Abstract
Rapid activation causes remodeling of atrial myocytes resembling that which occurs in experimental and human atrial fibrillation (AF). Using this cellular model, we previously observed transcriptional upregulation of proteins implicated in protein misfolding and amyloidosis. For organ-specific amyloidoses such as Alzheimer’s disease, preamyloid oligomers (PAOs) are now recognized to be the primary cytotoxic species. In the setting of oxidative stress, highly-reactive lipid-derived mediators known as γ-ketoaldehydes (γ-KAs) have been identified that rapidly adduct proteins and cause PAO formation for amyloid β1-42 implicated in Alzheimer’s. We hypothesized that rapid activation of atrial cells triggers oxidative stress with lipid peroxidation and formation of γ-KAs, which then rapidly crosslink proteins to generate PAOs. To investigate this hypothesis, rapidly-paced and control, spontaneously-beating atrial HL-1 cells were probed with a conformation-specific antibody recognizing PAOs. Rapid stimulation of atrial cells caused the generation of cytosolic PAOs along with a myocyte stress response (e.g., transcriptional upregulation of Nppa and Hspa1a), both of which were absent in control, unpaced cells. Rapid activation also caused the formation of superoxide and γ-KA adducts in atriomyocytes, while direct exposure of cells to γ-KAs resulted in PAO production. Increased cytosolic atrial natriuretic peptide (ANP), and the generation of ANP oligomers with exposure to γ-KAs and rapid atrial HL-1 cell stimulation, strongly suggest a role for ANP in PAO formation. Salicylamine (SA) is a small molecule scavenger of γ-KAs that can protect proteins from modification by these reactive compounds. PAO formation and transcriptional remodeling were inhibited when cells were stimulated in the presence of SA, but not with the antioxidant curcumin, which is incapable of scavenging γ-KAs. These results demonstrate that γ-KAs promote protein misfolding and PAO formation as a component of the atrial cell stress response to rapid activation, and they provide a potential mechanistic link between oxidative stress and atrial cell injury.
Keywords: preamyloid oligomers, amyloidosis, atrial HL-1 cells, oxidant stress, atrial natriuretic peptide, γ-ketoaldehyde, levuglandin
1. INTRODUCTION
Atrial fibrillation (AF) is a progressive disorder, with remodeling during rapid atrial activation that increases arrhythmia susceptibility. There is abundant evidence linking oxidative stress to the pathogenesis and progression of AF [1, 2]. Risk factors that promote AF, as well as inflammation and activation of the renin-angiotensin-aldosterone system, cause oxidative damage in humans [1-3]. Oxidative injury is evident in the atria of patients with permanent AF [4], and superoxide production is increased in both experimental and human AF [5, 6]. While pharmacologic agents with antioxidant properties have shown promise in experimental AF models, clinical trials to date have largely been disappointing [7].
Previously, we showed that atrial cells rapidly stimulated in culture undergo remodeling similar to that observed in human AF [8]. Importantly, transcriptional profiling in paced cells exhibited substantial concordance with changes seen in vivo [9]. Unexpectedly, we observed conserved transcriptional upregulation for proteins implicated in amyloidosis, including numerous heat-shock proteins that serve as molecular chaperones to prevent protein misfolding and aggregation.
It is increasingly recognized that proteotoxicity such as amyloid plays an important role in disease pathogenesis, especially for aging-related degenerative disorders such as Alzheimer’s disease [10, 11]. For systemic amyloidoses (e.g., with multiple myeloma), organ dysfunction is caused by the large quantity of amyloid fibrils that are deposited [12]. In contrast, for organ-limited amyloidoses such as Alzheimer’s disease and type II diabetes, mature amyloid deposits have no correlation with the state of disease advancement. Rather, soluble protein aggregate intermediates are now recognized as the primary cytotoxic species that correlate with disease phenotype [10, 11]. Preamyloid oligomers (PAOs) cause cell injury and/or death by multiple mechanisms, including endoplasmic reticulum stress, increased cytoplasmic calcium concentration, mitochondrial injury with oxidative stress, reduced protein clearance, and probable cell membrane pore formation [13]. PAO complexes derived from different proteins possess a common structural epitope related to the peptide backbone that is irrespective of amino acid sequence, enabling the development of confirmation-specific antibodies. Importantly, a critical distinction between soluble protein oligomers and amyloid deposits is that the oligomers do not possess the structure required for binding of amyloid-detecting dyes such as Congo red, and hence they are not visible by standard amyloid staining methods. Recent studies have demonstrated the presence of PAOs in experimental and human heart failure [14, 15]. However, the role of these cytotoxic complexes in atrial pathophysiology has not been explored.
In the setting of oxidative injury and cyclooxygenase activation, arachidonic acid can undergo oxygenation and structural rearrangement to generate γ-ketoaldehyde compounds given the trivial name of isolevuglandins or isoketals [16-18]. γ-Ketoaldehydes (γ-KAs) are the most reactive products of lipid peroxidation identified to date, and they rapidly adduct to lysine residues of proteins to form stable adducts and intermolecular crosslinks [19-21]. γ- KA adducts are increased in a number of pathologic conditions, including Alzheimer’s disease, that are linked to oxidative injury and inflammation [22, 23]. Recent evidence demonstrates that these highly-reactive compounds can directly promote formation of PAOs derived from amyloid β1-42, the highly fibrillogenic peptide involved in the development of Alzheimer’s disease [24]. In addition, molecular scavengers have been discovered that rapidly and irreversibly react with and inactivate γ-KAs, thus preventing them from reacting with and damaging proteins [17, 25]. Salicylamine (SA) is a member of a family of phenolic amines that act as highly-effective γ-KA scavengers. Importantly, SA prevents the development of cognitive deficits in a mouse model of Alzheimer’s disease [26].
Given the evidence implicating oxidative stress in the development of both Alzheimer’s disease and AF, common pathophysiologic mechanisms may be operative for these seemingly disparate disorders. We hypothesized that rapid activation of atrial myocytes triggers a cellular stress response that includes oxidative injury and the generation of γ-KAs, to promote protein misfolding and PAO formation.
2. MATERIALS AND METHODS
2.1 Atrial HL-1 Cell Culture and Stimulation
Atrial HL-1 myocytes were grown as described previously [8]. Nearly confluent HL-1 cells were subjected to rapid stimulation for 6hr at 5Hz (18V, 4ms) using a C-Pace cell culture stimulator (Ion Optix Corp) in the absence and presence of treatment, with spontaneously-beating control cells cultured in parallel. Optimization of pacing conditions was performed as described in Supplementary Data, to ensure reproducible stimulation of atrial HL-1 cells at 5 Hz, a rate that causes electrophysiologic and transcriptional remodeling [8, 9].
2.2 Immunocytochemistry: Preamyloid Oligomers
Rapidly-stimulated and control spontaneously-beating cells were subjected to immunocytochemistry using a rabbit polyclonal antibody (A-11; 1:200, EMD Millipore) recognizing the conformational epitope common to all preamyloid oligomers [11, 27], as detailed in the Supplementary Data.
2.3 Image Acquisition
Cells were imaged using a 40X/1.3 Plan-Neofluar objective on a confocal microscope (LSM-510, Carl Zeiss). Fluorescence images were acquired at 0.5μm focus intervals with a confocal pinhole set to 1 Airy unit, thus optimizing contrast and resolution. The Alexa Fluor 488-labeled A-11 and TO-PRO-3 were excited at 488 nm and 633 nm, respectively, and detected with 505-550 nm band pass and 650 nm long pass filters, respectively. DIC images were acquired as a separate scan and registered with the corresponding fluorescence images.
2.4 Detection of Superoxide Production
Atrial HL-1 cells were subjected to rapid stimulation as described above, except that for the final 30min, Claycomb medium was replaced with 50mM Krebs-HEPES buffer (in mM: NaCl 145; KCl 4.86; NaH2PO4 5.7; CaCl2 0.54; MgSO4 1.22; glucose 5.5; pH 7.4) containing 10μM dihydroethidium (DHE, Molecular Probes). Cells were washed with PBS and fixed with 3% PFA in PBS as usual. In some experiments, tempol (10μM, Enzo Life Sciences) was included in the buffer.
2.5 PAO Formation by γ-Ketoaldehydes
Atrial HL-1 myocytes were incubated with the synthetic γ-KA 15-E2-isolevuglandin/isoketals [28] (E2-IsoKs; 1μM) for 6hr in HBSS medium (Mediatech, Inc). Cells were fixed with 4% PFA in PBS for 10min and permeabilized with 0.1% Triton in PBS for 7min. Unreacted aldehyde groups were blocked with 50mM NH4Cl, while non-specific antigen binding sites were blocked using Power block (BioGenex) for 1hr. Immunostaining was performed using the A-11 antibody as described above. After incubation with E2-IsoKs, exclusion of Trypan blue was also used to determine cell viability.
2.6 Immunocytochemistry: γ-Ketoaldehyde Adducts
Rapidly-stimulated and control spontaneously-beating cells were subjected to immunocytochemistry using an anti-γ-KA-lysyl adduct single-chain antibody (D11 ScFv) as described previously [29] and in the Supplementary Data.
2.7 Immunocytochemistry: Atrial Natriuretic Peptide
ANP immunostaining of atrial HL-1 cells was performed using a primary rabbit polyclonal anti-α-ANP (1-28) antibody (1:200, Phoenix Pharmaceuticals, Inc.) and donkey anti-rabbit Alexa 488-conjugated secondary antibody (1:500, Molecular Probes), using the protocol described above for A-11. Immunostaining was visualized by using confocal microscopy.
2.8 Western Analysis
Cell lysate from both rapidly-stimulated and control atrial HL-1 cells was diluted with LDS sample buffer, denatured at 70°C for 10min, and electrophoresed onto a 4-12% gradient gel (Life Technologies). Proteins were then transferred onto a PVDF membrane (0.2μm pore size, BioRad) and exposed overnight to either rabbit A-11polyclonal antibody (1:500) or rabbit anti-α-ANP (1-28) polyclonal antibody (1:500, Phoenix Pharmaceuticals, Inc.). After removal of primary antibodies, membranes were incubated with HRP-conjugated secondary antibody (1:5000, goat anti-rabbit, Jackson ImmunoResearch) for 1hr. Membranes were stripped (Restore stripping buffer; Thermo Scientific) for 15min at room temperature and reprobed with anti-β-actin mouse monoclonal antibody (1:5000, Sigma) as a protein loading control. An enhanced chemiluminescent kit (Pierce ECL Western Blotting Substrate) was used for detection of protein bands.
2.9 α-ANP Peptide Oligomer Formation
Synthetic α-ANP peptide (1-28) (SLRRSSCFGGRMDRIGAQSGLGCNSFRY- disulfide bond [C7-C23]) was synthesized by RS Synthesis, LLC. To test for ANP oligomerization, peptide (10μM) was prepared in PBS buffer (pH 7.4) and incubated at room temperature for 6 days (positive control), while a separate sample was incubated for 24hr with either synthetic E2-IsoKs (2 molar equivalents) or vehicle (DMSO). After incubation, Western analysis was performed using the anti-α-ANP antibody (1:500) and goat anti-rabbit HRP-conjugated secondary antibody (1:5000, Jackson ImmunoResearch). E2-IsoKs were prepared as described previously [28].
2.10 mRNA Extraction and Real-Time Quantitative RT(q)-PCR
mRNA was extracted from cells, analyzed, and subjected to cDNA synthesis, and expression of Nppb and Hspa1a was evaluated by q-PCR as described previously [9] and in the Supplementary Data.
2.11 Data Analysis
Following normalization for 18S, the average Ct (cycle threshold) value for control cells was subtracted from that for paced cells. Fold change was defined as the residual ΔΔCt value for paced cells, using the 2−ΔΔCt method, with an unpaired t-test used to determine statistically significant changes. Nonparametric analysis with a Kruskal-Wallis test for the ΔΔCt values was used to evaluate for a drug effect for each gene. Pairwise comparisons for vehicle and either salicylamine or curcumin exposure were performed with a Wilcoxon rank sum test.
3. RESULTS
3.1 Formation of Preamyloid Oligomers in Rapidly-paced Atrial HL-1 Cells
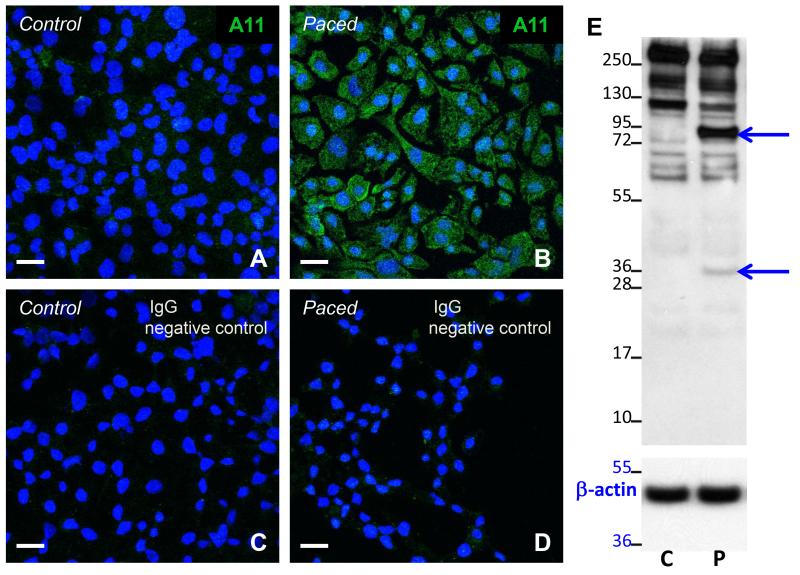
To test the hypothesis that rapid activation of atrial cells triggers protein misfolding and generation of PAOs, both rapidly-paced and control, spontaneously-beating atrial HL-1 cells were probed with a conformation-specific anti-oligomer antibody, A-11, recognizing the structural epitope common to all PAOs. As shown in Figure 1, rapid pacing caused the appearance of diffuse cytoplasmic immunoreactive protein (indicated by the green color in panel B) that was absent in control cells (panel A). Immunostaining was also absent when the primary antibody was omitted or IgG was substituted (Figure 1C and 1D), demonstrating the specificity of immunolabeling using A-11. Western blotting was also performed using A-11 for control and paced cells [30]. As shown in Figure 1E, rapid stimulation led to the appearance of bands at 34 and 95 kDa, representing different-sized PAOs (n=3 separate experiments), not present in lysate for control cells. Collectively these data indicate that rapid stimulation of atrial cells causes PAO formation. Given that these protein complexes are cytotoxic, these findings identify PAOs as potential candidates to promote myocyte injury during atrial cell remodeling.
Figure 1. PAO formation following rapid stimulation of atrial HL-1 cells.
Exposure of rapidly-paced cells to the anti-oligomer antibody A-11 revealed diffuse cytosolic immunostaining (B) that was absent in control, unpaced cells (A). Immunolabeling was not detected when IgG was substituted for A-11 (C, D). Scale bars = 20 microns. (E) Using A-11, Western analysis of cell lysate (20μg) from control (C) and rapidly-stimulated (P) atrial HL-1 cells demonstrates the development of bands at 34 and 95 kDa (arrows) with pacing. To control for protein loading, the blot was stripped and reprobed with an anti-β actin antibody (lower panel).
3.2 Generation of Oxidative Stress with Rapid Activation
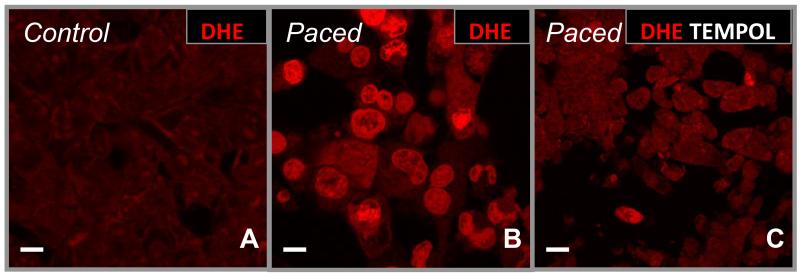
Reactive intermediates generated during oxidative stress can modify, cross-link, and aggregate proteins. Given that superoxide (O2·−) production is increased in animal models and human AF, we hypothesized that this reactive oxygen species (ROS) would also accumulate in rapidly-stimulated atrial HL-1 cells. In the presence of DHE (a non-fluorescent probe that becomes highly fluorescent after reacting with O2·−), spontaneously beating atrial HL-1 cells generated minimal O2·− under control conditions (Figure 2A); however, with rapid activation, O2·− production is markedly enhanced (Figure 2B). In the presence of the small molecule cell membrane-permeable superoxide dismutase mimetic tempol, O2·− levels were essentially reduced to baseline (Figure 2C), demonstrating the specificity of the DHE-generated fluorescent signal. These findings confirm that, as in vivo, rapid activation of atrial HL-1 cells leads to the production of ROS.
Figure 2. Production of superoxide (O2·−) with rapid stimulation.
After 6hr of rapid stimulation in culture, O2·− production by atrial HL-1 cells was detected by dihydroethidium (DHE; 10μM; B, indicated by the bright red color) that is not present in control, spontaneously-beating cells (A). Superoxide generation was essentially abolished under these conditions by co-incubation of cells with the cell-permeable superoxide dismutase mimetic tempol (C). Scale bars = 10 microns.
3.3 Role of γ-Ketoaldehydes in PAO Formation
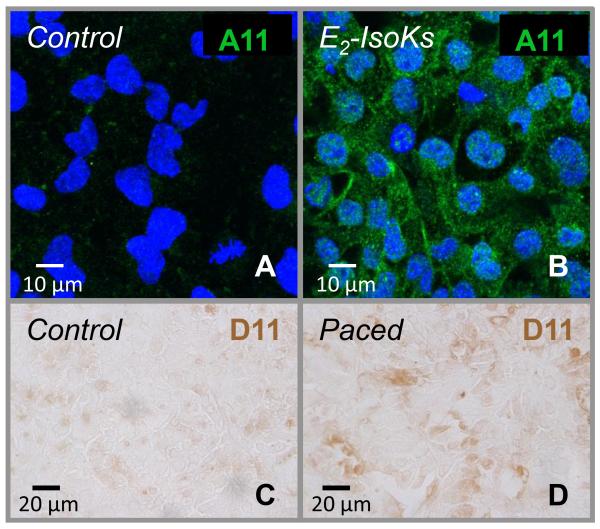
As described above, γ-KAs are highly-reactive products of oxidative stress that can crosslink proteins to initiate their aggregation. To investigate whether these compounds could play a role in generating PAOs in our experimental preparation, atrial HL-1 cells were incubated with a physiologic concentration of a synthetic E-series γ-KA (E2-IsoKs; 1μM) for 6 hr, followed by immunostaining with A-11. As illustrated in Figure 3A and 3B, exposure to E2-IsoKs led to the production of cytosolic PAOs similar to that observed with rapid pacing. Furthermore, immunostaining using an anti-γ-KA lysyl adduct single chain antibody (D11 ScFv) demonstrated the presence of abundant adducts in rapidly-stimulated but not control cells (Figure 3C and 3D). Taken together, these results are highly suggestive that γ-KAs can serve as a mechanistic link between oxidative stress and PAO formation in our experimental preparation.
Figure 3. Evidence for a role of γ-ketoaldehydes in PAO formation for paced atrial HL-1 cells.
After incubation with synthetic isolevugland in/isoketals (E2-IsoKs; 1 μM) for 6hr, immunofluorescent labeling with A-11 demonstrated abundant PAO formation in unstimulated atrial HL-1 cells (top row). Immunoreactivity using the anti-γ-KA adduct antibody (D11 ScFv) is shown in control and paced HL-1 cells (bottom row). Positivity (indicated by brown-colored DAB substrate) is evident in the paced cells.
3.4 Atrial Natriuretic Peptide and PAO Formation
Rapid activation of atrial cells causes atrial granule release, leading to elevated concentrations of natriuretic peptides such as ANP in plasma. ANP is known to be fibrillogenic (i.e., to form amyloid fibrils), and this hormone is primarily responsible for the development of aging-related cardiac amyloidosis in the atrium, a condition known as isolated atrial amyloidosis [31, 32]. We have previously demonstrated upregulation of Nppa, which encodes ANP, in rapidly-stimulated atrial HL-1 cells, with release of natriuretic peptides into the culture medium [9]. Therefore, immunostaining for ANP was performed on control and rapidly-paced atrial HL-1 cells. Figure 4B demonstrates increased intracellular production of ANP following rapid activation, with a diffuse cytosolic distribution resembling PAO formation. To test the hypothesis that ANP could contribute to PAO formation under these circumstances, purified ANP peptide was incubated with E2-IsoKs, followed by Western blotting using an anti-αANP antibody. As shown in Figure 4C, incubation of the peptide with E2-IsoKs led to oligomer formation (lane 2), detected as bands of increased molecular size (predominantly at ~10 kDa), compared to monomeric ANP (3kDa; lane 3). Analogous to amyloid β1-42, Figure 4C also demonstrates that ANP can spontaneously generate PAOs during incubation at room temperature for 6 days (lane 1), illustrating the fibrillogenic nature of this peptide. Finally, Western analysis of cell lysate from control and paced cells was performed for ANP. As shown in Figure 4D, the blot demonstrates the appearance of bands with rapid stimulation having nearly the identical size as the new bands that appeared on the A-11 Western blot (32 and 95 kDa; Figure 1E) and the predominant oligomer-related band (10 kDa) formed during the in vitro experiments with purified ANP shown in panel C. These data link both γ-ketoaldehydes and rapid pacing to the production of ANP oligomers, and they provide strong support for the hypothesis that ANP-derived oligomers are a significant component of the PAOs that form with rapid stimulation of atrial HL-1 cells.
Figure 4. Increased production of atrial natriuretic peptide (ANP) and ANP oligomers in rapidly-paced atrial HL-1 cells.
Immunofluorescent labeling with an anti-αANP antibody demonstrated a significant increase of ANP production in atrial HL-1 cells with rapid stimulation (B) compared to unpaced (control) cells (A). Scale bars = 10 microns. (C) Using the same antibody, Western analysis was performed on samples of purified ANP peptide (10μM) following incubation (at room temperature) for 6 days (lane 1), incubation for 24hr (lane 3), and incubation for 24hr in the presence of E2-IsoKs (lane 2). The development of ANP oligomers in lanes 1 and 2 is indicated by the appearance of higher molecular weight species, predominantly at ~10 kDa. (D) Using the anti-αANP antibody, Western analysis of cell lysate from control (C) and rapidly-stimulated (P) atrial HL-1 cells indicates the development of bands with pacing that are nearly identical in size (32 and 95 kDa) to the pacing-induced bands shown in Figure 1E, as well as a 10 kDa band similar to the oligomer-related band in panel C.
3.5 Effect of the γ-Ketoaldehyde Scavenger Salicylamine
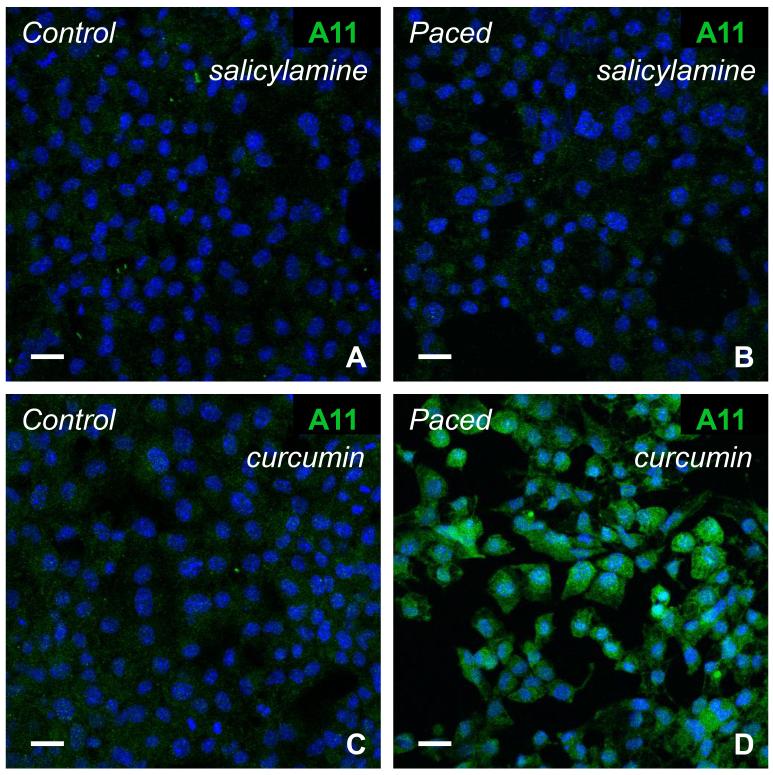
To confirm an essential role for γ-KAs in atriomyocyte PAO formation, cells were rapidly paced in the absence and presence of the γ-KA scavenger salicylamine. As illustrated in Figure 5A and B, pacing-induced PAO formation was essentially eliminated when salicylamine was present in the culture medium.
Figure 5. Salicylamine inhibited cytosolic PAO formation in rapidly-paced atrial HL-1 cells, while curcumin did not.
Immunostaining with the A-11 antibody was minimal for control (A) and rapidly-paced (B) cells in presence of the γ-ketoaldehyde scavenger salicylamine (100μM). However, abundant PAO formation was detected for paced cells when cultured with the antioxidant curcumin (20μM; C, D). Scale bars = 20 microns.
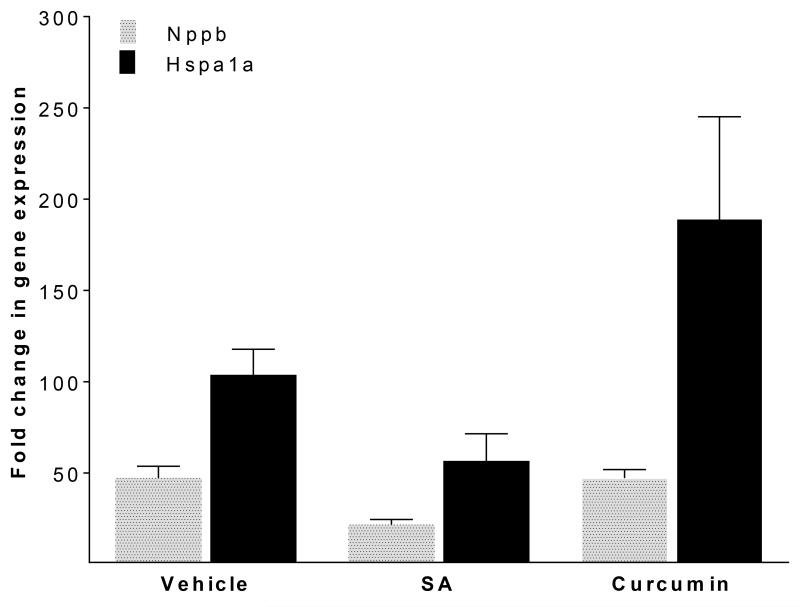
We have previously shown that rapid activation of atrial cells causes a myocyte stress response, a component of which includes transcriptional upregulation of Nppb and Hspa1a (encoding heat shock protein 70; Figure 6, left bars) [9]. However, in the presence of salicylamine, this response was blunted (Figure 6, middle bars; see Supplementary Data), providing additional evidence to support a cytoprotective response when γ-KAs are scavenged.
Figure 6. Effect of salicylamine (SA) and curcumin on transcriptional upregulation of Nppb and Hspa1a with rapid pacing.
Real-time quantitative RT-PCR results are expressed as mean fold change for Nppb and Hspa1a for atrial HL-1 cells subjected to rapid stimulation for 6hr (n = 6; fold change expressed as the residual value calculated from paced and control cells ΔΔCt values). In the absence of SA or curcumin, mRNA expression was up-regulated for Nppb (ΔCtcontrol 18.56±0.91 vs. ΔCtpaced 13.67±0.68) and Hspa1a (ΔCtcontrol 21.00±0.22 vs. ΔCtpaced 15.19±0.31) with rapid-pacing (p=0.002 and p<0.001, respectively; see Supplementary Data). For Nppb, the group effect for drug was significant (p=0.047), while the group effect for Hspa1a was not (p=0.097). However, pairwise comparisons (performed with a Wilcoxon rank sum test) did not demonstrate significant changes for Nppb or Hspa1a with either salicylamine or curcumin.
To investigate the specificity of the effects of SA, similar experiments were conducted using the antioxidant/anti-inflammatory compound curcumin [33]. This drug prevents soluble oligomer aggregation and fibril formation for amyloid β1-42 in vitro and in vivo [34]. However, unlike salicylamine, curcumin lacks an amine group, so that it cannot scavenge γ-KAs. In the presence of curcumin, rapid stimulation of atrial HL-1 cells resulted in robust generation of PAOs (Figure 5C and 5D) similar to that observed under drug-free conditions, as well as activation of stress response genes (Figure 6, right bars). Collectively, these data establish a critical role for γ-KAs in PAO formation in rapidly-activated atrial myocytes.
4. DISCUSSION
The basic molecular processes that promote atrial remodeling and disease progression during AF are not well understood. In the present study, we have identified a novel pathologic component of the myocyte stress response that occurs during rapid activation of atrial cells, namely the generation of protein oligomers. Our findings also indicate that a fundamental molecular mechanism driving the formation of these protein complexes is the production of highly-reactive γ-ketoaldehydes by ROS (Figure 7). The capacity of PAOs to cause injury of cells (including cardiomyocytes) is well documented [13]. Thus, these data support a growing body of evidence that “proteotoxicity” can occur in the heart and contribute to cardiac dysfunction in humans [2, 35].
Figure 7. Hypothesis linking rapidly-activated atrial cells, PAO formation, and atrial pathology/arrhythmogenesis, compared with the known process of isolated atrial amyloidosis.
Rapid activation of atrial cells causes oxidative stress, with superoxide generation. As a consequence of lipid peroxidation, highly-reactive γ-ketoaldehydes are formed that rapidly crosslink cellular proteins. This initiates protein misfolding and oligomerization in atrial cells, with ANP a principal component of PAOs. Protein oligomers can directly cause myocyte injury/death, or they can coalesce with aging to form amyloid fibril deposition in atrial myocardium. This process, known as isolated atrial amyloidosis, is associated with infiltrative structural damage in the atrium and an increased risk of atrial fibrillation.
A wide variety of physiologic and pathophysiologic factors can promote protein misfolding, including alterations in cellular temperature, mechanical strain, and oxidative stress [35]. In response, these stimuli trigger the production of molecular chaperones designed to protect newly-synthesized or damaged proteins from aggregation. Among these chaperones, heat shock proteins are particularly abundant in cardiac tissue, and we have previously shown that they are among the most highly-upregulated genes during rapid activation of atrial HL-1 cells [9]. Expression of heat shock proteins is increased in the atria of both animal models and patients with AF [36-38], while pharmacologic upregulation appears to be protective [38]. These data imply a prominent role for protein misfolding in vivo, and thus proteotoxicity, as a novel potential mechanism of myocyte injury that could increase AF susceptibility (Figure 7). Because PAOs are not visible by standard amyloid staining methods, the identification of these cytotoxic protein oligomers has become possible only recently with the development of appropriate antibodies, such as A-11. Moreover, as demonstrated in the brain, PAOs can cause organ dysfunction in the absence of demonstrable cellular pathology [39].
For degenerative disorders related to aging, factors such as impaired protein homeostatic mechanisms and acquired mutations can contribute to protein misfolding [35]. Senile amyloidosis can develop exclusively in the atria, known as isolated atrial amyloidosis [31, 40, 41]. The incidence of this atrial-specific disorder increases with age, exceeding 90% in the ninth decade of life. In several studies, isolated atrial amyloidosis has been linked to an increased risk of AF [31, 32, 41]. Given that PAOs are intermediate complexes in the formation of amyloid fibrils (Figure 7), it is logical that they should be detectable in human atria, which we have recently demonstrated [42]. Moreover, amyloid fibrils in atrial amyloidosis are primarily composed of ANP, and our data demonstrate that ANP is a likely component of PAOs in rapidly-stimulated atrial HL-1 cells. Based on the organ-specific nature of isolated atrial amyloidosis, it is likely that as for the brain in Alzheimer’s disease, PAOs play a role in atrial pathophysiology.
Increasingly, toxic protein oligomers have been recognized to play a role in experimental and human cardiac disease, in particular cardiomyopathy [35]. αB-Crystallin (CryAB) is a small heat shock protein in the heart that binds the intermediate filament desmin. Mutations in CryAB cause a cardiomyopathy characterized by PAO formation that are toxic to proteasomes and implicated in myocardial dysfunction [15, 35]. Recent data indicate that PAOs can be detected in ventricular tissue of patients with systolic and/or diastolic dysfunction in the setting of dilated, hypertensive, and hypertrophic cardiomyopathy [14, 43, 44]. Given our data that rapid activation induces protein misfolding in atrial myocytes as well, we hypothesize that mechanical strain is a stimulus for PAO formation in cardiomyocytes.
Oxidative stress has been linked to both Alzheimer’s disease and AF, supporting the concept that common pathophysiologic mechanisms may be present for these diseases. Upstream therapy with drugs having antioxidant properties (e.g., statins, omega (n)-3 polyunsaturated fatty acids, and RAAS inhibitors) have not proven beneficial for primary or secondary prevention of AF [7, 45]. An important omission in nearly all of the studies is that the ability of the intervention to reduce ROS was not measured. Thus, one potential explanation is insufficient potency of existing drugs. Indeed, recent studies have shown that therapeutically-used doses of both vitamin E [46, 47] and fish oil [48] are ineffective to reduce in vivo measures of oxidative stress. This limitation of existing antioxidants has led to the concept of identifying molecular scavengers that specifically target critical mediators of atrial oxidant injury. Reactive aldehyde products of fatty acid oxidation have recently been identified as strong candidates for this pivotal role, given their capacity to react extremely rapidly with lysyl residues and covalently modify, crosslink, and aggregate proteins, as well as DNA [17, 21, 49-51]. Additionally, γ-KA adducts have been identified in affected regions of the brain in patients with Alzheimer’s disease [52]. γ-KAs have been shown to directly promote the formation of soluble oligomers derived from amyloid β1-42 [24], while inactivation of these reactive γ-KAs with the small molecule scavenger SA prevented development of cognitive impairment in a mouse model of Alzheimer’s disease [26]. A recent study has identified a required role for γ-KAs in T cell-mediated hypertension in mice, and SA (referred to as 2-hydroxybenzylamine or 2-HOBA) was effective to lower blood pressure [53]. Our data, demonstrating an inhibitory effect of salicylamine, support a similar mechanism for γ-KAs in PAO formation in rapidly-paced atrial HL-1 cells.
Conclusion
Our results identify preamyloid oligomers as a novel component of myocyte stress remodeling in response to rapid atrial cell activation, and they identify γ-KAs as a critical molecular component of this pathophysiologic process. Future studies in vivo are needed to determine whether these ROS-generated mediators could represent a novel therapeutic target in the treatment of AF. Nonetheless, PAO formation by γ-KAs provides a potential mechanistic link between oxidative stress, atrial cell injury, and AF susceptibility.
Supplementary Material
ACKNOWLEDGEMENTS
None declared.
FUNDING
This work was supported by a grant from the National Institutes of Health [HL096844]; the National Institute of General Medical Sciences at the National Institutes of Health [T32 GM007569]; the American Heart Association, Southeast Affiliate [2160035]; and the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number UL1 TR000445. Confocal microscopy and image analysis were performed through the Vanderbilt Cell Imaging Shared Resource, which is also supported by the National Institutes of Health [CA68485, DK20593, DK58404, DK59637 and EY08126]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- AF
atrial fibrillation
- PAO
preamyloid oligomer
- γ-KAs
γ-ketoaldehydes
- ANP
atrial natriuretic peptide
- SA
salicylamine
- RAAS
renin-angiotensin-aldosterone system
- PFA
paraformaldehyde
- PBS
phosphate buffered saline
- BSA
bovine serum albumin
- q-PCR
real-time quantitative RT-PCR
- Ct
cycle threshold
- CryAB
αB-crystallin
- DHE
dihydroethidium
- DAB
3,3-diaminobenzidine
- ROS
reactive oxygen species
- E2-IsoKs
E2-isolevuglandin/isoketals
Footnotes
CONFLICTS OF INTEREST
None declared.
REFERENCES
- [1].Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–13. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- [2].De Jong AM, Maass AH, Oberdorf-Maass SU, Van Veldhuisen DJ, Van Gilst WH, Van Gelder IC. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89:754–65. doi: 10.1093/cvr/cvq357. [DOI] [PubMed] [Google Scholar]
- [3].Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115:135–43. doi: 10.1016/j.ijcard.2006.04.026. [DOI] [PubMed] [Google Scholar]
- [4].Mihm MJ, Yu F, Carnes CA, Reiser PJ, McCarthy PM, Van Wagoner DR, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–80. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- [5].Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–36. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- [6].Dudley SC, Jr., Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–73. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- [7].Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: primary prevention. Europace. 2011;13:308–28. doi: 10.1093/europace/eur002. [DOI] [PubMed] [Google Scholar]
- [8].Yang Z, Shen W, Rottman JN, Wikswo JP, Murray KT. Rapid stimulation causes electrical remodeling in cultured atrial myocytes. J Mol Cell Cardiol. 2005;38:299–308. doi: 10.1016/j.yjmcc.2004.11.015. [DOI] [PubMed] [Google Scholar]
- [9].Mace LC, Yermalitskaya LV, Yi Y, Yang Z, Morgan AM, Murray KT. Transcriptional remodeling of rapidly stimulated HL-1 atrial myocytes exhibits concordance with human atrial fibrillation. J Mol Cell Cardiol. 2009;47:485–92. doi: 10.1016/j.yjmcc.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Klein WL, Krafft GA, Finch CE. Targeting small Aβ oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–24. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- [11].Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology. 2006;66:S74–S78. doi: 10.1212/01.wnl.0000192103.24796.42. [DOI] [PubMed] [Google Scholar]
- [12].Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15:384–96. doi: 10.1038/nrm3810. [DOI] [PubMed] [Google Scholar]
- [13].Guerrero-Munoz MJ, Castillo-Carranza DL, Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem Pharmacol. 2014;88:468–78. doi: 10.1016/j.bcp.2013.12.023. [DOI] [PubMed] [Google Scholar]
- [14].Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, et al. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci U S A. 2004;101:10132–6. doi: 10.1073/pnas.0401900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, et al. Expression of R120G-αB-crystallin causes aberrant desmin and αB-crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. doi: 10.1161/hh1301.092688. [DOI] [PubMed] [Google Scholar]
- [16].Salomon RG, Miller DB, Zagorski MG, Coughlin DL. Solvent induced fragmentation of prostaglandin endoperoxides. New aldehyde products from PGH2 and novel intramolecular 1,2-hydride shift during endoperoxide fragmentation in aqueous solution. J Am Chem Soc. 1984;106:6049–60. [Google Scholar]
- [17].Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, et al. Pyridoxamine analogues scavenge lipid-derived gamma-ketoaldehydes and protect against H2O2-mediated cytotoxicity. Biochemistry. 2006;45:15756–67. doi: 10.1021/bi061860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Salomon RG, Subbanagounder G, Singh U, O’Neil J, Hoff HF. Oxidation of low-density lipoproteins produces levuglandin-protein adducts. Chem Res Toxicol. 1997;10:750–9. doi: 10.1021/tx970016b. [DOI] [PubMed] [Google Scholar]
- [19].Iyer RS, Ghosh S, Salomon RG. Levuglandin E2 crosslinks proteins. Prostaglandins. 1989;37:471–80. doi: 10.1016/0090-6980(89)90096-8. [DOI] [PubMed] [Google Scholar]
- [20].Brame CJ, Salomon RG, Morrow JD, Roberts LJ. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. 1999;274:13139–46. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- [21].Boutaud O, Ou JJ, Chaurand P, Caprioli RM, Montine TJ, Oates JA. Prostaglandin H2 (PGH2) accelerates formation of amyloid β1-42 oligomers. J Neurochem. 2002;82:1003–6. doi: 10.1046/j.1471-4159.2002.01064.x. [DOI] [PubMed] [Google Scholar]
- [22].Boutaud O, Andreasson KI, Zagol-Ikapitte I, Oates JA. Cyclooxygenase-dependent lipid-modification of brain proteins. Brain Pathol. 2005;15:139–42. doi: 10.1111/j.1750-3639.2005.tb00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roychowdhury S, McMullen MR, Pritchard MT, Li W, Salomon RG, Nagy LE. Formation of γ-ketoaldehyde-protein adducts during ethanol-induced liver injury in mice. Free Radic Biol Med. 2009;47:1526–38. doi: 10.1016/j.freeradbiomed.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boutaud O, Montine TJ, Chang L, Klein WL, Oates JA. PGH2-derived levuglandin adducts increase the neurotoxicity of amyloid β1-42. J Neurochem. 2006;96:917–23. doi: 10.1111/j.1471-4159.2005.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Amarnath V, Amarnath K, Amarnath K, Davies S, Roberts LJ. Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem Res Toxicol. 2004;17:410–5. doi: 10.1021/tx0300535. [DOI] [PubMed] [Google Scholar]
- [26].Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, et al. Treatment with a γ-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. J Alzheimers Dis. 2011;27:49–59. doi: 10.3233/JAD-2011-102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- [28].Amarnath V, Amarnath K, Masterson TS, Davies S, Roberts LJ. A simplified synthesis of the diasteromers of levuglandin E2. Synthetic Communications. 2005;35:397–408. [Google Scholar]
- [29].Davies SS, Talati M, Wang X, Mernaugh RL, Amarnath V, Fessel J, et al. Localization of isoketal adducts in vivo using a single-chain antibody. Free Radic Biol Med. 2004;36:1163–74. doi: 10.1016/j.freeradbiomed.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [30].Kayed R, Canto I, Breydo L, Rasool S, Lukacsovich T, Wu J, et al. Conformation dependent monoclonal antibodies distinguish different replicating strains or conformers of prefibrillar Aβ oligomers. Mol Neurodegener. 2010;5:57. doi: 10.1186/1750-1326-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rocken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106:2091–7. doi: 10.1161/01.cir.0000034511.06350.df. [DOI] [PubMed] [Google Scholar]
- [32].Leone O, Boriani G, Chiappini B, Pacini D, Cenacchi G, Martin SS, et al. Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation. Eur Heart J. 2004;25:1237–41. doi: 10.1016/j.ehj.2004.04.007. [DOI] [PubMed] [Google Scholar]
- [33].Epstein JA. Currying favor for the heart. J Clin Invest. 2008;118:850–2. doi: 10.1172/JCI34650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- [35].Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction--Alzheimer’s disease of the heart? N Engl J Med. 2013;368:455–64. doi: 10.1056/NEJMra1106180. [DOI] [PubMed] [Google Scholar]
- [36].Vitadello M, Ausma J, Borgers M, Gambino A, Casarotto DC, Gorza L. Increased myocardial GRP94 amounts during sustained atrial fibrillation: a protective response? Circulation. 2001;103:2201–6. doi: 10.1161/01.cir.103.17.2201. [DOI] [PubMed] [Google Scholar]
- [37].Yang M, Tan H, Cheng L, He M, Wei Q, Tanguay RM, et al. Expression of heat shock proteins in myocardium of patients with atrial fibrillation. Cell Stress Chaperones. 2007;12:142–50. doi: 10.1379/CSC-253R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brundel BJ, Henning RH, Ke L, Van G I, Crijns HJ, Kampinga HH. Heat shock protein upregulation protects against pacing-induced myolysis in HL-1 atrial myocytes and in human atrial fibrillation. J Mol Cell Cardiol. 2006;41:555–62. doi: 10.1016/j.yjmcc.2006.06.068. [DOI] [PubMed] [Google Scholar]
- [39].Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Seldin DC, Skinner M. Arthritis accompanying systemic diseases. In: Harris ED, Budd RC, Genovese MC, Firestein GS, Sargent JS, Sledge CB, editors. Kelley’s Textbook of Rheumatology. Elsevier Sanders; Philadelphia: 2005. pp. 1697–704. [Google Scholar]
- [41].Steiner I, Hajkova P. Patterns of isolated atrial amyloid: a study of 100 hearts on autopsy. Cardiovasc Pathol. 2006;15:287–90. doi: 10.1016/j.carpath.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [42].Sidorova TN, Mace LC, Wells KS, Yermalitskaya LV, Su PF, Shyr Y, et al. Quantitative imaging of preamyloid oligomers, a novel structural abnormality, in human atrial samples. J Histochem Cytochem. 2014;62:479–87. doi: 10.1369/0022155414535782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gianni D, Li A, Tesco G, McKay KM, Moore J, Raygor K, et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation. 2010;121:1216–26. doi: 10.1161/CIRCULATIONAHA.109.879510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Despa S, Margulies KB, Chen L, Knowlton AA, Havel PJ, Taegtmeyer H, et al. Hyperamylinemia contributes to cardiac dysfunction in obesity and diabetes: a study in humans and rats. Circ Res. 2012;110:598–608. doi: 10.1161/CIRCRESAHA.111.258285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: review of clinical evidence and implications for European Society of Cardiology guidelines. Part II: secondary prevention. Europace. 2011;13:610–25. doi: 10.1093/europace/eur023. [DOI] [PubMed] [Google Scholar]
- [46].Roberts LJ, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, et al. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med. 2007;43:1388–93. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Blumberg JB, Frei B. Why clinical trials of vitamin E and cardiovascular diseases may be fatally flawed. Commentary on “The relationship between dose of vitamin E and suppression of oxidative stress in humans”. Free Radic Biol Med. 2007;43:1374–6. doi: 10.1016/j.freeradbiomed.2007.08.017. [DOI] [PubMed] [Google Scholar]
- [48].Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J Am Coll Cardiol. 2014;64:1441–8. doi: 10.1016/j.jacc.2014.07.956. [DOI] [PubMed] [Google Scholar]
- [49].Davies SS, Amarnath V, Montine KS, Bernoud-Hubac N, Boutaud O, Montine TJ, et al. Effects of reactive g-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 2002;16:715–7. doi: 10.1096/fj.01-0696fje. [DOI] [PubMed] [Google Scholar]
- [50].Murthi KK, Friedman LR, Oleinick NL, Salomon RG. Formation of DNA-protein cross-links in mammalian cells by levuglandin E2. Biochemistry. 1993;32:4090–7. doi: 10.1021/bi00066a034. [DOI] [PubMed] [Google Scholar]
- [51].Carrier EJ, Amarnath V, Oates JA, Boutaud O. Characterization of covalent adducts of nucleosides and DNA formed by reaction with levuglandin. Biochemistry. 2009;48:10775–81. doi: 10.1021/bi9015132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zagol-Ikapitte I, Masterson TS, Amarnath V, Montine TJ, Andreasson KI, Boutaud O, et al. Prostaglandin H2-derived adducts of proteins correlate with Alzheimer’s disease severity. J Neurochem. 2005;94:1140–5. doi: 10.1111/j.1471-4159.2005.03264.x. [DOI] [PubMed] [Google Scholar]
- [53].Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–56. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.