Summary
Objectives
Bacteria within the Acinetobacter calcoaceticus-Acinetobacter baumannii (ACB) complex commonly cause nosocomial infection and are often multidrug resistant. Advances in genospecies typing allow for speciation within the ACB complex; however, little is known about the effect of genospecies on patient outcomes.
Methods
Adult patients with ACB complex bacteremia from Jan 2005–Oct 2012 were included. Bacterial isolates were speciated by rpoB gene sequence analysis, and clinical data were collected.
Results
Of 147 patients with ACB complex bacteremia, 116 had A. baumannii (78.9%), 28 had A. pittii (19.0%), and 3 had A. nosocomialis (2.0%). A. baumannii bacteremia was associated with greater comorbidity and was more frequently multidrug resistant (79% vs. 16%, p < 0.01). Multidrug resistant A. baumannii but not susceptible A. baumannii was associated with worse outcomes compared to non-baumannii ACB complex bacteremia. Neither multidrug resistance nor genospecies was an independent predictor of mortality, but receipt of appropriate therapy was associated with decreased risk of mortality (OR, 0.13; 95% CI, 0.04–0.44; p < 0.01).
Conclusions
A. baumannii bacteremia is associated with worse clinical outcomes than non-baumannii ACB complex bacteremia. The difference, however, appears to be related to multidrug resistance and attendant receipt of appropriate therapy rather than genospecies.
Keywords: Multidrug resistance, bacteremia, Acinetobacter calcoaceticus, Acinetobacter baumannii, genome
Introduction
Acinetobacter baumannii is a common cause of nosocomial infections, particularly pneumonia and catheter-related bloodstream infections, and often causes clonal outbreaks in critically ill patients.1–3 Infections with A. baumannii have been associated with high attributable mortality and increased hospital length of stay.4 In addition, multidrug resistance is often a predictor of poor clinical outcomes in patients infected with A. baumannii.5 However, much of the difference in patient outcomes due to multidrug resistance may be explained by delays in the receipt of active antimicrobial therapy.6,7
The Acinetobacter genus currently comprises 34 species, with the Acinetobacter calcoaceticus-Acinetobacter baumannii (ACB) complex including four phenotypically related species: A. baumannii, A. calcoaceticus, A. pittii (formerly Acinetobacter genospecies 3), and A. nosocomialis (formerly Acinetobacter genospecies 13TU).8,9 Conventional microbiologic phenotypic identification cannot reliably distinguish these species and, as such, they are often reported clinically as “Acinetobacter calcoaceticus-Acinetobacter baumannii complex.” Advances in molecular genotyping and whole genome sequencing have made possible delineation of individual Acinetobacter species within clinical isolates of the ACB complex.
All species except A. calcoaceticus have been implicated in human disease. Infection with different ACB complex species may be associated with different risk factors and produce different clinical outcomes.10–16 In some studies, infection with A. baumannii has been associated with increased mortality10–13 while in others, the presence of multidrug resistance or inappropriate initial therapy is associated with increased mortality rather than genospecies.15,16 In addition, A. baumannii appears to be resistant to more classes of antimicrobial agents than other species in the ACB complex.17 Due to the potential differences in epidemiology, antimicrobial resistance, and clinical outcomes among members of the ACB complex, the current practice of reporting ACB complex infection without further speciation may be inadequate and lead to inferior clinical care.
Many of the existing studies on the impact of ACB complex genospecies on clinical outcomes were conducted at a small number of medical centers in Taiwan and gave heterogeneous results.10,11,13,15,16 Only one study has been conducted in the U.S., and it included a high proportion of carbapenem-susceptible isolates (93%), making it difficult to draw conclusions from this study about a predominantly MDR A. baumannii population.14 In addition, most of these studies used 16S rRNA internal transcribed spacer (ITS) gene sequencing for speciation.10,11,13,15,16 16S rRNA sequencing may be insufficient for differentiating closely related bacterial species, especially when certain regions of the gene are included.18,19 Housekeeping gene sequence analysis can provide better resolution of species differences because these genes evolve faster than 16S rRNA genes.18,20 Although a number of genes have been investigated for Acinetobacter speciation, the rpoB gene is among the best described for this purpose.20–24
In this study, we investigated the clinical characteristics, antimicrobial susceptibility patterns and outcomes associated with ACB complex bacteremia at a large, academic medical center in the United States. To more accurately describe Acinetobacter genospecies variability in our population, we used the rpoB gene sequence for speciation.
Methods
Study population
This was a retrospective cohort study conducted at Northwestern Memorial Hospital (NMH), an approximately 900-bed tertiary-care academic medical center located in Chicago, IL. All adult patients (≥ 18 years old) with ACB complex cultured from 1 or more blood cultures between January 2005 – October 2012 were identified through the Clinical Microbiology Laboratory and were eligible for inclusion in the study. Patients whose blood cultures were felt to represent contamination and who were not treated for active infection were excluded. Only the first positive blood culture from patients with two or more positive blood cultures during a single hospital admission was included. This study was approved by the Institutional Review Board at Northwestern University with a waiver of informed consent.
Microbiologic studies
Phenotypic identification of blood cultures to the level of the ACB complex was performed in the Clinical Microbiology Laboratory using the Vitek2 system (bioMérieux, Marcy l’Etoile, France). Susceptibilities for most antibiotics also were performed using the Vitek2 system. Etests (bioMérieux) were used to determine colistin and tigecycline susceptibilities, and disk diffusion was used to determine piperacillin-tazobactam, minocycline and doxycycline susceptibilities. All susceptibilities were interpreted according to Clinical and Laboratory Standards Institute guidelines.25 Minimum inhibitory concentrations (MICs) for tigecycline were interpreted according to the U.S. FDA breakpoints for Enterobacteriaceae, with MICs ≤ 2 μg/mL considered susceptible.26 Frozen stocks of all bacterial isolates were maintained at −80°C.
Species identification
Isolates were streaked onto blood agar plates from the frozen stocks, and a single colony was inoculated into Luria-Bertani (LB) broth and grown overnight at 37°C. Genomic DNA was extracted from overnight cultures using either a Qiagen EZ1 Advanced robotic workstation (Valencia, CA) or a Promega Maxwell 16 instrument (Madison, WI) according to the manufacturer’s instructions. Genomic DNA libraries were prepared and barcoded using the Nextera XT kit (Illumina, San Diego, CA). Equal amounts (200 ng) of each DNA library were pooled together and run on either an Illumina HiSeq 2000 system with 100-bp paired end reads or on an Illumina MiSeq system with 250-bp paired end reads. Sequencing was performed by the University of Maryland Institute for Genome Sciences, blinded to all clinical data. Raw sequence reads were then assembled de novo using Ray v2.3.0.27 Low-quality assemblies, defined as a sequence size of assembled contigs ≥ 500 bp totaling more than 4.5 M bp, were excluded.
Further analysis of the whole genome sequencing data from our cohort will be reported separately, but for the purposes of this study the rpoB gene sequence was used to determine the genospecies of the blood isolates.20,22 Zone 1 rpoB sequences were identified from the assembled whole genome sequences using an in-house in silico PCR script.28 Briefly, assembly contigs were screened to identify sequence flanked by the primer sequences Ac696F (TAYCGYAAAGAYTTGAAAGAAG) and Ac1093R (CMACACCYTTGTTMCCRTGA) as described by La Scola et al.24 In silico “amplified” sequences were identified by BLAST alignment against the NCBI nucleotide collection database.29 Patients with bacteremia caused by mixed Acinetobacter genospecies were excluded.
Data collection
Data on demographics, underlying diseases, age-adjusted Charlson co-morbidity score,30 laboratory testing, infection characteristics, treatment, and clinical outcomes were collected retrospectively from chart review. Sources of bacteremia were identified in accordance with Centers for Disease Control and Prevention/National Health Safety Network (CDC/NHSN) guidelines.30,31 If no source or multiple possible sources were identified, the bacteremia was labeled as primary.
Polymicrobial bacteremia was defined as growth of any other bacteria in the same culture from which ACB complex was grown. Immunosuppression was defined as underlying immunosuppressive disease, receipt of antineoplastic drugs or other immunosuppressive agents in the previous 4 weeks, or receipt of corticosteroids at an equivalent dose of ≥ 10 mg of prednisone for more than 3 days in the previous 2 weeks. Neutropenia was defined as an absolute neutrophil count ≤ 500 cells/mm3. Hepatic dysfunction was defined as any liver function test value ≥ 3 times the upper limit of normal at the time of culture. Renal dysfunction was defined as history of chronic kidney disease, current dialysis, or an increase in serum creatinine of 0.5 mg/dl or 50% at the time of culture. Multidrug resistance was defined as non-susceptibility to at least one agent in three or more antimicrobial categories.32 Healthcare-associated infection (HAI) was defined according to the CDC/NSHN surveillance definition.30 Appropriate antibiotic treatment was defined as use of one or more antibiotics to which the isolate was susceptible. Time to appropriate therapy, time to negative cultures, hospital length of stay following positive culture, and both 14-day and 30-day mortality were also recorded.
Statistical analysis
For clinical and demographic characteristics, categorical variables were compared using the Fisher’s exact test and continuous variables were compared using the Mann-Whitney U test or Student’s t-test, as appropriate. The associations between clinical outcomes for patients with susceptible and MDR A. baumannii and susceptible and MDR non-baumannii ACB complex bacteremia were compared using the Kruskal–Wallis one-way analysis of variance (ANOVA) or Fisher’s exact test. Post-hoc tests using the Mann-Whitney U test or Fisher’s exact test and a Bonferroni-adjusted α level for pair-wise comparisons were performed if the results of the initial Kruskal-Wallis one-way ANOVA or Fisher’s exact test were significant. For the endpoint of 30-day mortality, variables that could plausibly be associated with the outcome and with a p-value of ≤ 0.1 in the univariate analysis were considered for entry into a multivariate logistic regression model using a forward stepwise method. Time to mortality was analyzed using the Kaplan-Meier survival analysis with the log-rank test. A p-value of < 0.05 was considered significant for all statistical tests. SPSS version 22.0 (Chicago, IL) was used for all statistical analyses.
Results
During the study period, 156 patients with ≥ 1 blood culture positive for the ACB complex were identified. Three patients with ACB complex bacteremia who were seen by an Infectious Diseases consultant and felt not to have active infection given a lack of SIRS criteria or other infectious symptoms at the time the culture was drawn were excluded. All three patients had prompt resolution of bacteremia without active antibiotic therapy. In addition, one patient whose culture contained mixed Acinetobacter genospecies and five patients whose cultures gave poor quality sequencing results despite multiple replicates and/or could not be speciated by rpoB analysis were excluded. Therefore, a total of 147 patients with ACB complex bacteremia were included: 116 A. baumannii (78.9%), 28 A. pittii (19.0%), and 3 A. nosocomialis (2.0%). Of note, there was one recognized outbreak of MDR A. baumannii during the study period, occurring between 2005–2006 and involving eight patients included in this study.
The clinical and demographic characteristics of patients with A. baumannii versus non-baumannii ACB complex bacteremia are listed in Table 1. Patients with non-baumannii ACB complex bacteremia were more likely to be female. Patients with A. baumannii bacteremia were more likely to have healthcare-associated infection, to require mechanical ventilation, to be in the ICU, to have a greater number of days of prior antibiotics, and to have diabetes and renal dysfunction. Most cases of bacteremia were associated with indwelling central catheters, although 11% of patients with A. baumannii bacteremia had a respiratory source. The proportions of A. baumannii and non-baumannii ACB complex identified among blood isolates were similar throughout the 7-year time period.
Table 1.
Clinical and demographic characteristics of patients with Acinetobacter calcoaceticus-Acinetobacter baumannii complex bacteremia.
| A. baumannii (n = 116) | Non-baumannii ACB complex (n = 31) | p-value | |
|---|---|---|---|
| Demographic | |||
| Age, mean ± (SD) | 53 (15) | 49 (19) | 0.20 |
| Sex, female | 49 (42) | 19 (61) | 0.07 |
| Infection characteristics | |||
| Source | |||
| Catheter | 78 (67) | 24 (77) | 0.38 |
| Respiratory | 13 (11) | 1 (3) | 0.30 |
| Wound or surgical site | 7 (6) | 1 (3) | 1.00 |
| Intra-abdominal | 8 (7) | 2 (7) | 1.00 |
| Urine | 2 (2) | 0 | 1.00 |
| Primary | 8 (7) | 3 (10) | 0.70 |
| Culture year | |||
| 2005–2007 | 46 (40) | 16 (52) | 0.16 |
| 2008–2010 | 44 (38) | 10 (32) | 0.36 |
| 2011–2012 | 26 (22) | 5 (16) | 0.24 |
| Healthcare-associated | 73 (63) | 12 (39) | 0.02 |
| Polymicrobial | 44 (38) | 15 (48) | 0.31 |
| Healthcare exposures | |||
| Central venous or arterial catheter | 103 (89) | 26 (84) | 0.54 |
| Mechanical ventilation | 46 (40) | 6 (19) | 0.04 |
| Prior antibiotic days, median (range) | 5 (0–105) | 0 (0–49) | 0.02 |
| ICU at onset | 58 (50) | 9 (29) | 0.04 |
| Clinical/laboratory | |||
| Age-adjusted Charlson comorbidity score, median (range) | 4 (0–12) | 4 (0–8) | 1.00 |
| History of hematopoietic stem cell transplant | 9 (8) | 4 (13) | 0.47 |
| History of solid organ transplant | 12 (10) | 2 (7) | 0.74 |
| Current immunosuppression | 61 (53) | 18 (58) | 0.69 |
| Neutropenia | 8 (7) | 3 (10) | 0.70 |
| Diabetes mellitus | 43 (37) | 5 (16) | 0.03 |
| Renal dysfunction | 60 (52) | 9 (29) | 0.03 |
| Liver dysfunction | 43 (37) | 7 (23) | 0.14 |
Data are No. (%) unless otherwise specified
ACB, Acinetobacter calcoaceticus-Acinetobacter baumannii complex; SD, standard deviation; ICU, intensive care unit
Table 2 lists the antimicrobial susceptibilities for A. baumannii and non-baumannii ACB complex isolates. When compared with non-baumannii ACB complex isolates, A. baumannii isolates exhibited decreased susceptibility to all antimicrobials tested except colistin, for which there was no significant difference in susceptibility (96% vs. 100%, p = 1.0). Only 24% of A. baumannii isolates were susceptible to tigecycline. Overall, A. baumannii isolates were also much more likely to be MDR (79% vs. 16%, p < 0.01).
Table 2.
Comparison of antimicrobial susceptibilities of Acinetobacter baumannii and non-baumannii Acinetobacter calcoaceticus-Acinetobacter baumannii complex isolates.
| No. of susceptible isolates/total no. isolates tested (%) | |||
|---|---|---|---|
| Antibiotic | A. baumannii | Non-baumannii ACB complex | p-value |
| Ampicillin-sulbactam | 47/105 (45) | 24/26 (92) | < 0.01 |
| Piperacillin-tazobactam | 15/111 (14) | 21/29 (72) | < 0.01 |
| Imipenem | 29/116 (25) | 29/31 (94) | < 0.01 |
| Cefepime | 23/116 (20) | 22/31 (71) | < 0.01 |
| Ciprofloxacin | 21/116 (18) | 28/31 (90) | < 0.01 |
| Amikacin | 45/116 (39) | 27/31 (87) | < 0.01 |
| Tobramycin | 55/116 (47) | 28/31 (90) | < 0.01 |
| Minocycline | 65/110 (59) | 26/29 (90) | < 0.01 |
| Doxycycline | 50/102 (49) | 24/27 (89) | < 0.01 |
| Tigecycline | 16/67 (24) | 17/18 (94) | < 0.01 |
| Colistin | 69/72 (96) | 18/18 (100) | 1.00 |
| Multidrug resistanta | 92/116 (79) | 5/31 (16) | < 0.01 |
Number of multidrug resistant isolates out of total (%).
To examine the impact of antimicrobial susceptibility in addition to genospecies, the clinical outcomes of patients with A. baumannii and non-baumannii ACB complex bacteremia were stratified by multidrug resistance (Table 3). There were no significant differences in outcomes between patients with susceptible A. baumannii and susceptible non-baumannii ACB complex or between patients with MDR A. baumannii and MDR non-baumannii ACB complex. However, MDR status was associated with higher 30-day and 14-day mortality in both the A. baumannii and non-baumannii ACB complex groups. In addition, patients with MDR A. baumannii were less likely to receive appropriate therapy than patients with susceptible A. baumannii. Of the 29 patients with MDR A. baumannii who did not receive appropriate therapy, 17 (59%) died within 48 hours, prior to susceptibility results returning.
Table 3.
Clinical outcomes of patients with Acinetobacter calcoaceticus-Acinetobacter baumannii complex bacteremia stratified by presence of multidrug resistance in A. baumannii isolates.
| Outcomea | Susceptible A. baumannii (n = 24) | MDR A. baumannii (n = 92) | Non-baumannii ACB complex (n = 31) | p-value | ||
|---|---|---|---|---|---|---|
| Overall | Susceptible A. baumannii vs. MDR A. baumannii | Susceptible A. baumannii vs. non-baumannii ACB complex | ||||
| Days to negative cultures, median (range)b | 2 (1–5) | 1 (1–7) | 2 (1–6) | 0.49 | ||
| Received appropriate therapy | 24 (100) | 63 (69) | 29 (94) | < 0.01 | < 0.01 | 0.50 |
| Days to appropriate therapy, median (range)c | 0.5 (0–6) | 2 (0–13) | 1 (0–4) | < 0.01 | < 0.01 | 0.93 |
| Hospital LOS postculture, median days (range) | 6 (2–38) | 11.5 (2–69) | 5 (0–28) | 0.01 | 0.09 | 0.12 |
| 30-day mortality | 2 (8) | 40 (44) | 4 (13) | < 0.01 | < 0.01 | 0.69 |
| 14-day mortality | 2 (8) | 33 (36) | 4 (13) | < 0.01 | 0.01 | 0.69 |
| Days to mortality, median (range) | 10.5 (6–15) | 2.5 (0–27) | 1.5 (1–5) | 0.40 | ||
Data are No. (%) unless otherwise specified
ACB, Acinetobacter calcoaceticus-Acinetobacter baumannii complex; MDR, multidrug resistant; LOS, length of stay
Statistical tests were also performed comparing outcomes between susceptible A. baumannii and susceptible non-baumannii ACB complex and also between MDR A. baumannii and MDR non-baumannii ACB complex. No significant differences were found (data not shown).
For 110 patients with repeat blood cultures.
For 117 patients receiving appropriate therapy
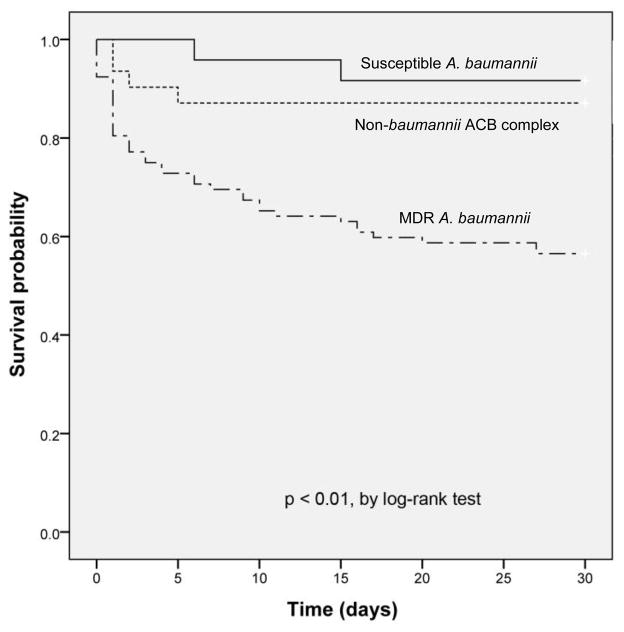
The univariate and multivariate logistic regression analyses for risk factors associated with 30-day mortality are shown in Table 4. In the multivariate logistic regression model, mechanical ventilation and renal dysfunction were significantly associated with an increased risk of mortality. Female sex and receipt of appropriate antibiotics were associated with a significantly decreased risk of mortality. Using 14-day mortality instead as the outcome did not change the multivariate model (data not shown). The Kaplan-Meier survival curves (Figure 1) demonstrated a significant difference at 30 days, with patients with MDR A. baumannii bacteremia having a decreased probability of survival in comparison to patients with susceptible A. baumannii bacteremia (log-rank test, p < 0.01) and patients with non-baumannii ACB complex bacteremia (log-rank test, p < 0.01). Patients with susceptible A. baumannii bacteremia did not have improved survival compared with patients with non-baumannii ACB complex bacteremia (log-rank test, p = 0.56).
Table 4.
Univariate and multivariate logistic regression analysis of risk factors associated with 30-day mortality in patients with Acinetobacter calcoaceticus-Acinetobacter baumannii complex bacteremia.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Risk factor | Survived (n = 101) | Died (n = 46) | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age, mean ± (SD) | 51 (17) | 56 (12) | 1.02 (1.00–1.05) | 0.06 | ||
| Sex, female | 54 (54) | 14 (30) | 0.38 (0.18–0.80) | 0.01 | 0.24 (0.08–0.75) | 0.01 |
| A. baumannii | 74 (73) | 42 (91) | 3.83 (1.26–11.7) | 0.02 | ||
| Source: | ||||||
| Catheter | 76 (75) | 26 (57) | 0.43 (0.21–0.89) | 0.02 | ||
| Respiratory | 3 (3) | 11 (24) | 10.27 (2.71–38.96) | < 0.01 | ||
| Primary | 10 (10) | 1 (2) | 0.20 (0.03–1.63) | 0.13 | ||
| Healthcare-associated | 46 (46) | 39 (85) | 6.67 (2.72–16.3) | < 0.01 | ||
| Multidrug resistance | 54 (54) | 43 (94) | 12.48 (3.63–42.85) | < 0.01 | ||
| Received appropriate therapy | 91 (90) | 25 (54) | 0.13 (0.06–0.31) | < 0.01 | 0.13 (0.04–0.44) | < 0.01 |
| Days to appropriate therapy, median (range) | 1 (0–13)a | 1 (0–5)b | 0.84 (0.64–1.12) | 0.24 | ||
| Mechanical ventilation | 16 (16) | 36 (78) | 19.13 (7.93–46.15) | < 0.01 | 20.7 (6.8–62.9) | < 0.01 |
| Prior antibiotic days, median (range) | 0 (0–42) | 12 (0–105) | 1.09 (1.05–1.13) | < 0.01 | ||
| Age-adjusted Charlson comorbidity score, median (range) | 3 (0–12) | 5 (0–11) | 1.16 (1.03–1.31) | 0.01 | ||
| Current immunosuppression | 46 (46) | 33 (72) | 3.04 (1.43–6.44) | < 0.01 | ||
| Diabetes mellitus | 27 (27) | 21 (46) | 2.3 (1.11–4.77) | 0.03 | ||
| Renal dysfunction | 35 (35) | 34 (74) | 5.34 (2.46–11.6) | < 0.01 | 4.21 (1.46–12.1) | < 0.01 |
| Liver dysfunction | 23 (23) | 27 (59) | 4.82 (2.28–10.19) | < 0.01 | ||
Data are No. (%) unless otherwise specified
OR, odds ratio; CI, confidence interval; SD, standard deviation; ICU, intensive care unit
For the 91 survivors who received appropriate therapy
For the 25 non-survivors who received appropriate therapy
Figure 1.
Kaplan-Meier survival curves for 147 patients with susceptible A. baumannii, MDR A. baumannii and non-baumannii Acinetobacter calcoaceticus-Acinetobacter baumannii complex bacteremia for 30 days post-infection.
Discussion
Infections due to A. baumannii are common in critically ill patients, are difficult to treat due to multidrug resistance, and are associated with high attributable mortality, particularly in the ICU.4,33 Earlier studies examining mortality in patients with Acinetobacter bacteremia did not differentiate among members of the ACB complex.33–36 More recent studies using sequence-based methods to identify Acinetobacter genospecies have demonstrated that the clinical characteristics of infected patients, antimicrobial susceptibility patterns, and clinical outcomes vary significantly for A. baumannii vs. non-baumannii ACB complex bacteremia.10,11,13,15
In this study, we used the rpoB gene sequence extracted from the whole genome sequence to demonstrate that 21% of blood cultures identified as ACB complex at a single tertiary care academic medical center in the U.S. are due to non-baumannii species. This is similar to the distribution reported in a lab-based study of Acinetobacter blood isolates from Taiwan.17 However, other studies from Taiwan and the U.S. found A. nosocomialis to be more common than A. pittii among non-baumannii ACB complex blood isolates.11,14,15 Most of these prior studies relied on 16S rRNA ITS sequencing to differentiate among Acinetobacter genospecies and, therefore, may not have accurately identified isolates to the genospecies level.10,11,13,15,16 An advantage of our study was the use of rpoB housekeeping gene sequence analysis for speciation. Housekeeping gene sequence analysis captures faster gene evolution rates and may more accurately differentiate among closely related Acinetobacter species.18,24 In a recent study comparing genotypic methods for speciation of a collection of 167 Acinetobacter clinical isolates, the accuracy of rpoB gene sequencing was 98.2%.37 This was superior to 16S rRNA gene sequencing (93.4%), gyrB multiplex PCR (77.2%) and the VITEK2 system (35.9%).37 Larger surveillance studies using rpoB gene sequence analysis or whole genome sequencing are needed to delineate further the factors influencing the global genospecies distribution of ACB complex blood isolates.
The clinical characteristics of our patients suggest that A. baumannii bacteremia may be more likely to occur in critically ill patients with multiple comorbidities, extensive prior antibiotic exposure, and requiring mechanical ventilation. Other studies have found similar associations.12,16 We also identified higher rates of antimicrobial resistance among A. baumannii isolates for all antimicrobials tested except colistin. The contrast was particularly evident for ciprofloxacin, with 90% susceptibility noted in non-baumannii ACB complex isolates compared with 18% in A. baumannii. This particular discrepancy in fluoroquinolone susceptibility has also been observed in other studies.11,17 Furthermore, a recent study of tigecycline in vitro antimicrobial susceptibility testing demonstrated significantly elevated MICs for A. baumannii with the Etest compared to broth microdiultion.38 Therefore, the low rate of tigecycline susceptibility among our isolates may have been influenced by the use of Etest for susceptibility testing.
It is also important to note that a significant proportion of our patients had polymicrobial infection. Polymicrobial infection is commonly associated with Acinetobacter bacteremia. In a large study of > 10,000 patients with Acinetobacter bacteremia at U.S. hospitals, 36% had polymicrobial bloodstream infection.39 This study found no differences in clinical outcomes between patients with monomicrobial and polymicrobial bloodstream infection. Lee et al. describe similar results in their study of ACB complex bacteremia, with 40.2% of A. baumannii, 32.5% of A. pittii, and 28.6% of A. nosocomialis bacteremia being polymicrobial.15 Similar to our study, this study did not find a statistically significant difference among the genospecies in the proportion of infections that were polymicrobial. Furthermore, a study examining the impact of carbapenem resistance and receipt of active antibiotic therapy on A. baumannii bacteremia failed to identify a statistically significant difference in the proportion of polymicrobial bacteremia between carbapenem-resistant and carbapenem-susceptible A. baumannii.40 These studies demonstrate that polymicrobial infection is unlikely to have a significant influence on clinical outcomes. Furthermore, any possible influence is likely to be similar in both A. baumannii and non-baumannii infections and both susceptible and multidrug resistant infections.
In our study, we demonstrated that MDR A. baumannii bloodstream infection is associated with worse outcomes compared to both susceptible A. baumannii and non-baumannii ACB complex bloodstream infection. There is no difference in outcomes between susceptible A. baumannii and susceptible non-baumannii ACB complex infection or between MDR A. baumannii and MDR non-baumannii ACB complex infection. A study in Taiwan involving 215 patients with ACB complex bacteremia also found that genospecies was not independently associated with mortality.15 In that study, there was a trend toward worse outcomes for patients with MDR A. baumannii. In contrast, other studies have identified genospecies as an independent predictor of mortality. Chiang et al. found that A. baumannii was independently associated with 14-day mortality in a cohort of 103 patients with solid tumors and ACB complex bacteremia.10 Park et al. also found that A. baumannii was an independent predictor of 30-day mortality among patients with ACB complex bacteremia at a university hospital in Korea.12 Likewise, Chusri et al. found that infection with non-baumannii ACB complex was associated with decreased 30-day mortality compared with carbapenem-susceptible A. baumannii.44 However, the first study did not include multidrug resistance as a variable in the analysis, the second study did not directly compare MDR A. baumannii to susceptible A. baumannii, and the third study only included 18 patients with bloodstream infection out of a total of 222. Furthermore, in the last study, only 56% of patients with carbapenem-susceptible A. baumannii received appropriate empirical antibiotic therapy, which may explain why these patients had worse outcomes compared to patients with non-baumannii ACB complex infection.44
Multidrug resistance may be more closely associated with mortality than genospecies because it directly correlates to receipt of appropriate therapy, a more accurate predictor of clinical outcome. In a recent study from Taiwan comparing imipenem-resistant to imipenem-susceptible ACB complex bacteremia, the authors found no difference in mortality between imipenem-susceptible A. baumannii and non-baumannii ACB complex bacteremia.16 They also found that inappropriate initial therapy was the greatest risk factor for mortality.16 Likewise, in our analysis, neither MDR status nor genospecies was a significant independent predictor of mortality but receipt of appropriate antibiotic therapy was associated with a significant decreased risk of mortality. Therefore, the increased frequency of multidrug resistance and concomitant delay in appropriate therapy may explain the increased mortality seen with A. baumannii bacteremia rather than variation in virulence among genospecies.
Our study had a number of limitations. First, although the number of patients in our cohort was similar to prior studies, our sample size was small and included patients from a single center. We had relatively low numbers of non-baumannii ACB complex isolates and few cases of A. nosocomialis bacteremia in particular, which may have impaired our ability to identify genospecies as a significant independent predictor of mortality. Second, the retrospective design of the study may have introduced bias from unidentified confounding variables. Despite controlling for comorbidity in the multivariate analysis, the substantial number of patients with MDR A. baumannii who died within 48 hours of the initial culture and, thus, did not receive appropriate antibiotic therapy may have affected outcomes. Finally, we used age-adjusted Charlson co-morbidity score rather than the Acute Physiology and Chronic Health Evaluation II score for classifying severity of underlying illness. We chose the Charlson score because almost half (46%) of our patients were not in the ICU at the time of bacteremia onset. However, this score may not have accurately represented the severity of illness of ICU patients. This also may account for the lack of independent association seen between age-adjusted Charlson score and mortality in our study.
In conclusion, we found that patients with A. baumannii bacteremia are more likely to be critically ill and have greater comorbidities and decreased survival compared with patients with non-baumannii ACB complex bacteremia. The difference in mortality is likely due to higher rates of multidrug resistance in A. baumannii that lead to delays in receipt of appropriate therapy. The evidence from our study and others suggests that we can no longer regard patients with ACB complex bacteremia as a homogeneous group. Prior estimates of attributable mortality from A. baumannii infection performed without differentiating species within the ACB complex underrepresent the impact of A. baumannii infection on clinical outcomes. Future studies investigating rapid PCR- and sequence-based diagnostics for earlier genospecies and antimicrobial susceptibility determination of ACB complex isolates and, thus, timelier administration of targeted antimicrobial therapy are likely to show improved patient outcomes.
Acknowledgments
The authors thank Dr. Chao Qi and Michael Malczynski from the Northwestern Memorial Hospital Clinical Microbiology Laboratory for assisting with the identification and retrieval of Acinetobacter bloodstream isolates. We thank Lisa Sadzewicz and Naomi Sengalamay at the University of Maryland Institute for Genome Sciences for assistance with the Illumina sequencing. We also thank Dr. Marc Scheetz, Associate Professor at Midwest University Chicago College of Pharmacy, for his assistance with the statistical analysis.
Funding
This work was supported by the National Institutes of Health (T32 AI095207, AI053674, AI075191, AI099269, AI04831 and AI088286) and by an Eleanor-Wood Prince intramural grant from Northwestern Memorial Hospital.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Transparency declarations
The authors have none to declare.
References
- 1.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–51. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Zembower T, Penugonda S, et al. Clinical outcomes of carbapenem-resistant Acinetobacter baumannii bloodstream infections: study of a 2-state monoclonal outbreak. Infect Control Hosp Epidemiol. 2010;31:1057–62. doi: 10.1086/656247. [DOI] [PubMed] [Google Scholar]
- 4.Falagas ME, Bliziotis IA, Siempos II. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: a systematic review of matched cohort and case-control studies. Crit Care. 2006;10:R48. doi: 10.1186/cc4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng WH, Liao CH, Lauderdale TL, et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis. 2010;14:e764–9. doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]
- 6.Lee YT, Kuo SC, Yang SP, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis. 2012;55:209–15. doi: 10.1093/cid/cis385. [DOI] [PubMed] [Google Scholar]
- 7.de Gouvea EF, Martins IS, Halpern M, et al. The influence of carbapenem resistance on mortality in solid organ transplant recipients with Acinetobacter baumannii infection. BMC Infect Dis. 2012;12:351. doi: 10.1186/1471-2334-12-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemec A, Krizova L, Maixnerova M, et al. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov (formerly Acinetobacter genomic species 13TU) Res Microbiol. 2011;162:393–404. doi: 10.1016/j.resmic.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Euzeby JP. List of Bacterial Names with Standing in Nomenclature: a folder available on the Internet. Int J Syst Bacteriol. 1997;47:590–2. doi: 10.1099/00207713-47-2-590. [DOI] [PubMed] [Google Scholar]
- 10.Chiang MC, Kuo SC, Chen SJ, et al. Clinical characteristics and outcomes of bacteremia due to different genomic species of Acinetobacter baumannii complex in patients with solid tumors. Infection. 2012;40:19–26. doi: 10.1007/s15010-011-0187-4. [DOI] [PubMed] [Google Scholar]
- 11.Chuang YC, Sheng WH, Li SY, et al. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with acinetobacter bacteremia. Clin Infect Dis. 2011;52:352–60. doi: 10.1093/cid/ciq154. [DOI] [PubMed] [Google Scholar]
- 12.Park KH, Shin JH, Lee SY, et al. The clinical characteristics, carbapenem resistance, and outcome of acinetobacter bacteremia according to genospecies. PLoS One. 2013;8:e65026. doi: 10.1371/journal.pone.0065026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YT, Kuo SC, Yang SP, et al. Bacteremic nosocomial pneumonia caused by Acinetobacter baumannii and Acinetobacter nosocomialis: a single or two distinct clinical entities? Clin Microbiol Infect. 2013;19:640–5. doi: 10.1111/j.1469-0691.2012.03988.x. [DOI] [PubMed] [Google Scholar]
- 14.Wisplinghoff H, Paulus T, Lugenheim M, et al. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect. 2012;64:282–90. doi: 10.1016/j.jinf.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Lee YC, Huang YT, Tan CK, et al. Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. J Antimicrob Chemother. 2011;66:1839–46. doi: 10.1093/jac/dkr200. [DOI] [PubMed] [Google Scholar]
- 16.Lee HY, Chen CL, Wu SR, Huang CW, Chiu CH. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med. 2014 doi: 10.1097/CCM.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 17.Ko WC, Lee NY, Su SC, et al. Oligonucleotide array-based identification of species in the Acinetobacter calcoaceticus-A. baumannii complex in isolates from blood cultures and antimicrobial susceptibility testing of the isolates. J Clin Microbiol. 2008;46:2052–9. doi: 10.1128/JCM.00014-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim A, Gerner-Smidt P, Liesack W. Phylogenetic relationship of the twenty-one DNA groups of the genus Acinetobacter as revealed by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:837–41. doi: 10.1099/00207713-47-3-837. [DOI] [PubMed] [Google Scholar]
- 19.Adams-Haduch JM, Onuoha EO, Bogdanovich T, et al. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol. 2011;49:3849–54. doi: 10.1128/JCM.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saeed S, Fakih MG, Riederer K, Shah AR, Khatib R. Interinstitutional and intrainstitutional transmission of a strain of Acinetobacter baumannii detected by molecular analysis: comparison of pulsed-field gel electrophoresis and repetitive sequence-based polymerase chain reaction. Infect Control Hosp Epidemiol. 2006;27:981–3. doi: 10.1086/507286. [DOI] [PubMed] [Google Scholar]
- 21.Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol. 2006;44:2974–6. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dijkshoorn L, Aucken H, Gerner-Smidt P, et al. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol. 1996;34:1519–25. doi: 10.1128/jcm.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemec A, De Baere T, Tjernberg I, Vaneechoutte M, van der Reijden TJ, Dijkshoorn L. Acinetobacter ursingii sp. nov. and Acinetobacter schindleri sp. nov. isolated from human clinical specimens. Int J Syst Evol Microbiol. 2001;51:1891–9. doi: 10.1099/00207713-51-5-1891. [DOI] [PubMed] [Google Scholar]
- 24.La Scola B, Gundi VA, Khamis A, Raoult D. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J Clin Microbiol. 2006;44:827–32. doi: 10.1128/JCM.44.3.827-832.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Institute CaLS. Twenty-third informational supplement. Wayne, PA: CLSI; 2013. Performance standards for antimicrobial susceptibility testing; p. M100-S23. [Google Scholar]
- 26.Tian GB, Adams-Haduch JM, Bogdanovich T, et al. Identification of diverse OXA-40 group carbapenemases, including a novel variant, OXA-160, from Acinetobacter baumannii in Pennsylvania. Antimicrob Agents Chemother. 2011;55:429–32. doi: 10.1128/AAC.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boisvert S, Laviolette F, Corbeil J. Ray: simultaneous assembly of reads from a mix of high-throughput sequencing technologies. J Comput Biol. 2010;17:1519–33. doi: 10.1089/cmb.2009.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camacho C, Coulouris G, Avagyan V, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [access 2014 June 18];CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2014 Available http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
- 31.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 33.Wareham DW, Bean DC, Khanna P, et al. Bloodstream infection due to Acinetobacter spp: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis. 2008;27:607–12. doi: 10.1007/s10096-008-0473-y. [DOI] [PubMed] [Google Scholar]
- 34.Cisneros JM, Reyes MJ, Pachon J, et al. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis. 1996;22:1026–32. doi: 10.1093/clinids/22.6.1026. [DOI] [PubMed] [Google Scholar]
- 35.Tseng YC, Wang JT, Wu FL, Chen YC, Chie WC, Chang SC. Prognosis of adult patients with bacteremia caused by extensively resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2007;59:181–90. doi: 10.1016/j.diagmicrobio.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Jang TN, Lee SH, Huang CH, Lee CL, Chen WY. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J Hosp Infect. 2009;73:143–50. doi: 10.1016/j.jhin.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Lee MJ, Jang SJ, Li XM, et al. Comparison of rpoB gene sequencing, 16S rRNA gene sequencing, gyrB multiplex PCR, and the VITEK2 system for identification of Acinetobacter clinical isolates. Diagn Microbiol Infect Dis. 2014;78:29–34. doi: 10.1016/j.diagmicrobio.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Marchaim D, Pogue JM, Tzuman O, et al. Major variation in MICs of tigecycline in Gram-negative bacilli as a function of testing method. J Clin Microbiol. 2014;52:1617–21. doi: 10.1128/JCM.00001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wisplinghoff H, Edmond MB, Pfaller MA, Jones RN, Wenzel RP, Seifert H. Nosocomial bloodstream infections caused by Acinetobacter species in United States hospitals: clinical features, molecular epidemiology, and antimicrobial susceptibility. Clin Infect Dis. 2000;31:690–7. doi: 10.1086/314040. [DOI] [PubMed] [Google Scholar]
- 40.Esterly JS, Griffith M, Qi C, Malczynski M, Postelnick MJ, Scheetz MH. Impact of carbapenem resistance and receipt of active antimicrobial therapy on clinical outcomes of Acinetobacter baumannii bloodstream infections. Antimicrob Agents Chemother. 2011;55:4844–9. doi: 10.1128/AAC.01728-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee NY, Lee HC, Ko NY, et al. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2007;28:713–9. doi: 10.1086/517954. [DOI] [PubMed] [Google Scholar]
- 42.Erbay A, Idil A, Gozel MG, Mumcuoglu I, Balaban N. Impact of early appropriate antimicrobial therapy on survival in Acinetobacter baumannii bloodstream infections. Int J Antimicrob Agents. 2009;34:575–9. doi: 10.1016/j.ijantimicag.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Kwon KT, Oh WS, Song JH, et al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother. 2007;59:525–30. doi: 10.1093/jac/dkl499. [DOI] [PubMed] [Google Scholar]
- 44.Chusri S, Chongsuvivatwong V, Rivera JI, et al. Clinical Outcomes of Hospital-Acquired Infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrob Agents Chemother. 2014;58:4172–9. doi: 10.1128/AAC.02992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]