Abstract
Aims
Recently, we described a series of pancreatic neuroendocrine tumors (PanNETs) featuring prominent stromal fibrosis, which we called sclerosing PanNET. In this study, we examined the pathologic, immunophenotypic, and clinical differences between sclerosing and non-sclerosing PanNETs.
Methods and Results
One hundred and six PanNETs were identified, of which, 15 (14%) were sclerosing NETs. Tissue microarrays containing 44 non-sclerosing and 5 sclerosing panNETs as well as sections from 10 additional sclerosing tumors were immunohistochemically labeled with serotonin, CDX2, CDH17 and islet 1. Sclerosing PanNETs were smaller in size (p=0.045) and more likely to show an infiltrative growth pattern (p< 0.001) compared to non-sclerosing PanNETs. They were frequently associated with a large pancreatic duct, causing duct stenosis. Additionally, we found significantly increased expression of the small intestinal NET markers serotonin, CDX2, and CDH17 in sclerosing PanNETs (p<0.001) compared with non-sclerosing PanNETs. No difference in clinical outcome was found; however, more sclerosing PanNETs were stage IIB or above (p=0.035), with lymph node metastasis being seen in 3 of 9 sclerosing PanNETs with a tumor size less than 2.0 cm.
Conclusions
Sclerosing PanNETs have distinct pathologic features and biomarker expression profiles. In addition, lymph node metastasis can be present even in small sclerosing PanNETs.
Keywords: Cadherin 17, serotonin, sclerosing, pancreatic neuroendocrine tumor
Introduction
Although rare, pancreatic neuroendocrine tumors (PanNETs) and small intestine neuroendocrine tumors (SINETs) are the second most common malignancies in the pancreas and small intestine, respectively[1-3]. PanNETs and SINETs share similar morphologies and both frequently metastasize to the liver[4]. Despite similarities, they differ in many aspects, including biological behavior, genetic basis, and biomarker expression.
Morphologically, PanNETs tend to show hypercellularity with minimal stromal fibrosis, while SINETs are associated with frequent stromal fibrosis, especially in deep invasion[5]. Serotonin expression is not generally documented in PanNETs and may mediate the fibrosis seen in SINETs[5]. MEN1, DAXX, ATRX, and the PI3K signaling pathway genes are frequently mutated in PanNETs but not in SINETs[6-10]. CDX2 is a transcription factor that functions in intestinal cell growth and differentiation by inducing transduction of proteins such as cadherin 17 (CDH17)[11]. Expression of both CDX2 and CDH17 is a feature of SINETs but absent in most PanNETs[12]. Unlike SINETs, PanNETs also express pancreatic and duodenal homeobox 1 (pdx1) and the transcription factor islet 1 gene product, islet 1[13].
Recently, we identified a group of PanNETs characterized by prominent stromal fibrosis and a frequent association with pancreatic duct strictures, which we called the sclerosing variant of PanNET or sclerosing PanNET[14-16]. Like SINETs, sclerosing PanNETs frequently express serotonin, but little is known regarding sclerosing PanNET biomarkers or their clinical/prognostic significance. This study examined the clinical and pathologic features of sclerosing PanNET, including expression of CDX2 and CDH17.
Materials and Methods
Patient selection
Between September 1998 and December 2013, 106 patients who underwent PanNET resection—distal pancreatectomy, partial pancreatectomy, or Whipple procedure—were identified from our pathology databases. Slides and pathology reports were reviewed for all cases, and the following pathologic features were documented: tumor size, tumor location, focality, tumor stage, lymphovascular invasion, perineural invasion, tumor necrosis, and growth pattern—infiltrative versus well-defined. Of the 106 cases, 15 (14%) were identified as sclerosing PanNETs, showing the previously described trabecular or trabecular-glandular pattern with interspersed prominent fibrosis (Figure 1A-B)[14-16]. Ki67 index and mitotic rate were available for all cases and the 2010 World Health Organization (WHO) grading system for PanNETs was used to classify tumors into grades 1 (Ki67 <3% and <2 mitoses/10 HPF), 2 (Ki67 3-20% or 2-20 mitoses/10 HPF), or 3 (Ki67 >20% or >20 mitoses/10 HPF). Patient demographics and clinical data were collected from the electronic medical record. Long-term survival status was determined by review of the medical records and through use of the social security death index. Three patients who died of surgical complications were excluded from overall survival analysis. This study was approved by Vanderbilt Institutional Review Board (IRB#101735, 08/20/2010).
Figure 1.
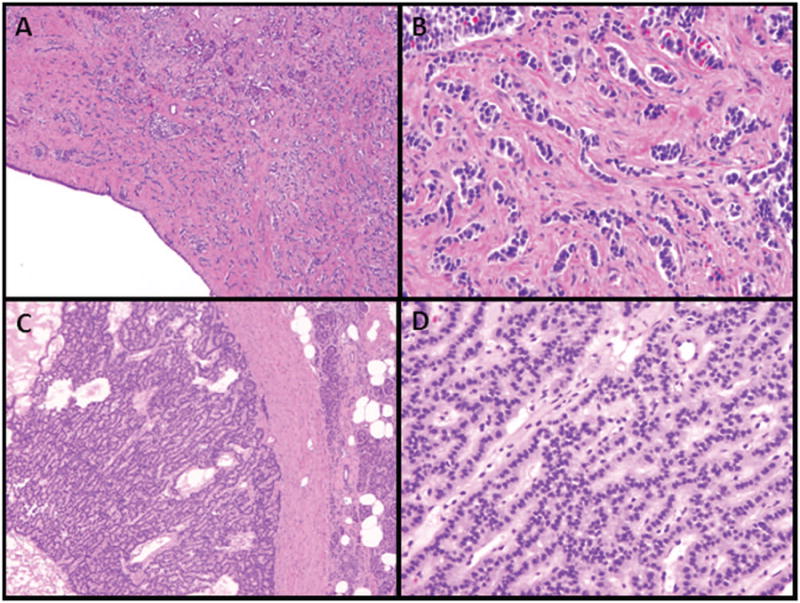
Examples of sclerosing and non-sclerosing pancreatic neuroendocrine tumors. A-B: A sclerosing pancreatic neuroendocrine tumor surrounding a pancreatic duct (A, original magnification 40×; B, 200×); B: A non-sclerosing, hypercellular pancreatic neuroendocrine tumor (C, original magnification 40×; D, 200×).
Immunohistochemistry
Two tissue microarrays (TMAs) containing 44 non-sclerosing PanNETs and 5 sclerosing PanNETs along with 10 additional sclerosing PanNET slides were immunohistochemically labeled with serotonin, CDX2, CDH17 and islet 1 primary antibodies (Table 1). 4 μm sections were cut and deparaffinized by routine methods. For antigen retrieval, sections were heated to 105°C for 20 minutes in pH 6.0 citrate buffer. Sections were cooled to room temperature, quenched with 3% H2O2 in sodium azide for 5 minutes, and incubated with primary antibodies. Antibody localization was performed using Dako Envision+ HRP-labeled polymer (DAKO), and stains were visualized by 5 minute incubation with diaminobenzidine.
Table 1. Details for antibodies used.
| Antibody | Source | Catalog# | Clonal | dilution |
|---|---|---|---|---|
| Serotonin | Dako (Carpinteria, CA) | M0758 | Mouse Monoclonal | 1:200 |
| CDX2 | Cell Signaling (Danvers, MA) | 3977 | Rabbit Polyclonal | 1:400 |
| CDH17 | Novus (Littleton, CO) | H00001015-M01 | Mouse Monoclonal | 1:1000 |
| Islet 1 | Abcam (Cambridge, MA) | ab124651 | Mouse Monoclonal | 1:400 |
Assessment of immunohistochemical stains was performed on cells demonstrating cytoplasmic staining by serotonin, membranous staining by CDH17, and nuclear staining by CDX2 and islet 1. A positive staining reaction was defined as >5% staining of tumor cells. CDH17 and CDX2 labeling was further graded into focally positive (<50%) and diffusely positive (>50%). TMA sections without significant tumor present were excluded from immunohistochemical evaluation.
Statistical analysis
Patients' demographics, clinical variables, and biomarker expressions were summarized using the median with the 25th and 75th percentiles (quartiles) for continuous variables. For categorical variables, frequency and percentages were shown. Patients' overall survival was defined as the time from PanNET resection to the date of all-cause death or last follow-up. Comparisons between non-sclerosing and sclerosing groups were conducted with Wilcoxon rank-sum test (continuous variables) and Pearson's Chi-squared test (categorical variables). The Kaplan-Meier method and Log-rank test were used to compare the overall survival between sclerosing and non-sclerosing groups and the CDH17 groups (positive vs. negative). Analyses were performed with R version 2[17], and statistical significance was based on two-sided tests at the 5% level.
Results
Fifteen of 106 (14%) cases were identified as sclerosing PanNETs, which included 9 males and 6 females (Table 1) with a median age of 56, ranging from 32 to 78 years. Of the 91 patients with non-sclerosing tumors, 13 (14%) had multiple endocrine neoplasia type 1 (MEN1) syndrome and 4 (4%) had Von Hipple Lindau disease. One of 15 patients with sclerosing tumors had an underlying syndrome—MEN1. Fifteen of 91 patients with non-sclerosing tumors were functional, which was defined by hormone-related symptoms and immunohistochemical stains for insulin, gastrin or glucagon. Tumor function was absent in all sclerosing PanNETs. Comparison of sclerosing versus non-sclerosing PanNETs found no statistically significant differences regarding gender, age, syndrome presence, or tumor functionality. Sclerosing tumors were significantly smaller in size compared to non-sclerosing PanNETs (median 2.5 cm, interquartile range 1.5 cm to 4.3 cm, n=91 versus median 1.5 cm, interquartile range 1.2 cm to 2.6 cm, n=15; p=0.045; see Table 2).
Table 2.
Comparison of non-sclerosing and sclerosing PanNETs.
| N | Non-Sclerosing N=91 | Sclerosing N = 15 | P-value | |
|---|---|---|---|---|
| Sex | 106 | 0.28 | ||
| Female | 55% (50) | 40% (6) | ||
| Male | 45% (41) | 60% (9) | ||
|
| ||||
| Age | 106 | 56 (44, 65) | 56 (45, 65) | 0.48 |
|
| ||||
| Syndrome | 106 | 0.25 | ||
| Yes | 19% (17) | 7% (1) | ||
| No | 81% (74) | 93% (14) | ||
|
| ||||
| Functional | 106 | 0.20 | ||
| Yes | 18% (15) | 0% (0) | ||
| No | 82% (76) | 100% (15) | ||
|
| ||||
| Size (cm) | 106 | 2.5 (1.5, 4.3) | 1.5 (1.2, 2.6) | 0.045 |
|
| ||||
| LVI | 106 | 0.48 | ||
| Yes | 31% (28) | 40% (6) | ||
| No | 69% (63) | 60% (9) | ||
|
| ||||
| PNI | 106 | |||
| Yes | 11% (10) | 27% (4) | 0.097 | |
| No | 89% (81) | 73% (11) | ||
|
| ||||
| Necrosis | 105 | |||
| Yes | 12% (11) | 0% (0) | 0.15 | |
| No | 88% (79) | 100% (15) | ||
|
| ||||
| Infiltrative | 106 | <0.001 | ||
| Yes | 28% (25) | 80% (12) | ||
| No | 72% (66) | 20% (3) | ||
|
| ||||
| Islet1 | 0.33 | |||
| Positive | 54 | 78% (31) | 64% (9) | |
| Negative | 22% (9) | 36% (5) | ||
|
| ||||
| Serotonin | 59 | < 0.001 | ||
| Positive | 4% (2) | 47% (7) | ||
| Negative | 96% (42) | 53% (8) | ||
|
| ||||
| CDX2 | < 0.001 | |||
| Positive | 59 | 11% (5) | 73% (11) | |
| Negative | 89% (39) | 27% (4) | ||
|
| ||||
| CDH 17 | 59 | < 0.001 | ||
| Positive | 36% (16) | 93% (14) | ||
| Negative | 64% (28) | 7% (1) | ||
|
| ||||
| WHO Grade | 106 | 0.6 | ||
| 1 | 45% (41) | 53% (8) | ||
| 2 | 39% (35) | 40% (6) | ||
| 3 | 16% (15) | 7% (1) | ||
|
| ||||
| Stage | 106 | 0.035 | ||
| I - IIA | 74% (67) | 47% (7) | ||
| IIB - V | 26% (24) | 53% (8) | ||
|
| ||||
| Survival | 103 | 0.43 | ||
| Alive | 88% (77) | 80% (12) | ||
| Dead | 12% (11) | 20% (3) | ||
Age and tumor size were summarized with median and quartiles (Wilcoxon rank sum test). P-values were from Wilcoxon rank sum test (continuous) and Pearson's Chi-squared test (categorical). PanNETs: pancreatic neuroendocrine tumors.
Microscopically, sclerosing PanNETs were composed of small nests, trabeculae, or single files of well-differentiated neuroendocrine tumor cells embedded in a prominent fibrous stroma (Figure 1A-B), whereas non-sclerosing PanNETs were always hypercellular tumors with minmal stroma (Figure 1C-D). Sections from 10 of the 15 sclerosing tumors demonstrated an associated stenotic pancreatic duct (Figure 1A). Unlike most non-sclerosing PanNETs, sclerosing variants were frequently associated with severe chronic pancreatitis in adjacent parenchyma. We found no differences in lymphovascular invasion, perineural invasion, or tumor necrosis between non-sclerosing and sclerosing groups. However, infiltrative growth patterns did vary by group, seen in 80% and 28% of sclerosing and non-sclerosing tumors, respectively (p<0.001, Table 2).
Immunohistochemical stains for islet 1, serotonin, CDX2 and CDH17 were performed on 44 non-sclerosing and 15 sclerosing PanNETs (Figure 2). Both groups showed expression of islet 1 (p=0.33, Table 2, Figure 2A-B). Consistent with previous studies, most of non-sclerosing PanNETs (2/44, 4.5%, Figure 2C) did not express serotonin, which was found in 7 of 15 (47%) sclerosing variants (p<0.001, Figure 2D). Similar to serotonin, 11 of 15 (75%) sclerosing PanNETs diffusely expressed CDX2 (Figure 2F). Only 5 of 44 (11%) non-sclerosing NETs (p<0.001, Figure 2E) showed CDX2 expression, with 3 showing focal staining and 2 diffuse staining. CDH17 was expressed in 16 of 44 (36%) non-sclerosing PanNETs (Figure 2G), 10 of which showed focal immunoreactivity. In contrast, CDH17 expression was present in 14 of 15 (93%) sclerosing cases (p<0.001), with 11 of them showing diffuse labeling (Figure 2H). Seven of 14 (50%) CDH17 positive sclerosing tumors also showed expression of serotonin and CDX2.
Figure 2.
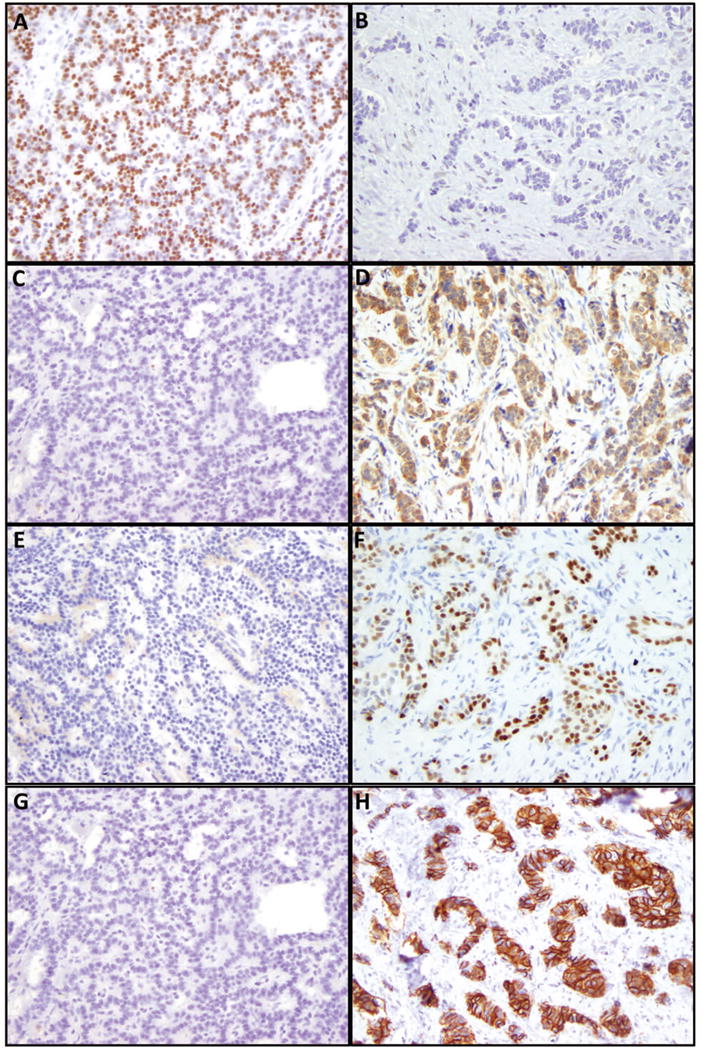
Expression of islet 1, serotonin, CDX2 and CDH17 in non-sclerosing and sclerosing pancreatic neuroendocrine tumors (original magnification 200×). A-B: Immunohistochemical labeling of islet 1 in non-sclerosing tumor (A) and sclerosing tumor (B); C-D: Immunohistochemical labeling of serotonin in non-sclerosing tumor (C) and sclerosing tumor (D); E-F: Immunohistochemical labeling of CDX2 in non-sclerosing tumor (E) and sclerosing tumor (F); G-H: immunohistochemical labeling of CDH17 in non-sclerosing tumor (G) and sclerosing tumor (H).
Expression of CDH17 in PanNETs, including both sclerosing and non-sclerosing tumors, tended to adversely affect patient overall survival; 8 of 28 (29%) patients died of disease or other causes in CDH17 positive group, whereas only 4 of 28 (14%) died in CDH17 negative group (Figure 3, p=0.094).
Figure 3.
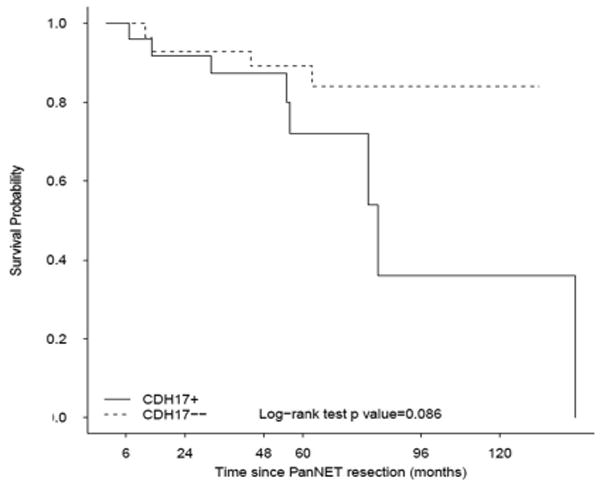
Overall survival by CDH17 expression.
According to the American Joint Committee on Cancer 7th edition, 8 of 15 (53%) sclerosing tumor cases were staged 2B or above and showed either lymph node (n=7) or distant metastasis (n=1). Three of the 7 cases with lymph node metastasis had a tumor size of less than 1.6 cm. Compared to sclerosing PanNETs, non-sclerosing tumors showed an earlier stage, with only 24 of 91 (26%) cases staged 2B or above (p=0.035). WHO grades were similar between groups (p=0.6). Median survival were 44 and 34 months for non-sclerosing and sclerosing PanNETs, respectively (p=0.13). Three of the 15 (20%) sclerosing cases died of the disease, whereas 11 of 88 (11%) died of the disease or other causes in non-sclerosing group (p=0.43). Although smaller in size, patients with sclerosing PanNETs did not have improved clinical outcomes over non-sclerosing PanNETs.
Discussion
Sclerosing PanNET is a recently described tumor characterized by decreased tumor cellularity, prominent stromal fibrosis, and associated pancreatic duct stenosis with subsequent chronic pancreatitis of the uninvolved pancreas. Compared to classical PanNET, our study suggests a more pronounced infiltrative growth pattern in the sclerosing variant as well as several biomarker expression differences typically associated with SINETs. In addition, patients with a sclerosing PanNET were more likely to present with a late stage disease (stage IIB or above) compared to those with a non-sclerosing tumor.
The morphology of sclerosing PanNET resembles that of SINET. In addition, sclerosing PanNETs metastasize to the liver in similar frequency to SINET. Therefore, biomarkers are needed to distinguish metastatic sclerosing PanNETs from SINETs. This study shows that immunohistochemical stains for CDX2, CDH17, and serotonin did not discriminate SINET from sclerosing PaNET. Other biomarkers such as islet 1, Pdx1 and PAX8 were originally thought to be highly specific for PanNETs; however, recent studies have shown that they are expressed in intestinal NETs[18-22].
CDH17, a calcium-dependent, membrane-associated glycoprotein, is expressed in the small intestine, colon, and pancreatic ducts[23, 24]. Previous studies have demonstrated direct regulation of CDH17 expression by CDX2 in normal, metaplastic and neoplastic epithelial cells of the gastrointestinal tract[11]. Co-expression of CDX2 and CDH17 is frequently observed in intestinal metaplasia/neoplasia in the gastrointestinal tract and pancreas[12, 25-27]. CDX2 and CDH17 expression has also been documented in Barrett's esophagus [26, 27]. In the pancreas, intestinal type intraductal papillary mucinous neoplasms also co-express CDX2 and CDH17[25]. We found 11 of 14 CDH17 positive sclerosing tumors co-expressed CDX2, suggesting an association of both proteins in these lesions. However, SINETs also frequently express both CDH17 and CDX2[12]. The morphologic and immunophenotypical similarities between sclerosing PanNET and SINETs suggest a possible shared carcinogenesis.
Overexpression of CDH17 has been associated with lymph node metastasis and poor prognosis in gastric cancer[28, 29], whereas reduced expression was associated with tumor dedifferentiation and shorter overall survival in colorectal cancer[30]. In PanNETs, CDH17 expression trended to a negative effect on patient survival; however, studies with a larger group of PanNET patients are needed to determine definitive prognostic role of CDH17 expression in these tumors.
Our data show that sclerosing PanNETs are smaller than non-sclerosing PanNETs, with an average dimension of 1.9 cm versus 3.5 cm, respectively. PanNET tumor size has been shown to impact prognosis [31]; however, we found similar survival rates in sclerosing PanNET versus non-sclerosing PanNETs, despite a significantly smaller tumor size seen in sclerosing PanNETs. Lymph node metastasis was found in 7 of 15 sclerosing tumors, 3 of which measured less than 2.0 cm in greatest dimension. In non-sclerosing PanNETs, nodal metastasis was only found in tumors larger than 2.0 cm. Median survival was 44 months for non-sclerosing and 34 months for sclerosing tumors. There were more tumors staged IIB or above in the sclerosing group than in the non-sclerosing group. However, a statistically significant difference in survival was not found and limitations in sample sizes as well as short follow-up time may have prevented detection of decreased survival in sclerosing lesions.
Aside from prognostic significance, the smaller size of sclerosing PanNET is an important feature that may lead to false negative imaging studies. Prominent tumor fibrosis in sclerosing PanNETs can cause pancreatic duct stenosis, consequently leading to upstream pancreatic duct dilation and chronic pancreatitis. In cases where imaging reveals liver metastasis without an obvious pancreatic mass and with chronic pancreatitis or pancreatic duct dilation, a sclerosing PanNET should be considered. When a small PanNET is detected by imaging studies, changes in adjacent pancreatic duct and background pancreas should be documented in detail. Presence of upstream pancreatic duct dilation and/or chronic pancreatitis may indicate a sclerosing PanNET. In such cases, a radical resection might be indicated due to the fact that sclerosing NETs can have lymph node metastasis even when they are less than 2.0 cm.
In summary, sclerosing PanNETs are rare, frequently associated with fibrosis, and express several biomarkers characteristically present in SINETs. In addition, surgical management of sclerosing PanNET may need to be more aggressive, since even small PanNETs can metastasize to lymph nodes.
Acknowledgments
This project is funded by NIH/NIDDK P30DK058404 (ZZ, TK and CS) and NIH/NCIP50CA095103(CS)
Footnotes
Conflict of Interest: none for each author
Author Contributions: Adam Johnson, Jesse Wright and Chanjuan Shi performed the research
Zhiguo Zhao and Tatsuki Komaya analyzed data
Alexander Parikh, Nipun Merchant and Chanjuan Shi designed the research study.
Adam Johnson and Chanjuan Shi wrote the paper
References
- 1.Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control. 2005;16:781–787. doi: 10.1007/s10552-005-3635-6. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–3500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 5.Druce M, Rockall A, Grossman AB. Fibrosis and carcinoid syndrome: from causation to future therapy. Nat Rev Endocrinol. 2009;5:276–283. doi: 10.1038/nrendo.2009.51. [DOI] [PubMed] [Google Scholar]
- 6.Bergsland EK. The evolving landscape of neuroendocrine tumors. Semin Oncol. 2013;40:4–22. doi: 10.1053/j.seminoncol.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Oberg K. The genetics of neuroendocrine tumors. Semin Oncol. 2013;40:37–44. doi: 10.1053/j.seminoncol.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Francis JM, Kiezun A, Ramos AH, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet. 2013;45:1483–1486. doi: 10.1038/ng.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banck MS, Kanwar R, Kulkarni AA, et al. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest. 2013;123:2502–2508. doi: 10.1172/JCI67963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinoi T, Lucas PC, Kuick R, Hanash S, Cho KR, Fearon ER. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology. 2002;123:1565–1577. doi: 10.1053/gast.2002.36598. [DOI] [PubMed] [Google Scholar]
- 12.Panarelli NC, Yantiss RK, Yeh MM, Liu Y, Chen YT. Tissue-specific cadherin CDH17 is a useful marker of gastrointestinal adenocarcinomas with higher sensitivity than CDX2. Am J Clin Pathol. 2012;138:211–222. doi: 10.1309/AJCPKSHXI3XEHW1J. [DOI] [PubMed] [Google Scholar]
- 13.Hermann G, Konukiewitz B, Schmitt A, Perren A, Kloppel G. Hormonally defined pancreatic and duodenal neuroendocrine tumors differ in their transcription factor signatures: expression of ISL1, PDX1, NGN3, and CDX2. Virchows Arch. 2011;459:147–154. doi: 10.1007/s00428-011-1118-6. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto S, Shi C, Hruban RH, et al. Small serotonin-producing neuroendocrine tumor of the pancreas associated with pancreatic duct obstruction. AJR Am J Roentgenol. 2011;197:W482–488. doi: 10.2214/AJR.10.5428. [DOI] [PubMed] [Google Scholar]
- 15.Shi C, Siegelman SS, Kawamoto S, et al. Pancreatic duct stenosis secondary to small endocrine neoplasms: a manifestation of serotonin production? Radiology. 2010;257:107–114. doi: 10.1148/radiol.10100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCall CM, Shi C, Klein AP, et al. Serotonin expression in pancreatic neuroendocrine tumors correlates with a trabecular histologic pattern and large duct involvement. Hum Pathol. 2012;43:1169–1176. doi: 10.1016/j.humpath.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 18.Schmitt AM, Riniker F, Anlauf M, et al. Islet 1 (Isl1) expression is a reliable marker for pancreatic endocrine tumors and their metastases. Am J Surg Pathol. 2008;32:420–425. doi: 10.1097/PAS.0b013e318158a397. [DOI] [PubMed] [Google Scholar]
- 19.Koo J, Mertens RB, Mirocha JM, Wang HL, Dhall D. Value of Islet 1 and PAX8 in identifying metastatic neuroendocrine tumors of pancreatic origin. Mod Pathol. 2012;25:893–901. doi: 10.1038/modpathol.2012.34. [DOI] [PubMed] [Google Scholar]
- 20.Agaimy A, Erlenbach-Wunsch K, Konukiewitz B, et al. ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Mod Pathol. 2013;26:995–1003. doi: 10.1038/modpathol.2013.40. [DOI] [PubMed] [Google Scholar]
- 21.Graham RP, Shrestha B, Caron BL, et al. Islet-1 is a sensitive but not entirely specific marker for pancreatic neuroendocrine neoplasms and their metastases. Am J Surg Pathol. 2013;37:399–405. doi: 10.1097/PAS.0b013e31826f042c. [DOI] [PubMed] [Google Scholar]
- 22.Chan ES, Alexander J, Swanson PE, Jain D, Yeh MM. PDX-1, CDX-2, TTF-1, and CK7: a reliable immunohistochemical panel for pancreatic neuroendocrine neoplasms. Am J Surg Pathol. 2012;36:737–743. doi: 10.1097/PAS.0b013e31824aba59. [DOI] [PubMed] [Google Scholar]
- 23.Takamura M, Sakamoto M, Ino Y, et al. Expression of liver-intestine cadherin and its possible interaction with galectin-3 in ductal adenocarcinoma of the pancreas. Cancer Sci. 2003;94:425–430. doi: 10.1111/j.1349-7006.2003.tb01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gessner R, Tauber R. Intestinal cell adhesion molecules. Liver-intestine cadherin Ann N Y Acad Sci. 2000;915:136–143. doi: 10.1111/j.1749-6632.2000.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 25.Morimatsu K, Aishima S, Kayashima T, et al. Liver-intestine cadherin expression is associated with intestinal differentiation and carcinogenesis in intraductal papillary mucinous neoplasm. Pathobiology. 2012;79:107–114. doi: 10.1159/000334269. [DOI] [PubMed] [Google Scholar]
- 26.Weimann A, Rieger A, Zimmermann M, et al. Comparison of six immunohistochemical markers for the histologic diagnosis of neoplasia in Barrett's esophagus. Virchows Arch. 2010;457:537–545. doi: 10.1007/s00428-010-0972-y. [DOI] [PubMed] [Google Scholar]
- 27.Weimann A, Zimmermann M, Gross M, Slevogt H, Rieger A, Morawietz L. CDX2 and LI-cadherin expression in esophageal mucosa: use of both markers can facilitate the histologic diagnosis of Barrett's esophagus and carcinoma. Int J Surg Pathol. 2010;18:330–337. doi: 10.1177/1066896910364228. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Yu JC, Kang WM, Wang WZ, Liu YQ, Gu P. The predictive effect of cadherin-17 on lymph node micrometastasis in pN0 gastric cancer. Ann Surg Oncol. 2012;19:1529–1534. doi: 10.1245/s10434-011-2115-3. [DOI] [PubMed] [Google Scholar]
- 29.Park SS, Kang SH, Park JM, et al. Expression of liver-intestine cadherin and its correlation with lymph node metastasis in gastric cancer: can it predict N stage preoperatively? Ann Surg Oncol. 2007;14:94–99. doi: 10.1245/s10434-006-9114-9. [DOI] [PubMed] [Google Scholar]
- 30.Kwak JM, Min BW, Lee JH, et al. The prognostic significance of E-cadherin and liver intestine-cadherin expression in colorectal cancer. Dis Colon Rectum. 2007;50:1873–1880. doi: 10.1007/s10350-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 31.Fortner JG, Klimstra DS, Senie RT, Maclean BJ. Tumor size is the primary prognosticator for pancreatic cancer after regional pancreatectomy. Ann Surg. 1996;223:147–153. doi: 10.1097/00000658-199602000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]


