basal media supplemented with 1% fetal calf sera for 24 hours, then stimulated with 100ng/ml IL-19 for the times indicated.
Bone Marrow derived macrophage
To generate BMDM, mice femurs and tibiae were flushed with sterile DMEM. After lysis of red blood cells, total BM cells were plated at a density of 3,5 × 106 cells per 10-cm Petri dish in 10 ml macrophage growth medium (complete DMEM medium with 10% FBS and 100ng/ml M-CSF (Peprotech Inc.) and were allowed to differentiate for 5–7 days. Cells were fed with additional 5 ml of growth medium on day 3. On day 7, cells were lifted with Versene 1× solution (GIBCO) at 37°C and were re-plated in 12- or 6-well plates (1 × 106 cells per ml per well (12-well) or 2 × 106 cells per 3ml per well (6-well)) in macrophage complete media (DMEM+10%FBS).
RNA extraction and quantitative RT-PCR
RNA from cultured cells, hind limb, or spleen was isolated and reverse transcribed into cDNA as we have described, and target genes amplified using an Eppendorf Realplex4 Mastercycler27,29. Multiple mRNAs (Ct values) were quantitated simultaneously by the Eppendorf software. Gene expression in IL-19-stimulated cultured human mvEC was performed using the human angiogenesis RT2 Profiler PCR array from SABiosciences as described by the manufacturer. Primer pairs were purchased from Integrated DNA Technologies, (Coralville, IA), SYBR green used for detection. The following primer pairs were used: Mouse GAPDH: F: GCAAGGACACTGAGCAAGAG, R: GGGTCTGGGATGGAAATTGT, Mouse Arginase 1:F: AAGAATGGAAGAGTCAGTGTGG, R: GGGAGTGTTGATGTCAGTGTG Mouse Arginase 2: F: CAGAAGGTGATGGAACAGACA, R: GCCAGTTTAGGGTCAAATGC Mouse Ym1: F: AGAGTGCTGATCTCAATGTGG, R: GGGCACCAATTCCAGTCTTAG Mouse KLF4: F: ACTTGTGACTATGCAGGCTG, R: ACAGTGGTAAGGTTTCTCGC Mouse VEGF-A: F: GGCAGCTTGAGTTAAACGAAC, R: TGGTGACATGGTTAATCGGTC Mouse IL-12p40: F: GTGAAGCACCAAATTACTCCG, R: AGAGACGCCATTCCACATG Human GAPDH: F: CGAGAGTCAGCCGCATCTT, R: CCCCATGGTGTCTGAGCG, Human IL-8: F: CCAGGAAGAAACCACCGGA, R: GAAATCAGGAAGGCTGCCAAG Human HGF: F: ATCAAATGTCAGCCCTGGAG, R:CCTCTGGATTGCTTGTGAAAC Human CXCL1: F: TGCTCCTGCTCCTGGTAG, R: CTTCTGGTCAGTTGGATTTGTC
Western blotting
Protein extracts from hind limb, cultured hmvEC and BMDM were made as described separated by SDS-PAGE, transferred to nitrocellulose membrane, incubated with a 1:4000 dilution of primary antibody (HGF, IL-8, CXCL1, VEGFA, IL-12, IL-19, CD68, GAPDH, AbCam, Inc), and a 1:7000 dilution of secondary antibody27,29. Equal loading of protein extracts on gels was verified by Ponceau S staining of the membrane, and blotting with the housekeeping protein anti-GAPDH (1:7000 dilution, Biogenesis, Inc.), and reactive proteins were visualized using enhanced chemiluminescence. The intensity of each band was quantitated using image analysis software (NIH Image, Frederick, MD). ELISA for VEGF-A was purchased from R&D, Inc, and VEGF-A in plasma from mice treated with either rIL-19 or saline was detected according to manufacturer’s instructions.
Statistical analysis
Results are expressed as mean ± SEM. Differences between groups were evaluated with the use of ANOVA or by paired t tests where appropriate. Interquartile range determined by the GraphPad Prism statistical analysis program as we described27,29. Differences were considered significant when p<0.05.
Results
IL-19 expression is induced in murine ischemic hindlimb
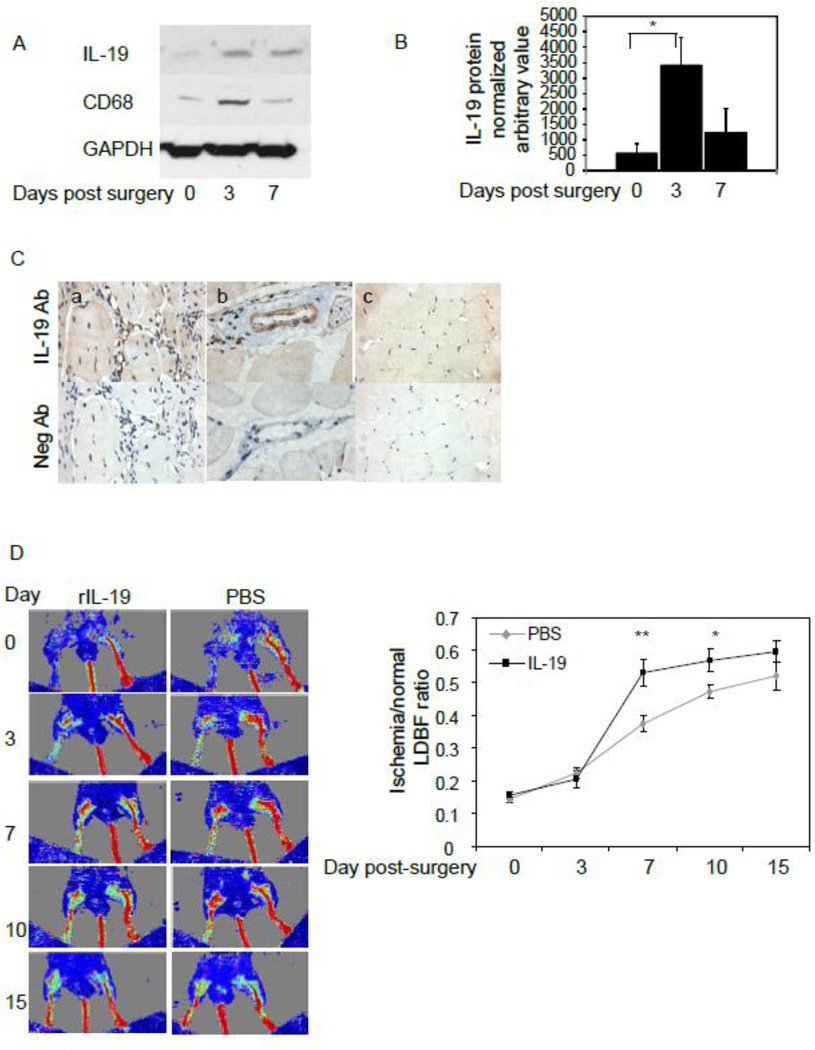
IL-19 expression is induced in angiogenic tissue24, and initial experiments validated IL-19 expression in ischemic murine hind limb. Figure 1A and B shows that IL-19 expression is very low, but detectible in non-ischemic hind limb control, but is significantly increased by 505.8% 3 days post-ligation surgery. IL-19 expression declines by 7 days post-ligation surgery but remains elevated above baseline levels. Macrophage are detected 3 days post-ligation, and decrease, but remain detectable 7 days post-ligation surgery. Immunohistochemistry was performed to determine the cell types which expresses IL-19 in ligated hind limbs. Figure 1C shows that IL-19 is expressed at low levels in skeletal muscle, medial VSMC, capillaries and infiltrating macrophage in ligated hind limbs.
Figure 1.
IL-19 expression in ischemic hind limbs and IL-19 injection increases reperfusion in wild-type mice. A. Representative immunoblot of expression of IL-19 and CD68 in ischemic hindlimb. Protein extracts from gastrocnemius muscle at various times post-ligation surgery were immunoblotted with anti-IL-19 antibody. B. Densiometric quantification of three independent western blots. Asterisk indicates significant difference quantitated from 3 different western blots (P<0.05). C. Representative photomicrographs of immunohistochemical detection of IL-19 in murine hindlimb. IL-19 is expressed in capillaries (a), smooth muscle cells (b), and skeletal muscle (c). D. wild-type C57BL/6 mice were subject to hind-limb ligation surgery, followed by injection with 10ng/g/day rIL-19 or an equal volume of PBS five days per week for 15 days. Blue color represents lower perfusion and red color represents higher perfusion D. Time course of quantitation of serial blood flow measurements were performed using LDPI over a period of 15 days and ratio of flow from ischemic/non-ischemic limb quantitated. N=13 mice per group, P<0.05 or 0.01 as indicated
IL-19 can accelerate neovascularization in vivo
Based on our prior study, we hypothesized that IL-19 could increase revascularization in ischemic hind limbs. In a first series of experiments, wild-type C57BL/6 mice were subject to hind-limb ligation surgery, followed by i.p. injection the day after surgery with 10ng/g/day rIL-19 or an equal volume of PBS five days per week for 15 days. Serial blood flow measurements were performed using LDPI over a period of 15 days. Figure 1C illustrates typical time-course of flow recovery in murine ischemic hind limbs. When quantitated, Figure 1D demonstrates a significantly increased perfusion ratio in mice injected with rIL-19 compared with PBS controls. The perfusion ratio increased by 47.0% at day 7 (37.4+/−2.4% vs 53.0+/−4.0%, for PBS and IL-19, respectively, n=13, P<0.01) in mice receiving rIL-19. It remained significantly higher (22%) at day 10 (47.3+/−2.0 vs 57.8+/−3.5 for PBS and IL-19, respectively, P<0.05).
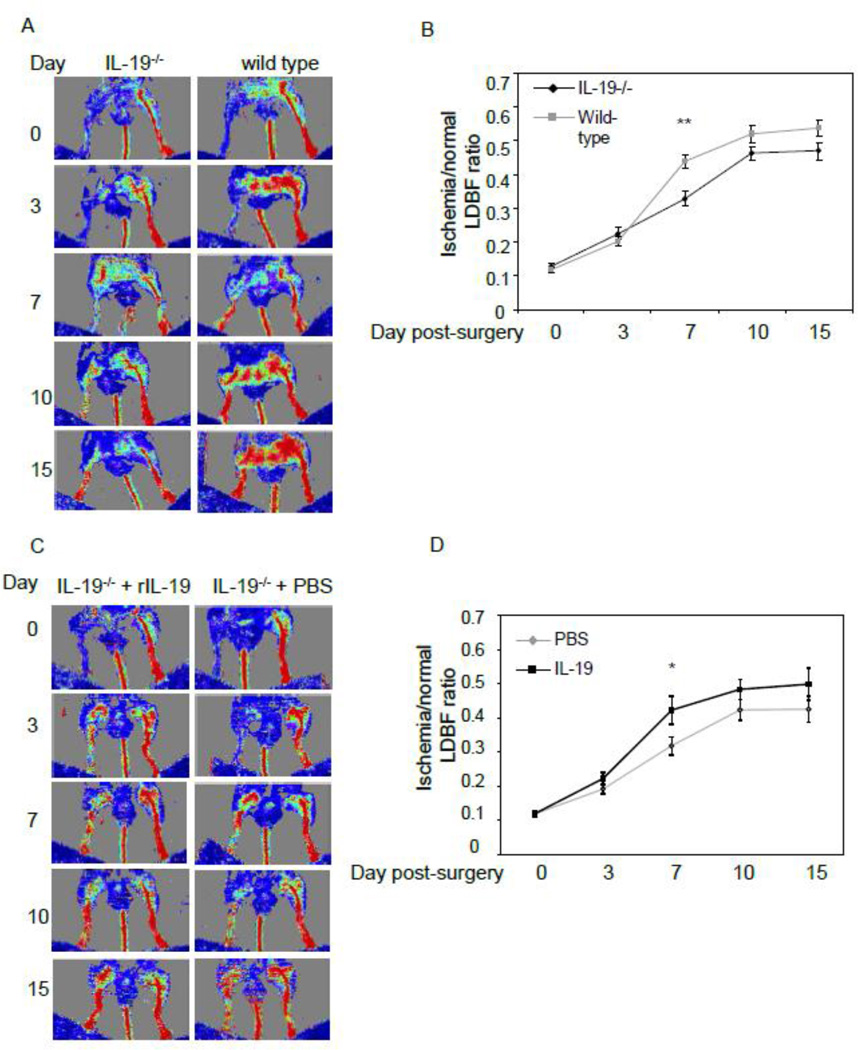
Lack of IL-19 decreases neovascularization
To determine the necessity of IL-19 in neovascularization, we next compared reperfusion in age matched IL-19−/− with wild-type littermates. Mice were ligated and Doppler imaged as described in the preceding section. Figure 2A demonstrates that mice lacking IL-19 revascularized more slowly than wild-type mice. The perfusion ratio in IL-19−/− was significantly decreased by 25.3% at day 7 (43.8+/−1.8% vs 32.7+/−2.2% for wild type and IL-19−/−, n=13, P<0.05). Reperfusion remained decreased at days 10 and 15, (P=0.08 and 0.06, respectively) (Figure 2B).
Figure 2.
A. Lack of IL-19 decreases reperfusion in C57BL/6 mice. Wild-type or IL-19−/− mice (both C57BL/6) were subject to hind-limb ligation surgery. B. Time course of quantitation of serial blood flow measurements were performed using LDPI over a period of 15 days and ratio of flow from ischemic/non-ischemic limb quantitated. N=13 mice per group, P<0.01 as indicated. C. IL-19 injection restores reperfusion in IL-19−/− mice. A. IL-19−/− mice wild-type were subject to hind-limb ligation surgery, followed by injection with 10ng/g/day rIL-19 or an equal volume of PBS five days per week for 15 days. D. Time course of quantitation of serial blood flow measurements were performed using LDPI over a period of 15 days and ratio of flow from ischemic/non-ischemic limb quantitated. N=14 mice per PBS, 13 mice for rIL-19, P<0.05.
To further confirm the role of IL-19 in regulation of neovascularization, two groups of IL-19 knockout mice were subject to femoral ligation. Some mice were injected i.p. with 10ng/g/day rIL-19 5 days per week, others with equivalent volume of PBS as controls, and reperfusion quantitated by LDPI as described in the preceding sections. IL-19−/− mice injected with rIL-19 had significantly increased reperfusion compared with PBS control mice (23.0%) at day 7 (42.3+/−4.0% vs 31.8+/−2.6% for rIL-19 and PBS, respectively, n=14, P<0.05) (Figures 2C and 2D). In non-ischemic hind limbs, capillary density remained the same between IL-19−/− and wild type controls. Since addition of IL-19 to IL-19−/− mice can increase, or rescue reperfusion, these data demonstrate IL-19 specificity and suggest that IL-19 can regulate perfusion of ischemic hind limbs.
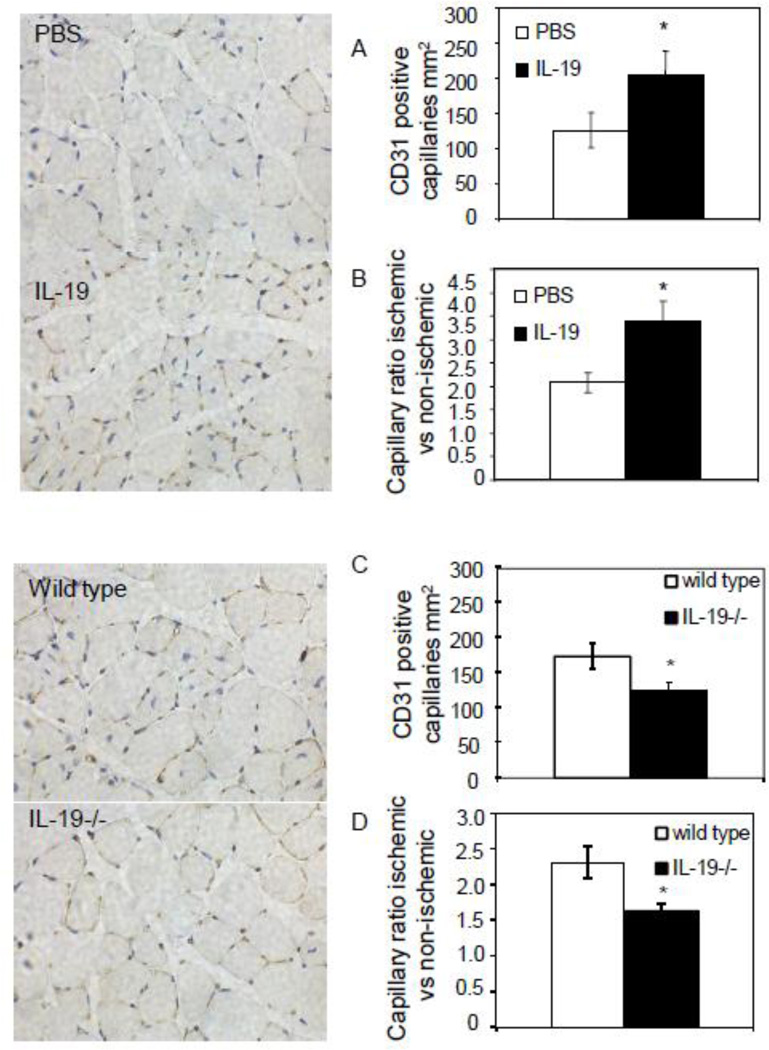
IL-19 expression regulates capillary vessel density
To gain further insight into potential mechanisms of IL-19 revascularization of ischemic hind limbs, capillary density in gastrocnemius muscle was determined by immunohistochemistry using anti-CD31 antibody. CD31-positive structures surrounding a lumen were quantitated. There was a significant increase in the number of capillaries in ligated arteries in mice injected with rIL-19 compared with controls (126.0+/−25 vs 203.15+/−34.5 per HPF for PBS and rIL-19, respectively P<0.05) (Figure 3A). Correspondingly, there was also a significant increase in the capillary ratio in ligated versus unligated control limbs in mice injected with rIL-19 (2.08+/−0.2 vs 3.38+/−0.4, for PBS and rIL-19, respectively P<0.05) (Figure 3B). Conversely, a significant decrease in capillary vessel density was observed in IL-19−/− versus wild-type controls (175.3+/−18.25 vs 126.1+/−12.05, P<0.05 for wild type and IL-19−/−, respectively) with a subsequent decrease in capillary ratio in ligated versus unligated control limbs (2.3+/−0.22 vs 1.63+/−0.95 for wild type and IL-19−/−, P<0.05 respectively) (Figures 3C and D). In non-ischemic hind limbs, capillary density remained the same between rIL-19 and PBS injected controls. Together, these data suggest IL-19 can regulate capillary density in ischemic hind limbs. The remainder of this study aimed to determine the cellular and molecular mechanisms for these effects.
Figure 3.
IL-19 regulates capillary density. Representative photomicrographs showing immunohistochemistry on gastrocnemius muscle using CD31 antibody at 15 days femoral artery ligation. A. The number of CD31 positive structures surrounding a lumen per mm2 in wild-type mice injected with rIL-19 or PBS. B. Capillary ratio ischemic/non-ischemic limbs in wild-type mice injected with rIL-19 or PBS. C. The number of capillaries in wild-type or IL-19 mice. D. Capillary ratio ischemic/non-ischemic limbs in wild-type or IL-19 mice. Capillaries in three representative 5 micron-thick stained tissue sections at least 75–100 microns apart per muscle, n=6 mice per group were quantitated, P<0.05. Photomicrographs are counter-stained with hematoxylin/eosin, magnification 400×
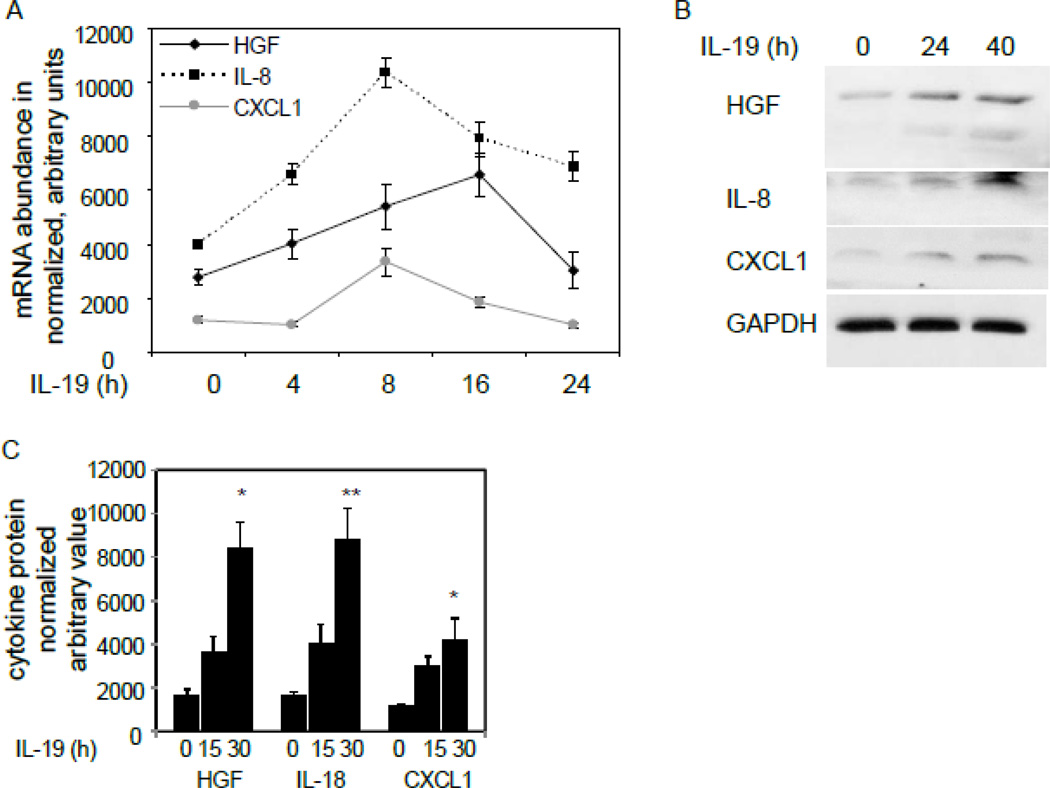
IL-19 induces angiogenic growth factor gene expression in human microvascular EC
Based on our prior study, we hypothesized that IL-19 would have direct effects on EC gene transcription. We treated human microvascular EC with IL-19 for 24 hours, and we examined gene induction with a commercially available qRT-PCR microarray as a screen (Supplemental Data Table 1). Of 96 possible angiogenesis-related genes, only three verifiable transcripts were up-regulated from 2.0 to 4.0-fold; IL-8, CXCL1, and HGF, all of which are pro-angiogenic and mitogenic for EC30. PCR array expression was verified by qRT-PCR in a time-course of IL-19-stimulated EC (Figure 4A), and was further validated at the protein level by western analysis in Figure 4B and quantitated by densiometry in 4C. This suggests that IL-19 has direct angiogenic effects on EC.
Figure 4.
IL-19 induces angiogenic gene expression in microvascular EC. A. Primary human microvascular EC were treated with IL-19 for various times, and gene induction quantitated by quantitative reverse transcription PCR. B. Representative immunoblot of cytokine protein expression in human microvascular EC. C. Densiometric quantification of three independent western blots. Asterisk indicates significant difference quantitated from 3 different western blots of IL-19 stimulated EC at 30 hours versus unstimulated EC (P<0.05).
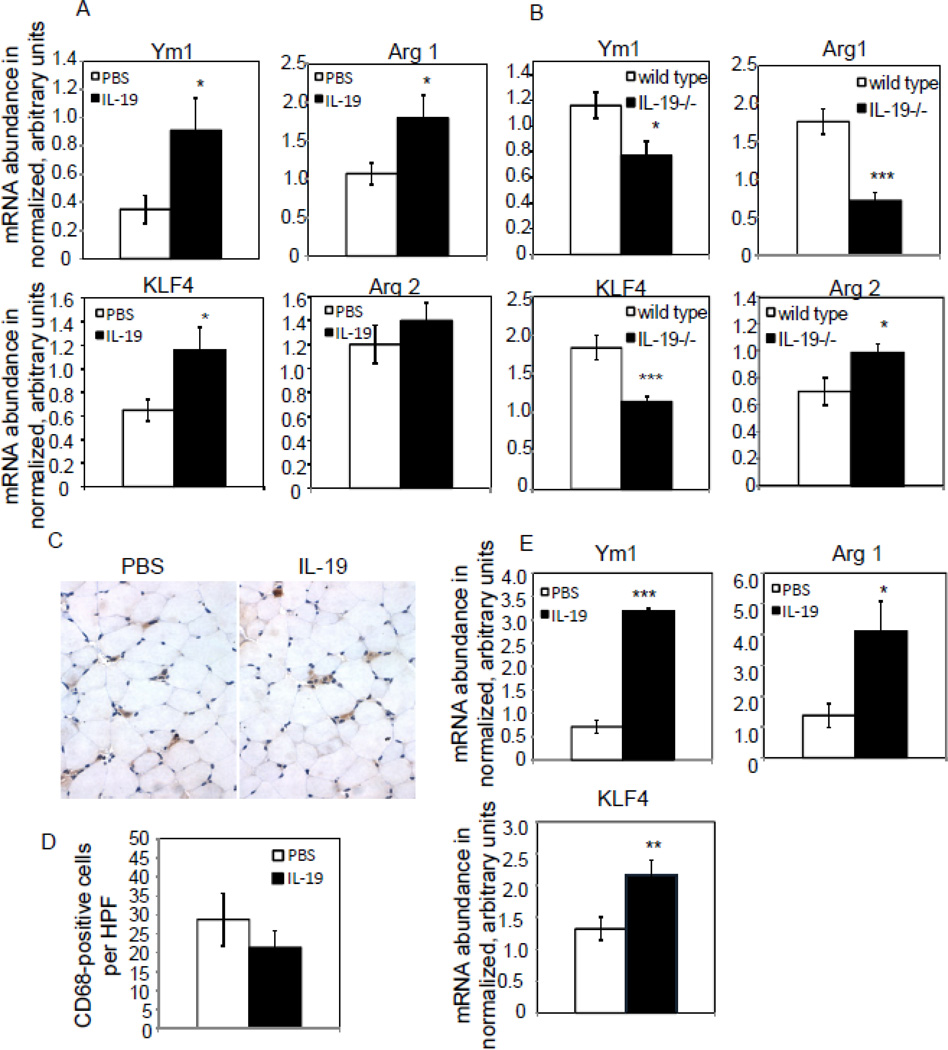
IL-19 alters macrophage M1/M2 phenotype in mice
It is recognized that M2 macrophage have pro-angiogenic effects. As IL-19 effects on macrophage differentiation are currently uncharacterized, we hypothesized that an additional mechanism of IL-19 pro-angiogenic effects were through macrophage polarization to M2. Spleen from mice subject to hind-limb ischemia were recovered upon euthanasia, and expression of macrophage phenotype markers quantitated by qRT-PCR. Figure 5A shows that mice injected with IL-19 expressed significantly increased expression of M2 markers Ym1, Arg 1, and KLF4 compared with saline-injected controls (0.91+/−0.23 vs 0.35+/−.01 for Ym1, 1.79+/−0.3 vs 1.07+/−0.14 for Arg 1, 1.16+/−0.19 vs 0.65+/−0.09 for KLF4, for PBS vs rIL-19, respectively, P<0.05 for all). There was no significant difference in expression of the M1 marker Arg 2 in wild-type mice injected with rIL-19. Similarly, IL-19−/− mice have significantly decreased expression of M2 phenotype markers compared with wild-type controls (0.77+/−0.11 vs 1.16+/−0.10 for Ym1, 0.72+/−11 vs 1.76+/−0.16 for Arg 1, 1.14+/−0.07 vs 1.83+/−0.16 for KLF4, for IL-19−/− and wild type, respectively, P<0.05 or 0.001 as indicated). IL-19−/− mice had significantly higher expression of the M1 marker Arg 2 compared with wild-type controls (0.70+/−0.1 vs 0.98+’ −0.06 for PBS and IL-19, respectively, P<0.05) (Figure 5B).
Figure 5.
IL-19 polarizes macrophage to the M2 phenotype. Spleen from mice subject to hind-limb ischemia were recovered upon euthanasia, and expression of macrophage phenotype markers quantitated by qRT-PCR. A. Wild type mice injected with rIL-19 express significantly more Ym1, Arg1, and KLF4 than those injected with PBS. B. Spleen from IL-19−/− mice express significantly less Ym1, Arg1, and KLF4 than spleen from wild type mice, and significantly more Arg2 compared with wild type mice. P<0.05 or 0.001 where indicated. C. Immunohistochemical staining for macrophage in ischemic hind limbs treated with PBS or IL-19. Representative photomicrograph is shown. D. There is no significant difference in macrophage infiltrate in ischemic hind limbs in rIL-19 and PBS injected mice. Results are mean of CD68 positive cells counted manually from 9 HPF from at least three different sections from 6 different mice per group. Red-brown indicates positive stain. E. IL-19 polarizes phenotype of infiltrating macrophage. Quantitative RT-PCR from ischemic hind limbs treated with either PBS or IL-19, 5 days post-surgery. P<0.05, 0.01, or 0.001 where indicated.
Immunohistochemistry on ischemic hind limbs using anti-CD68 antibody indicated that total macrophage infiltrate was not significantly different between IL-19 and saline-treated mice (28.76+/−6.9 vs 21.39+/−4.3 positive cells per HPF (Figure 5C, D). To determine IL-19 effect on localized macrophage phenotype at the site of angiogenesis, we performed quantitative RTPCR on hind-limbs from mice ligated 5 days earlier. Figure 5E shows that mice treated with IL- 19 have significantly increased expression of the M2 markers Ym1 and Arg 1 compared with mice injected with saline (0.71+/−0.13 vs 3.18+/−0.05 for Ym1, 1.38+/−0.39 vs 4.11+/−0.96 for Arg 1, and 1.32+/−0.18 vs 2.16+/−.24 for KLF, for PBS vs rIL-19, respectively, P<0.05, 0.01, or 0.001 as indicated) (Figure 5D.) Together, these data strongly suggest that IL-19 can polarize macrophage to the M2 phenotype in cultured macrophage, in vivo in spleen, and locally at the site of angiogenesis.
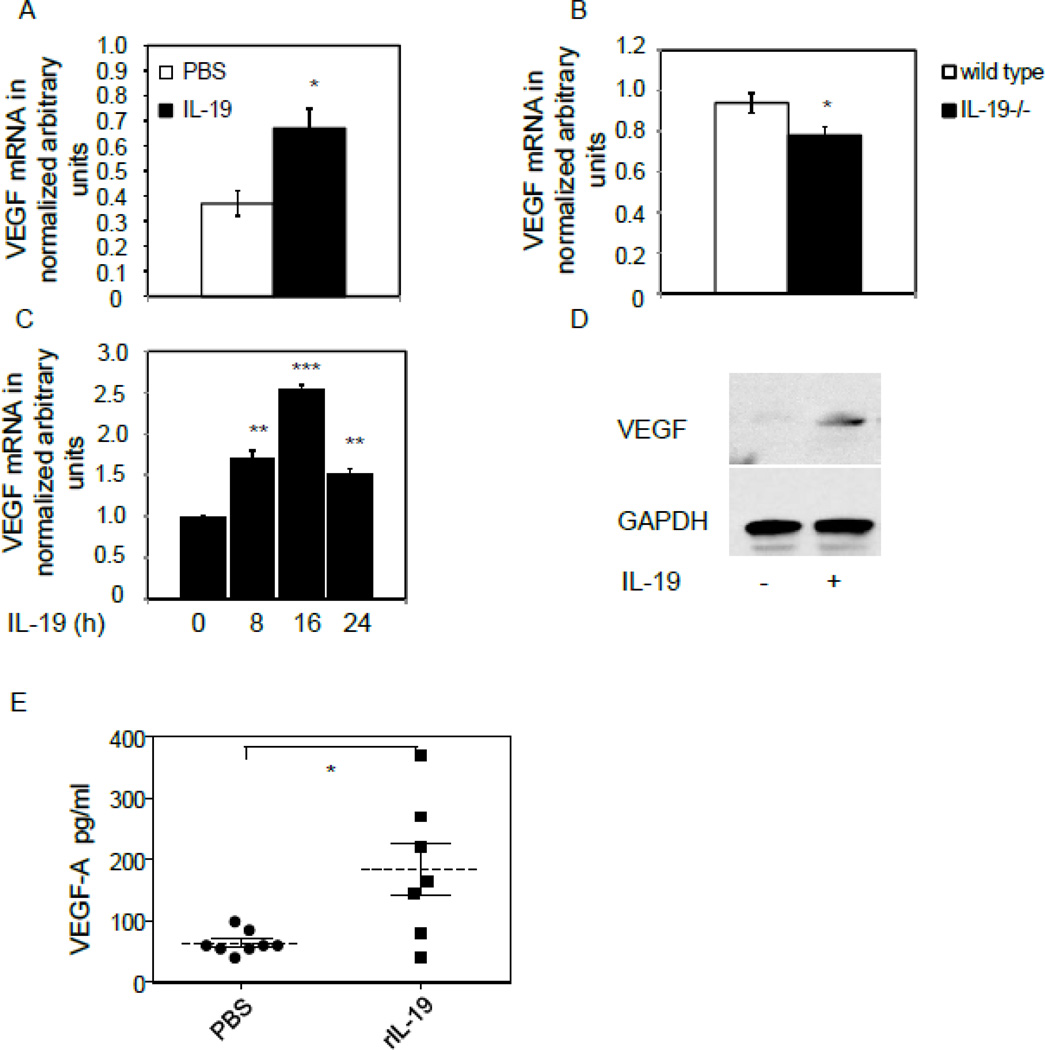
IL-19 induces synthesis of VEGF-A in macrophage
M2 macrophage are reparative and purported to elaborate angiogenic cytokines. We hypothesized that IL-19 could induce expression of VEGF in macrophage. RNA was isolated from spleen from mice subject to hind limb ischemia, and Figure 6A shows that VEGF-A mRNA expression was significantly increased in mice treated with rIL-19 (0.37+/−0.05 vs 0.67+/−0.08 for PBS and IL-19, respectively, P<0.05). Similarly, significantly less VEGF-A was detected in spleen from IL-19−/− mice (0.94+/−0.04 vs 0.78+/−0.04 for wild type and IL-19−/−, respectively, P<0.05) (Figure 6B). Although IL-19 was unable to induce VEGF expression in human EC, VEGF-A mRNA and protein are induced in BMDM (0.99+/−0.1 vs 2.54+/−0.04 for unstimulated and 16 hour stimulation, respectively, P<0.001) (Figures 6C,D).
Figure 6.
IL-19 regulates angiogenic gene expression in macrophage. A–D, IL-19 induces expression of VEGF-A in macrophage. A. Spleen from wild type mice injected with rIL-19 express significantly more VEGF-A mRNA than spleen from those injected with PBS. B. Spleen IL-19−/− mice express significantly less VEGF-A mRNA than spleen from wild type mice. C. Murine BMDM were pre-treated with IL-19 for the indicated times, and VEGF-A mRNA quantitated by qRT-PCR. D. Representative immunoblot of murine BMDM treated with IL-19 for 24 hours. Asterisks indicate significance P<0.05, 0.01, or 0.001, respectively. E., IL-19 increases systemic VEGF-A abundance in plasma from ligated mice. Plasma was isolated from mice injected with IL-19 or PBS controls, and subject to ELISA to quantitate systemic VEGF-A abundance. N=7 mice per group, P<0.05.
To determine if the VEGF-A produced by spleen macrophage could elevate systemic levels of VEGF-A, plasma was recovered from mice at the time of tissue recovery and subject to ELISA. Figure 6E shows that mice injected with rIL-19 had significantly more plasma VEGF-A compared with control mice (64.25+/−6.0 vs 184.30+/−42.7 pg/ml for PBS and rIL-19, respectively, P<0.05). Together, these data suggest that IL-19 can induce VEGF-A expression in macrophage, and this may represent an additional angiogenic mechanism utilized by IL-19.
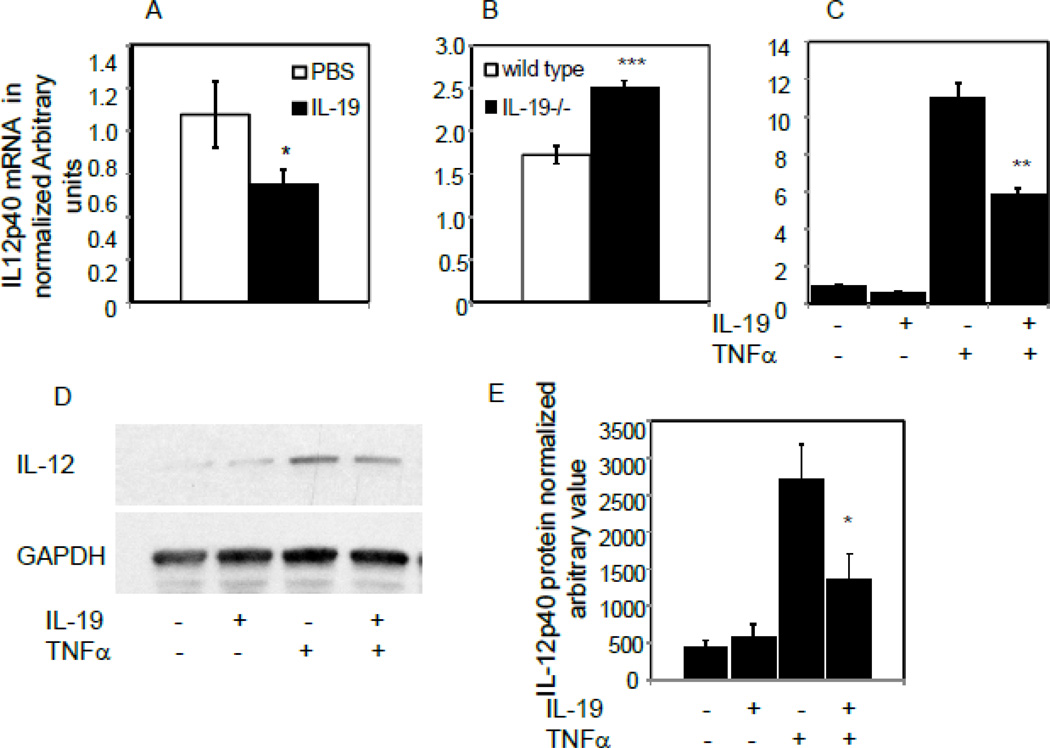
IL-19 decreases IL-12β expression in macrophage
In addition to being pro-inflammatory, Interleukin-12 is also potently anti-angiogenic7, and several experiments were undertaken to determine IL-19 effects on IL-12 expression. IL-12 expression is restricted to leukocytes, and we were unable to detect IL-12 mRNA in EC, which pointed to IL-19 effects on macrophage. Initially, IL12p40 mRNA was quantitated from spleen from mice subject to hind-limb ischemia. IL-12p40 mRNA expression was significantly decreased in spleen from wild-type mice injected with rIL-19 (1.02+/−0.18 vs 0.64+/−0.08 for PBS and IL-19, respectively, P< 0 05) (Figure 7A). Conversely, IL-12p40 expression is significantly increased in spleen from IL-19−/− mice compared with wild-type controls (1.72+/−0.10 vs 2.50+/−0.09 for wild type and IL-19−/−, respectively) (Figure 7B). In a third experiment, BMDM were isolated from wild-type mice and pre-treated with IL-19 prior to stimulation with TNFα. Figures 7C and 7D show that 8 hour pretreatment with IL-19 could significantly decrease TNFα-driven IL-12 mRNA and protein expression in BMDM an average of 55.8% (Figure 7E). This suggests that macrophage-derived IL-12 is regulated by IL-19, and IL-12 down regulation may represent a further potential pro-angiogenic mechanism utilized by IL-19.
Figure 7.
IL-19 decreases expression of IL-12 in macrophage. A. Spleen from wild type mice injected with rIL-19 express significantly less IL-12p40 mRNA than spleen from those injected with PBS. B. Spleen IL-19−/− mice express significantly more IL-12p40 mRNA than spleen from wild type mice. C. IL-19 decreases TNFα-driven IL-12p40 mRNA expression. Murine BMDM were pre-treated with IL-19 for 8 hours, then stimulated with TNFα for four hours. IL-12p40 mRNA was quantitated by qRT-PCR. D. Representative immunoblot of murine BMDM pretreated with IL-19 for 8 hours, then stimulated with TNFα for 24 hours. E. Densiometric quantification of three independent western blots. Asterisk indicates significant difference quantitated from 3 different western blots, P<0.05.
Discussion
In a previous report using cell culture and ex vivo approaches, we determined that IL-19 had angiogenic potential. The present study extends those data to show that IL-19 can increase perfusion of ligated murine hind-limbs by multiple mechanisms and effects on multiple target cells. A major point of novelty is that unlike other Th2 interleukins, IL-19 appears to have pro-angiogenic effects in ligated murine hind limbs.
IL-19 expression is rapidly induced 3 days post ligation surgery, consistent with reports indicating that transcripts related to immune function were induced 1–3 days post-ligation surgery26,31. IL-19 expression also correlates with macrophage infiltration. While considered to be part of the IL-10 sub-family which includes IL-20, IL-22 and IL-24, IL-19 has dissimilar effects on angiogenesis from IL-10, as injection of rIL-19 increases hind limb perfusion. In contrast, angiogenesis and capillary density in ischemic murine hind limbs was increased in IL10−/− mice, while IL-10 gene transfer into these mice reversed this process8. Furthermore, ligation of IL-19−/− hind limbs allowed us to confirm an significant role for IL-19 in regulation of neovascularization as the perfusion ratio in ligated hind limbs from these mice were significantly less than wild type mice. An important role for IL-19 was validated as injection of recombinant IL-19 into these mice restored perfusion ratio to near wild type levels.
Laser Doppler flow velocity strongly correlates with capillary density32, and capillary density increases in areas of acute ischemia33. IL-19 levels significantly correlated with capillary density in recovering ischemic hind limbs as capillary density was significantly decreased in mice receiving rIL-19 compared with saline controls, and significantly less in IL-19−/− mice compared with wild type littermates. Capillary density in non-ischemic hind limbs was not significantly different for any of the experimental cohorts compared with control groups, suggesting ischemic conditions, or perhaps inflammation is necessary for IL-19 angiogenic effects. Together these data strongly support a previously unrecognized central role for IL-19 in angiogenesis in ischemic hind limbs, and imply therapeutic potential for IL-19 as a modality for peripheral vascular disease.
It was important to identify molecular mechanisms for these effects. Angiogenesis occurs when activated EC sprout from pre-existing blood vessels. We have previously shown that IL-19 is chemotactic for EC, promotes cell spreading and migration, activates multiple signaling proteins including p44/42 and the GTPase Rac1, is mitogenic for EC, promotes tube-like structure formation on Matrigel by HUVECs, and microvessel formation in the mouse aortic ring assay24. Together, these data suggested that IL-19 would have direct effects on angiogenic gene expression in EC. We determined that IL-19 induced expression of IL-8, CXCL1, and HGF in human microvascular EC. Interestingly, mRNA for recognized angiogenic factors such as FGF, VEGF, and HIF1α, among others, were not increased by IL-19, which is consistent with our previous report indicating that IL-19 did not induce FGF, or VEGF expression in human mvEC24. HGF is a potent EC mitogen, and several studies have shown HGF is angiogenic in ischemic mouse and rabbit hind limbs34–36. While HGF is mitogenic for EC, it has no effect on VSMC proliferation, which is consistent with our previous reports showing that IL-19 does not induce VSMC proliferation37. CXCL1 and IL-8 are VSMC growth factors. CXCL1 and CXCL8 (IL-8) are chemokines of the CXC family and not only chemotactic for leukocytes, but also regulate EC motility, proliferation, and viability, and both poses angiogenic properties38,39. IL-19 induction of these two cytokines are consistent with studies in other cell systems which suggest that Th2 cytokines preferentially over Th1 cytokines could induce expression of CXC chemokines leading to EC tube formation40. However, none of these studies have been reported to induce angiogenesis in ischemic hind limbs. Because IL-19 did not significantly enhance FGF or VEGF expression, together with our previous report indicating that IL-19 did not increase FGF or VEGF-induced EC proliferation suggests that at least in cultured mvEC, IL-19 utilizes an angiogenic pathway which excludes FGF or VEGF. This is the first to report that IL-19 can directly induce expression of angiogenic factors from human microvascular EC. Induction of these cytokines in EC indicates direct, angiogenic effects of IL-19 on the vasculature, and provides one potential mechanism for IL-19 angiogenic properties.
Macrophage display phenotypic modulation and can be classified as M1 or M2 based on their activation state. The M2 anti-inflammatory, or “wound healing” pathway is induced by IL-10, IL-4, and IL-1341. M2 macrophage secrete EC growth and angiogenic factors such as VEGF and CXCL1, and is considered to promote wound repair and neovascularization42–45. Elimination of circulating monocytes do not reduce reperfusion in ligated limbs, but introduction of exogenously polarized macrophage do46. While IL-19 is upregulated in macrophages after infection, to the best of our knowledge, nothing has been published regarding IL-19 effects on macrophage polarization. The present study is the first to demonstrate that in wild type mice, IL-19 can induce expression of Ym1, Arg1, and KLF4, all markers of the M2 phenotype47. No significant decrease in M1 profile was noted in these mice. Consistent with this, IL-19−/− mice displayed significantly lower levels of these M2 markers, and a significantly higher level of the M1 marker Arg2, suggesting that genetic deletion of IL-19 polarizes macrophage to the M1 phenotype. While we noted not significant difference in macrophage infiltrate into ligated hindlimbs between rIL-19 and PBS control mice, this study does demonstrate that IL-19 can polarize macrophage at the site of angiogenesis in the ischemic hindlimb. In this regard, IL-19 is somewhat similar to other Th2 interleukins IL-4 and IL-13, which are typical stimuli for polarizing macrophage to the M2 phenotype47,48. Similarly, macrophage in IL-10−/− mice are more M1 polarized compared with wild type mice8. A limitation to the present study is that it cannot definitively determine the extent that IL-19 pro-angiogenic activity depends on macrophage compared with EC stimulation. Future studies are required in which monocyte/macrophage populations are depleted and IL-19 angiogenic effects assessed.
VEGF-A is a primary driver of neovascularization and induces angiogenic gene expression, proliferation and differentiation of endothelial cells49. VEGF is expressed in macrophage50 where its expression presumably drives angiogenesis in a juxtacrine fashion in local EC. Although hypoxia is a strong stimulus for VEGF expression, numerous cytokines can induce VEGF production51. IL-19 can induce VEGF-A expression in splenic and cultured BMDM macrophage, indicating that hypoxia is not necessary for IL-19 driven VEGF expression in macrophage. Systemic VEGF-A concentrations are also significantly increased in mice injected with IL-19 compared with controls. It is interesting that IL-19 did not induce VEGF expression in cultured human microvascular EC, again suggesting complex and cell specific effects of IL-19 on angiogenic and inflammatory responses. When taken together, IL-19 polarization of macrophage to M2, together with induction of VEGF-A in these cells suggests a second potential mechanism for IL-19 angiogenic properties.
IL-12 is a potent, pro-inflammatory cytokine expressed in leukocytes. It induces IFNγ expression and drives the Th1 pro-inflammatory response7. Importantly for the present study, IL-12 is also a powerful anti-angiogenic agent, and induction of IFNγ by lymphocytes decreases (CAM) expression in EC52. The IL-12 receptor is not expressed on EC, so its negative angiogenic effects are likely indirect. Not surprisingly, IL-12−/− mice have accelerated angiogenesis, and preclinical studies have focused on IL-12 for anti-tumor therapy53. Interestingly, IL-4 and IL-13, both considered Th2 cytokines, up regulate IL-12, but IL-10, the prototypical Th2 interleukin, negatively regulates IL-12 production54, underscoring the complexity of Th2 regulation of immune responses and angiogenesis. Consistent with published reports indicating leukocyte-specific expression, we were unable to induce IL-12 expression in EC, suggesting that IL-19 down regulation of IL-12 is a macrophage-specific phenomenon. Since IL-12 is pro-inflammatory and anti-angiogenic, and IL-19 is antiinflammatory and pro-angiogenic, a novel reciprocal expression/functional relationship may exist between IL-19 and IL-12. Future studies are necessary to elucidate the precise molecular mechanisms for this regulation. EC do not directly respond to IL-12, and IL-12 anti-angiogenic effects are surmised to be indirect as a result of its effects on leukocyte gene expression55. In one important study, it was found that IL-12 stimulation of mouse spleen cells and human mononuclear blood cells in co-culture with EC induced expression of chemokines which inhibited EC proliferation and angiogenic gene expression56. This suggests anti-angiogenic leukocyte-endothelial cross talk mediated is by IL-12; within this context, IL-19 expression would modify this this cross-talk toward a more pro-angiogenic phenotype. Consequently, IL-19 inhibition of IL-12 expression in macrophage suggests a third potential pro-angiogenic mechanism for IL-19.
In summary, injection of IL-19 can increase, and lack of IL-19 can decrease reperfusion and angiogenesis in ischemic hind limbs. Potential mechanisms include direct angiogenic effects on EC, macrophage M2 polarization and VEGF-A induction, and indirect effects by reduction of IL-12 expression in macrophage. This work not only suggests that IL-19 has therapeutic potential, but implies that IL-19 may play a paracrine role in EC/macrophage cross talk in angiogenesis.
Supplementary Material
Highlights.
Injection of recombinant Interleukin-19 increases reperfusion in ligated hind limbs
Absence of IL-19 decreases reperfusion in ligated hind limbs
IL-19 induces angiogenic gene expression in human endothelial cells
IL-19 induces M2 phenotype and VEGF-A expression in macrophage
IL-19 may represent a pro-angiogenic therapeutic
Acknowledgments
Funding:
This work was supported by grants HL115575 and HL117724 from the National Heart Lung, and Blood Institute of the National Institutes of Health, and Grant 13GRNT1685003 from the American Heart Association to MVA. K.G was supported by American Heart Association post-doctoral fellowship 11POST7530001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The author(s) declare no competing financial interests or any other conflict of interest.
References
- 1.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in Physiology and pathophysiology of Vascular Disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 2.Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 3.Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45(Suppl A):A48–A56. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J. Immunol. 2001;167:1644–1653. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 5.Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1 alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. FASEB J. 2002;16:1471–1483. doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am. J. Pathol. 2002;161:125–134. doi: 10.1016/S0002-9440(10)64164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 8.Silvestre JS, Mallat Z, Duriez M, Tamarat R, Bureau MF, Scherman D, Duverger N, Branellec D, Tedgui A, Levy BI. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ Res. 2000;87:448–452. doi: 10.1161/01.res.87.6.448. [DOI] [PubMed] [Google Scholar]
- 9.Walch L, Massade L, Dufilho M, Brunet A, Rendu F. Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis. 2006;187:285–291. doi: 10.1016/j.atherosclerosis.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Haas CS, Amin MA, Allen BB, Ruth JH, Haines GK, 3rd, Woods JM, Koch AE. Inhibition of angiogenesis by interleukin-4 gene therapy in rat adjuvant-induced arthritis. Arthritis Rheum. 2006;54:2402–2414. doi: 10.1002/art.22034. [DOI] [PubMed] [Google Scholar]
- 11.Lee YW, Eum SY, Chen KC, Hennig B, Toborek M. Gene expression profile in interleukin-4-stimulated human vascular endothelial cells. Mol Med. 2004;10:19–27. doi: 10.2119/2004-00024.lee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura Y, Nitto T, Inoue T, Node K. IL-13 Attenuates Vascular Tube Formation Via JAK2-STAT6 Pathway. Circ. J. 2008;72:469–475. doi: 10.1253/circj.72.469. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh MY, Chen WY, Jiang MJ, Cheng BC, Huang TY, Chang MS. Interleukin-20 promotes angiogenesis in a direct and indirect manner. Genes Immun. 2006;7:234–242. doi: 10.1038/sj.gene.6364291. [DOI] [PubMed] [Google Scholar]
- 14.Tritsaris K, Myren M, Ditlev SB, Hübschmann MV, van der Blom I, Hansen AJ, Olsen UB, Cao R, Zhang J, Jia T, Wahlberg E, Dissing S, Cao Y. IL-20 is an arteriogenic cytokine that remodels collateral networks and improves functions of ischemic hind limbs. Proc Natl Acad Sci U S A. 2007;104:15364–15369. doi: 10.1073/pnas.0707302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heuzé-Vourc'h N, Liu M, Dalwadi H, Baratelli FE, Zhu L, Goodglick L, Põld M, Sharma S, Ramirez RD, Shay JW, Minna JD, Strieter RM, Dubinett SM. IL-20, an anti-angiogenic cytokine that inhibits COX-2 expression. Biochem Biophys Res Commun. 2005;333:470–475. doi: 10.1016/j.bbrc.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 16.Bréchot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, Alanio-Bréchot C, Durand M, Philippe J, Silvestre JS, Van Rooijen N, Corvol P, Nicoletti A, Chazaud B, Germain S. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One. 2008;3:e3950. doi: 10.1371/journal.pone.0003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, Vazquez N, Pestka S, Donnelly RP, Kotenko SV. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher G. Interleukin-19: Multiple roles in immune regulation and disease. Cytokine GrowthFactor Rev. 2010;21:345–352. doi: 10.1016/j.cytogfr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Sabat R, Wallace E, Endesfelder S, Wolk K. IL-19 and IL-20: two novel cytokines with importance in inflammatory diseases. Expert Opin Ther Targets. 2007;5:601–612. doi: 10.1517/14728222.11.5.601. [DOI] [PubMed] [Google Scholar]
- 21.Oral H, Kotenko S, Yilmaz M, Mani O, Zumkehr J, Blaser K, Akdis C, Akdis M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–388. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher G, Eskdale E, Jordan W, Peat J, Campbell J, Boniotto M, et al. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. International Immunopharmacology. 2004;4:615–626. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and Related Cytokines and Receptors. Ann. Rev. Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 24.Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV. The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:167–175. doi: 10.1161/ATVBAHA.110.214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 26.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol. 2004;43:474–482. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Ellison S, Gabunia K, Richards JM, Kelemen SE, England RN, Rudic D, Azuma YT, Munroy MA, Eguchi S, Autieri MV. IL-19 Reduces Ligation-Mediated Neointimal Hyperplasia by Reducing Vascular Smooth Muscle Cell Activation. Am J Pathol. 2014;184:2134–2143. doi: 10.1016/j.ajpath.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 29.Cuneo AA, Herrick D, Autieri MV. IL-19 Reduces VSMC Activation by Regulation of mRNA Regulatory Factor HuR and Reduction of mRNA Stability. J Mol Cell Cardiol. 49:647–654. doi: 10.1016/j.yjmcc.2010.04.016. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 31.Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L. Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice. Physiol Genomics. 2002;11:263–272. doi: 10.1152/physiolgenomics.00110.2002. [DOI] [PubMed] [Google Scholar]
- 32.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 33.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. J Mol Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura Y, Morishita R, Nakamura S, Aoki M, Moriguchi A, Matsumoto K, Nakamura T, Higaki J, Ogihara T. A vascular modulator, hepatocyte growth factor, is associated with systolic pressure. Hypertension. 1996;28:409–413. doi: 10.1161/01.hyp.28.3.409. [DOI] [PubMed] [Google Scholar]
- 35.Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, Taiji M, Noguchi H, Takeshita S, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379–1384. doi: 10.1161/01.hyp.33.6.1379. 996. [DOI] [PubMed] [Google Scholar]
- 36.Taniyama Y, Morishita R, Aoki M, Nakagami H, Yamamoto K, Yamazaki K, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hindlimb ischemia models: preclinical study for treatment of peripheral arterial disease. Gene Ther. 2001;8:181–189. doi: 10.1038/sj.gt.3301379. [DOI] [PubMed] [Google Scholar]
- 37.Tian Y, Sommerville LJ, Cuneo A, Kelemen SE, Autieri MV. Expression and Suppressive Effects of Interleukin-19 on Vascular Smooth Muscle Cell Proliferation, Signaling, and Development of Intimal Hyperplasia. Am J Pathol. 2008;173:901–909. doi: 10.2353/ajpath.2008.080163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72:9–18. [PMC free article] [PubMed] [Google Scholar]
- 39.Zaja-Milatovic S1, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23:1399–1407. doi: 10.14670/hh-23.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda A, Fukuda S, Matsumoto K, Saito H. Th1/Th2 cytokines reciprocally regulate in vitro pulmonary angiogenesis via CXC chemokine synthesis. Am J Respir Cell Mol Biol. 2008;38:168–175. doi: 10.1165/rcmb.2007-0162OC. [DOI] [PubMed] [Google Scholar]
- 41.Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187:3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- 42.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80:705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- 45.Ligresti G, Aplin AC, Zorzi P, Morishita A, Nicosia RF. Macrophage-derived tumor necrosis factor alpha is an early component of the molecular cascade leading to angiogenesis in response to aortic injury. Arterioscler Thromb Vasc Biol. 2011;31:1151–1159. doi: 10.1161/ATVBAHA.111.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jetten N, Donners MM, Wagenaar A, Cleutjens JP, van Rooijen N, de Winther MP, Post MJ. Local delivery of polarized macrophages improves reperfusion recovery in a mouse hind limb ischemia model. PLoS One. 2013;8(7):e68811. doi: 10.1371/journal.pone.0068811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantovani A. Sica Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 50.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Müller KH, Sharkey AM, Smith SK. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–489. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF. EXS. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS. 1997;79:233–269. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- 52.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 53.Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X, Gri G, Wysocka M, Kim JE, Liu L, Liao F, Farber JM, Pestka S, Trinchieri G, Lee WM. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9:25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 54.Du C, Sriram S. Mechanism of inhibition of LPS-induced IL-12p40 production by IL-10 and TGFbeta. J Leukoc Biol. 1998;64:92–97. doi: 10.1002/jlb.64.1.92. [DOI] [PubMed] [Google Scholar]
- 55.Duda DGM, Sunamura L, Lozonschi T, Kodama S, Egawa G, Matsumoto H, Shimamura K, Shibuya K, Takeda S. Matsuno. 2000. Direct in vitro evidence and in vivo analysis of the antiangiogenesis effects of interleukin 12. Cancer Res. 2000;60:1111–1116. [PubMed] [Google Scholar]
- 56.Strasly M, Cavallo F, Geuna M, Mitola S, Colombo MP, Forni G, Bussolino F. IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J Immunol. 2001;166:3890–3899. doi: 10.4049/jimmunol.166.6.3890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.