Abstract
Trogocytosis was originally thought to be restricted to the interaction of cells of the immune system and interactions of these cells with cancer cells. Such membrane exchanges are probably a general process in cell biology, and membrane exchange has been demonstrated to occur between non-immune cells within an organism. Herein, we report that membrane and protein exchange, consistent with trogocytosis, between Trypanosoma cruzi (both the Brazil and Tulahuen strains) and the mammalian cells it infects. Transfer of labeled membrane patches was monitored by labeling of either parasites or host cells, i.e. human foreskin fibroblasts and rat myoblasts. Trypomastigotes and amastigotes transferred specific surface glycoproteins to the host cells along with membranes. Exchange of membranes between the parasite and host cells occurred during successful invasion. Extracellular amastigotes did not transfer membrane patches and heat killed trypomastigotes were did not transfer either membranes or proteins to the host cells. Membrane exchange was also found to occur between interacting epimastigotes in cell-free culture and may be important in parasite-parasite interactions as well. Further studies should provide new insights into pathogenesis and provide targets for therapeutic intervention.
Keywords: Trypanosoma cruzi, Chagas disease, trogocytosis
1. INTRODUCTION
Trypanosoma cruzi can cause infection in over 100 species of mammals. In vitro, it is able to infect virtually any nucleated cell. In humans, T. cruzi causes Chagas disease. The major consequences of infection are acute myocarditis, vasculitis, chronic cardiomyopathy and gastrointestinal disorders [1, 2]. The parasite employs a variety of mechanisms to infect mammalian cells and distinct strategies to facilitate their survival in these infected cells. The multitude of invasive strategies employed by T. cruzi varies widely between strains and isolates and represents an important obstacle in the development of suitable chemotherapy. T. cruzi has several life cycle stages namely: bloodstream and metacyclic trypomastigotes, which do not replicate but infect mammalian cells; amastigotes, which replicate within host cells; and epimastigotes, which are found in insects and replicate extracellularly, but do not infect host cells [2]. It has recently been appreciated that there are both intracellular and extracellular amastigotes. The infectivity of extracellular amastigotes to mammalian cells depends on the strain of T. cruzi and the type of mammalian cell [3]. Extracellular amastigotes may represent up to 10% of circulating parasite forms during acute infection in mice [4, 5].
Interacting cells have been reported to exchange membranes and associated proteins by: absorption [6], uptake of 50–90 nm vesicular exosomes [7, 8], membrane tunnels or nanotube structures [9, 10], plasma membrane bridges [11], cell-contact-dependent intercellular transfer of intracellular proteins [12, 13] and trogocytosis [14, 15]. Trogocytosis can transfer molecules between interacting cells bi-directionally or to cells to which they are conjugated by exchange of plasma membrane fragments between themselves. The transferred membrane and associated molecules becomes part of the recipient cell. Trogocytosis occurs when cells are in tight physical contact and is often mediated by a ligand receptor interaction. Furthermore, the process of trogocytosis is fast, and can occur between completely unrelated host cells. Transferred materials include not only membrane lipids but also proteins. Originally, it was thought that trogocytosis only occurred with cells of the immune system; as such constantly moving cells exhibit multiple transient interactions with other cell types and have a significant opportunity to transfer molecules [16-19]. Recent studies, however, indicate that cells in other tissues can also exchange proteins with each other and neighboring cells. This more widespread recognition of trogocytosis suggests that this may be a general process in cell biology and an essential component in the control of various cellular systems. Trogocytosis requires physical cell-to-cell contact as a selectively permeable transwell membrane, which prevents physical contact, can completely inhibit transfers [20].
Trogocytosis was reported, in 2014, to occur between two unrelated eukaryotic organisms, namely Entamoeba histolytica and host cells [21]. In this paper, we report the transfer of membrane lipids and surface protein molecules between trypomastigotes and amastigotes of T. cruzi and the mammalian cells it infects. Furthermore, the presented data indicate that membrane exchange also occurs between interacting epimastigotes of T. cruzi in cell-free culture. As intercellular membrane transfer in vivo is difficult to detect, intravital imaging techniques and molecular tagging was used to demonstrate membrane and protein transfer in T. cruzi. The presence of this trogocytosis-like process extends the mechanisms by which these parasites interact with host cell pathways.
2. MATERIALS AND METHODS
2.1. Reagents
Tissue culture reagents were purchased from Invitrogen (Carlsbad, CA). Plasticware was purchased from Costar (Cambridge, MA). Mouse monoclonal antibodies 2H11, and 2C2 directed against trypomastigote-specific surface glycoprotein SSP-1 and amastigote specific surface glycoprotein SSP-4 respectively of T. cruzi were a generous gift of Dr. Norma W. Andrews (Department of Cell Biology and Molecular Genetics, University of Maryland, USA) to our laboratory [4, 22]. Alexa-Fluor-488 conjugated goat anti-mouse IgG and DAPI were purchased from Molecular probes (Carlsbad, CA), goat serum was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were of the highest grade available.
2.2. Cell lines and parasite culture conditions
The rat myoblast cell line (L6E9) and human foreskin fibroblast (HFF) cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS). Cells were grown in 25 mm diameter cover glass in a 6-well plate at humidified 37°C, 5% CO2 incubator. Trypomastigotes (Brazil and Tulahuen strains) were obtained from the supernatant of infected monolayers of L6E9 cells as previously described [23]. The Tulahuen strain was maintained by syringe passage in A/J mice (Jackson Laboratories, Bar Harbor, ME) while the Brazil strain was maintained in C3H/HeJ mice (Jackson Laboratories) [24]. Animal experiments were performed under an approved protocol from our Institutional Animal Care and Use Committee (IACUC). The multiplicity of infection was kept at ~2:1 [23]. Parasitism was determined by fixing the infected cells with methanol and staining with Giemsa. Epimastigotes were grown in liver infusion tryptose (LIT) medium containing 10% FBS at 28°C.
2.3. Preparation of cells and parasites for trogocytosis
Parasite life cycle stages (e.g. epimastigotes, extracellular amastigotes and trypomastigotes) and mammalian host cells (e.g. HFF and L6E9), were labeled with PKH 26 (Sigma-Aldrich, St Louis, MO) according to the manufacturers' protocols. When the transfer of label from host cells to parasites was evaluated, host cells (1×106) were seeded on cover glass, cultured overnight, and labeled with PKH 26 when they were at 60 to 70% confluence. Unlabeled T. cruzi were then placed on top of the host cell monolayer to bind or infect depending upon the parasite life cycle stage employed. Conversely, to evaluate transfer of label from parasites to host cells, 1×108 trypomastigotes or epimastigotes were labeled with PKH 26 and added to unlabeled host cells growing on a cover glass. Since trypomastigotes of T. cruzi naturally infect host cells, these forms were simply added to the cultures. Since epimastigotes and round form epimastigotes do not infect cells, to promote interaction, in some experiments, a short centrifugation was performed before replating the cells. Transfer of membranes or proteins was monitored at time intervals of 2, 6, 12, 24, 48 and 72 hrs. At each time-point, each sample was fixed with 4% paraformaldehyde (EMS, Hatfield, PA) for 10 min, followed by washing four times with PBS. Nuclei were stained with 300 nM DAPI for 5 minutes and following two rinses with PBS, each coverglass was mounted on slide with Prolong Gold Antifade Reagent (Life Technologies, Grand Island, NY). When dead parasites were required they were heat-killed at 90°C in a water bath for 20 minutes. Microscopic examination of heat killed parasites demonstrated that they remained intact after this treatment.
2.4. Immunofluorescence
PFA fixed infected cells or parasites were washed twice with PBS and blocked in 10 % goat serum in TBS containing 0.1 % Triton X-100 in PBS-T for 30 min. Samples were then incubated with mouse monoclonal anti-T. cruzi trypomastigote specific 2H11 and amastigote specific 2C2 antibodies for 1 hour, washed three times in PBS-T, and stained with anti-mouse secondary antibody conjugated to AlexaFluor 488 (Molecular Probes, Carlsbad, CA) for 1 hr. Secondary antibody alone was used as a negative control and no auto-fluorescence were detected with host cells or the parasites used in this study.
2.5. Image acquisition and processing
Transfer of membrane patches to the parasite stages was observed either in Nikon Diaphot epifluorescent microscope using either a 60x or 100× dry objectives. Immunofluorescence images were acquired with a 60x (1.4) oil Olympus objective on an Olympus 1×71 inverted microscope containing automated excitation and emission filter wheels. Data were collected through a CoolSNAP HQ cooled charge-coupled device (Photometrics, Roper Scientific, Tucson, AZ) camera regulated by Meta Morph (Molecular Devices, Sunnyvale, CA) software. Exposure times (100 ms) and brightness adjustments (image normalization) were kept constant for images from different cell types. Care was taken to minimize exposure time for each field of view to reduce photobleaching and phototoxicity. Data were analyzed with Image J 1.39u (National Institutes of Health public domain; http://rsb.info.nih.gov/ij/) and Adobe Photoshop CS2 version 9.0.2 (Adobe Systems, San Jose, CA).
2.6. Statistical analysis
All experiments were performed in triplicate (e.g. 3 samples for each time point for each experiment) and each experiment was replicated at least four times (e.g. each time point had a minimum of 12 samples examined). At least 20 fields were examined for each coverglass examined. Statistical analysis was performed using SPSS (version 12) employing Student's t test or binominal analysis. Images presented in this paper are representative images of a typical experiment.
3. RESULTS
3.1. Transfer of membrane patches from trypomastigotes and amastigotes to cells
In order to examine transfer of membrane patches from trypomastigotes, we labeled trypomastigotes with PKH 26, a dye that binds membrane phospholipids and displays bright red fluorescence when bound into membrane phospholipids. PKH 26 labeling has been used extensively in trogocytotic assays to monitor membrane exchange between interacting cells; the diluent used for PKH 26 labeling does not result in any fluorescence in cells (Fig. 1A). Moreover, PKH 26 labeling does not alter cell growth and division (data not shown). PKH 26 labeled human foreskin fibroblasts (HFF) and rat myoblasts (L6E9) control cells lost fluorescence after 72 hours post labeling because of redistribution of cell surface membranes during cellular growth (Fig. 1B). Labeled trypomastigotes were used to infect HFF cells and the transfer of labeled lipids from the parasite to the cells at different time points was monitored. Transferred lipids were detected as early as 2 hours post-infection and the rate of transfer increased up to 6 hours post-infection as observed by fluorescence microscopy (p < 0.05). At 24 hours post-infection, red fluorescent was clearly evident on the surface of infected cells (Fig. 1C, D) and red amastigotes transformed from trypomastigotes was clearly visible inside infected cells (Fig. 1E). Fluorescence then declined as PKH 26 dye was lost over time Figure 1F). The fluorescence decreased because the dye was distributed into the parasite membranes, replicating cells and to expanding infected cells to accommodate the parasites.
Figure 1. Transfer from trypomastigotes of Brazil and Tulahuen strains labeled with PKH 26 to the infected HFF cells monitored for 24 and 48 hours.
A: HFF cells incubated with diluent only (control) did not stain the membranes.
B: HFF cells incubated with PKH 26 showed punctuate staining.
C: When labeled trypomastigotes were added to the unlabeled HFF cells, the transfer of labeled membrane lipids was observed from the trypomastigote of the Tulahuen strain.
D: When labeled trypomastigotes were added to the unlabeled HFF cells, the transfer of labeled membrane lipids were observed from the trypomastigote of the Brazil strain.
E: At 24 hours post-infection, red amastigotes transformed from trypomastigotes was clearly visible inside infected cells by phase contrast coupled to fluorescence microscopy (60X).
F: Fluorescence intensity declined as PKH 26 dye was lost over time (48 hr). Scale bar: 25μ
In order to study membrane exchange during T. cruzi infection, we evaluated the ability of the Tulahuen (class I) and Brazil (class II) strains of this parasite for their ability to transfer membrane lipids and surface protein to HFF and L6E9 cell lines. Both the Tulahuen strain (Fig. 1C) and the Brazil strain (Fig. 1D) were able to transfer lipids at a very fast rate and none of the strains displayed any evidence of toxicity due to labeling with PKH 26. The L6E9 cell line demonstrated the same pattern as HFF cells (data not shown). Phase-contrast coupled to fluorescence microscopy confirmed transfer of labeled lipids from the amastigotes to the cells (60X, Fig. 1E) as after 48 hours post-infection, the red labeling from the amastigotes was transferred to cells (Fig. 1F).
T. cruzi is known to interact with several cell surface receptors prior to invasion of the cells. Successful invasion depends on T. cruzi surface protein and cell membrane protein interaction, which distinguish it from simple T. cruzi adhesion, which does not result in internalization [25, 26]. The aim of the current study was not to identify specific receptors but to identify if membrane exchange occurred when successful invasion was established. The phenomenon of membrane transfer is specific to T. cruzi, as the closely related kinetoplastid T. brucei and apicomplexan Toxoplasma gondii do not transfer membrane patches to interacting cells (data not shown). We used long slender form T. brucei trypomastigotes in our experiment.
3.2. Transfer of membrane patches from cells to trypomastigotes and amastigotes
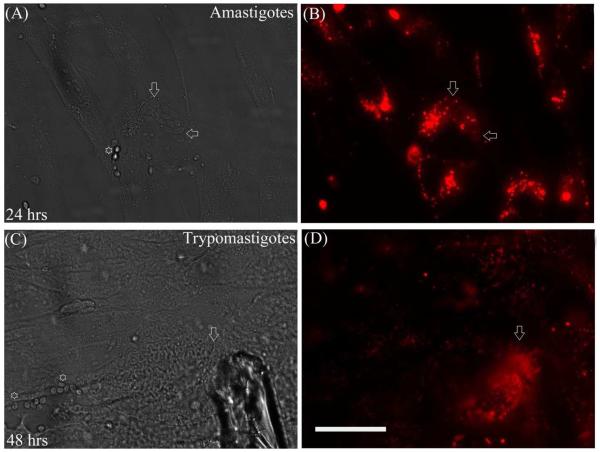
In order to examine the transfer of membrane patches from the host mammalian cells to the infecting parasites, we labeled HFF cells with PKH 26 dye and infected them with unlabeled trypomastigotes. Twenty four hours after infection labeled amastigotes were clearly visible inside the infected cells (Fig. 2B). Figure 2A shows a phase-contrast image of amastigotes inside HFF (arrow), as compared to the brightly reflecting extracellular amastigotes (EA) (* in Fig. 2A). Forty-eight hours post-infection, it was possible to observe labeled trypomastigotes derived from amastigotes indicating preservation of the transferred membrane patches during amastigote to trypomastigote differentiation (Fig. 2D). PKH 26 labeled HFF and L6E9 lost their punctuate labeling after 48 hours. However, infected cells were much brighter as internalized membranes like those in the amastigotes were subjected to less membrane recycling than the plasma membrane of the mammalian cells. These experiments were replicated six times with identical results (p<0.05) and a representative figure is presented. From all of the replicates, it was clear extracellular amastigotes which did not invade cells neither transferred membranes nor received any patches from the mammalian cells, in spite of the fact that they remain tightly bound to the cell surface. These experiments strongly suggest that trogocytosis in T. cruzi infection is an active process and does not occur between non-invading live parasites and mammalian cells (compare between Fig. 2A & B and Fig. 2C & D).
Figure 2. The transfer of membrane patches from cells to trypomastigotes and amastigotes were observed when PKH 26 labeled HFF cells was infected with unlabeled trypomastigotes.
A: The brightly reflecting extracellular amastigotes (EA *) and the intracellular amastigotes (arrow) were clearly visible in phase contrast microscopy
B: Twenty-four hours post-infection, labeled amastigotes were visible inside the infected cells.
C and D: The EA binds tightly to the cell but do not get internalized. EAs do not transfer membranes to the bound cells (compare between A & B and C & D). D: Forty-eight hours post-infection, labeled trypomastigotes derived from amastigotes was observed indicating transfer of membranes during amastigote to trypomastigote differentiation. Scale bar: 25μ; arrows point to trypomastigotes.
3.3. Transfer of membrane patches is cell contact dependent
To study whether physical contact was necessary for membrane transfer, a transwell apparatus was used. This approach has been used to demonstrate trogocytosis in other systems. PKH 26 labeled trypomastigotes were co-cultured with either HFF or L6E9 cells but separated from these host cells by either a 0.4 μM or 3 μM transwell inserts. Parasites were either placed in transwell inserts (experimental groups) or directly overlaid (control group) onto host cells for 24 and 48 hours. Transwell inserts with a 0.4 μM filter did not allow passage/contact of trypomastigotes with host cells and completely abrogated membrane patch transfer to host cells (Fig. 3A). On the other hand, Transwell inserts with 3 μM filters which restrictively allowed passage/contact of trypomastigotes with host cells, reduced membrane patch transfer to host cells (Fig.3B) as compared to the situation when parasites were directly overlaid over the cells (Fig. 3C). These experiments demonstrated similar results with either the Brazil or Tulahuen strains (Fig. 3 Brazil strain shown). The results of these experiments indicate that transfer of membrane patches from parasites to the host cells were cell-contact dependent and that this could not occur with soluble or insoluble factors that could pass though a 0.4 μM filter (e.g. exosomes).
Figure 3. Membrane transfer requires cell to cell contact and live parasites.
A: Transfer of membrane patches is cell contact dependent as PKH 26 labeled trypomastigotes separated by 0.4 μM filters from the HFF cells were unable to transfer membrane patches to the host cells.
B and C: While PKH 26 labeled trypomastigotes separated by 3 μM filters were able to transfer membrane to HFF cells (B) to a much lesser extent as compared to direct infection control (C). These experiments demonstrated similar results with either the HFF or L6E9 cells infected with either the Brazil or Tulahuen strains of T. cruzi (Figure 4 Brazil strain shown).
D: When heat-killed Tulahuen trypomastigotes were overload onto L6E9 host cells membrane transfer was not observed. Scale bar: 25μ
3.4. Live parasite interaction with cells necessary for transfer of membrane patches
In order to examine whether the membrane transfer is an active process driven by specific interaction between the parasites and the cells, heat-killed parasites were overlaid onto host cells. Trypomastigotes of heat killed (90oC for 20 minutes) Brazil or and Tulahuen strains were observed under microscope for cell integrity, added to the cultured HFF or L6E9 cells, and then these cultures were incubated for 24 hours at 37oC. No membrane transfer was detected with heat killed parasites, indicating active invasion is necessary for trogocytosis (Fig. 3D, Tulahuen with L6E9 shown). These experiments complement the observations seen with extracellular amastigotes (Fig. 2A & B), which being non-infective forms do not display membrane transfer to host cells. Extracellular amastigotes do; however, bind to the host cells strongly and despite this binding the fluorescent intensity of these labeled extracellular amastigotes remained the same for 48 hours post-contact. Membrane exchange also depended on the membrane integrity/viability of the parasites as cross-linking proteins on parasite by treatment with paraformaldehyde for 10 minutes also strongly inhibited transfer (data not shown).
3.5. Exchange of membrane patches also co-transfer associated surface proteins
Since trogocytosis in the immune system is known to transfer membrane anchored proteins during membrane swapping, the ability of T. cruzi trypomastigote and amastigote forms to transfer highly expressed surface glycoproteins to the host cells was evaluated. Transfer was evaluated in a time-course experiment from 1 to 48 hours using immunofluorescence microscopy (Figure 4). Mouse monoclonal antibody directed against trypomastigote surface glycoprotein (SSP-1) and an antibody directed against amastigote glycoprotein (SSP-4) were used to evaluate transfer of anchored surface proteins (4). At 1 hour post-infection, trypomastigotes were mostly seen outside the cells with limited to no invasion. During this time, fluorescence was mainly localized at the site of infection. However, by 6 and 24 hours, when most of the trypomastigotes were inside the cells, a redistribution of SSP-1 was observed possibly along with the membrane patches. We could not simultaneously stain parasites with PKH 26 and SSP-1 because detergent permeabilization of cells prior to antibody incubation released the PKH 26 dye from the plasma membrane. After 48 hours, fluorescence was not visible, as the parasites no longer express SSP-1 when they convert to amastigotes and, it is likely that any transferred trypomastigote specific glycoprotein was degraded by host cell machinery (Fig. 4). Intracellular amastigotes were visible at 24 hours post-infection and transfer of SSP-4 proteins was observed at the cell membrane. Extracellular amastigotes sticking to the cell membrane were visible at 6 hours and throughout the time-course. Extracellular amastigotes that failed to infect cells did not transfer SSP-4 to the host cell as in the case with PKH 26 staining. At 24 hours post-infection, amastigote nests were clearly visible and fluorescence could be observed even after 72 hours (data not shown). Interestingly, intracellular amastigotes were observed only in host cells that had acquired SSP-4.
Figure 4. Transfer of highly expressed T. cruzi surface glycoprotein along with membrane patches was observed from 1 to 48 hours by immunofluorescence microscopy.

Mouse monoclonal antibody directed against trypomastigote surface glycoprotein (SSP-1) and an antibody directed against amastigote glycoprotein (SSP-4) were used to evaluate transfer of anchored surface protein. At 6 and 24 hours post infection, when trypomastigotes were inside the cells, we could detect SSP-1 on the cell surface as punctuate staining, indicating possible transfer of SSP-1 during invasion from trypomastigotes to the host cells. At 48 hours post-infection, SSP-1 fluorescence was not visible, as the parasites no longer express SSP-1 in the amastigote stage or because of degradation of SSP-1 by host cell machinery (upper panel). Similarly, we observed transfer of amastigote protein SSP-4 from amastigotes to the cell at 24 hours post-infection with maximum intensity at 48 hours post-infection when amastigotes were dividing and being released from the cells. Extracellular amastigotes sticking to the cell membrane were visible at 6 hours and throughout the time-course. Scale bar: 25μ
We also surface labeled the target cell with biotin using the EZ-Link Sulfo-NHS-Biotin and Biotinylation Kits (Thermo Scientific, data not shown), and were able to observe a bulk transfer of proteins in membrane patches between the parasite and the host cells. However, since biotin labeling of targets gave a very high non-specific background, we used parasite specific antibodies as our primary method for confirming protein transfer in these trogocytosis experiments.
3.6. Exchange of membrane patches between epimastigotes
Epimastigotes are known to form rosettes while growing in cell-free culture conditions. It has long been assumed that by rosette formation, they exchange materials between themselves or that this rosette formation could even be instrumental in quorum sensing. In order to examine whether epimastigotes can exchange membranes between themselves, we labeled one epimastigote culture with PKH 26 counterstained with DAPI and then mixed this culture with epimastigotes labeled with epimastigotes expressing GFP constitutively in their cytoplasm. Triple labeling with three dyes staining the membrane, cytoplasm and the nucleus was performed to increase the contrast of the microscopic results. After 24 hrs of co-incubation, we obtained chimeric red-green epimastigotes expressing both labeled membranes and GFP (Fig. 5, examples are present in mixed rosette and chimeric panel) (p<0.05). We also performed similar experiments when epimastigotes solely labeled with DAPI was mixed with PKH 26 labeled epimastigotes and obtained chimeric epimastigotes (data not shown). We performed these experiments with both the Tulahuen and Brazil strains and obtained similar results. Data employing the Brazil strain is shown in Fig. 5. Co-culture of differentially labeled epimastigotes clearly demonstrated that epimastigotes share their membranes with other epimastigotes either during the rosette formations or during lateral interactions.
Figure 5. Membrane exchange between epimastigotes was observed when differentially labeled epimastigotes were mixed together.

We mixed PKH 26 labeled epimastigotes, counterstained with DAPI with epimastigotes that is constitutively expressing GFP. After 24 hours of co-culture, we obtained chimeric epimastigotes expressing both labeled membrane and GFP. Double labeling experiments, demonstrated that epimastigotes share their membranes with other epimastigotes either during the rosette formations or during lateral interactions. These experiments yielded similar results when performed with either Tulahuen or Brazil strains of T. cruzi. Scale bar: 20μ
4. DISCUSSION
The phenomenon of membrane exchange (i.e trogocytosis) may originally have been a symbiotic arrangement between two cells, but later evolved as a mechanism of intercellular communication and intra-cellular communication (signal transduction) and/or a mechanism for initiating behavioral changes in recipient cells. Trogocytosis is a well-documented mode of cell communication and this suggests that the phenotype of a functionally mature cell not only depends on its genetic repertoire, but is further modified by its cellular surroundings and cell-cell interactions. The preservation of trogocytosis in the host parasite relationship demonstrates another mechanism by which parasites can exploit their host cells.
During immune responses, trogocytosis plays an important role in modifying the behavior of interacting B and T cells with antigen presenting cells. Studies in various systems indicate that this membrane exchange is fairly common among different cell types. Trogocytotic mechanisms are now believed to be involved during embryonic development to create a functional network of neighboring cells. In drosophila, trans-endocytosis, is employed during signaling of Notch [27,28], BOSS [29] and eye development [29]. In mammalian cells, during neural development and axonal guidance, Epherin B proteins are exchanged by a process of bidirectional trans-endocytosis [30]. It has been suggested that the acquisition of oocyte membrane fragments by sperm may help in fertilization [31]. It has also been reported that glycosyl-phosphatidylinositol (GPI)-anchored proteins are transferred across the homotypic interactions seen between HeLa cells [32].
Trogocytosis can also occur without ligand receptor stimulation [33, 34]. It has been reported that trogocytosis can be bidirectional as observed between dendritic and T cells in murine system [35-39]. It is quite likely that trogocytosis is an ancient mechanism of cell to cell communication and adaptation. There is experimental evidence that indicates that the trogocytotic mechanism varies between conjugated cell types and how strongly cells interact. B-cells are particularly trogocytotic, as they strongly bind to their conjugated partners and this interaction is further stabilized by cytoskeleton membranes [36, 37]. In NK cells, trogocytosis occurs through signaling in acceptor cells [38]. In T cells, trogocytosis is triggered by several costimulatory molecules and co-receptors. In some cases, fused membrane bridges also help in trogocytosis [39]. Actin polymerization inhibitors are known to disrupt trogocytosis in T cells, but not in B cells [40]. Furthermore, trogocytosis by T cells is an active process that is inhibited at 4°C, whereas in B cells, the process is passive and is independent of temperature [40]. Ttrogocytosis has also been suggested to be a mechanism by which anti-cancer monoclonal antibodies (rituximab, trastuzumab, cetuximab or monoclonal antibody T101) bound to their cognate receptors is shed from the malignant cells[41]. For example, it has been demonstrated that therapeutic monoclonal antibodies interact with CD20 on malignant B cells and mAb-CD20 immune complexes were removed and internalized by effector cells via a trogocytosis mechanism [41].
To our knowledge, this manuscript is the first report that a trogocytosis-like mechanism occurs in the unicellular protozoan parasite, T. cruzi and mammalian cells. The implication of this “membrane and proteome mixing” is important because it opens up possibilities by which parasites can scavenge essential proteins from their host cells that they otherwise lack or are incapable of producing because of genetic streamlining. Ralston et al [21] clearly demonstrated that trogocytosis by E. histolytica significantly contributes to killing of host cells and tissue invasion and destruction. However, in the case of T. cruzi, we do not know the precise reason for the transfer of surface molecules from the parasite and the host cells and vice versa. Acquisition of different molecules through trogocytosis by parasites or host cells may directly or indirectly change their phenotype and probably provides a survival advantage for the parasite. Furthermore, such trogocytosed proteins likely contribute to the pathogenesis of these infections.
One can speculate that the membrane and protein transfer we observed may be due to some other mechanism besides trogocytosis; however, the kinetics of the observed transfer, necessity of live cell contact and active cellular mechanisms involved strongly favor a trogocytotic transfer mechanism. The transfer could be detected within an hour, a time window that precludes processes including exocytosis and plasma membrane bridges. We postulate that trogocytosis have a broader function in the life cycle of the parasite and in the establishment of infection. Since, lipids are the most energetically demanding components to synthesize, it is also possible that trypomastigotes while infecting host cells scavenge a large portion of host cell’s membranes in order to utilize it during transformation to amastigote form and subsequent replication. As a consequence of this process, the parasites may utilize trogocytosis as a mechanism to scavenge essential proteins from the host for their own survival and modulation of host immune reaction towards them. This includes proteins that are not transcribed by the parasites as they lack the genes for them. Parasites could either use host-derived proteins as shields to protect themselves from the immune system or use the acquired proteins, to develop a functional regulatory system that was otherwise not possible. It may also be possible that host primed parasites could act as quorum sensors to limit their multiplication and pathogenicity.
We believe that T. cruzi-host trogocytosis is not meant for “non-specific” acquisition of membrane fragments or proteins, but is an essential adaptation that helps the parasite to survive in the host for a prolonged length of time. Any imbalance in parasite life cycle in the infected host might result in high parasitemia which may be detrimental in maintenance of chronic infection associated with T. cruzi infection. Thus it is tempting to speculate that via capture of essential host proteins by trogocytosis, the parasites utilize them for their survival, limiting immune mechanisms and inflammation. Past research from our laboratory indicates that T. cruzi has an eicosanoid (signaling lipids essential in inflammation and immunity) synthetic pathway [42, 43]; however, several of the eicosanoid synthetic enzymes such as PLA2 and COX are not predicted in the T. cruzi genome database despite biochemical and immunologic evidence of their being found in this parasite (unpublished data). It is possible that trogocytosis is the mechanism by which this parasite acquires these eicosanoid pathway enzymes from its host cells. Investigations are ongoing in our laboratory using PLA2 and COX as a tool to investigate whether trogocytosis is involved in this synthetic pathway in this parasitic protist.
ACKNOWLEDGEMENTS
This study was supported by Scientist Development Grant from the National affiliate of the American Heart Association (SDG 0735252N) to SM, NIH grants AI-076248 to HBT and AI-103450 to HH and CNPq and FAPEMIG grants to FSM. The authors express their gratitude to Dr. Norma W. Andrews, Department of Cell Biology and Molecular Genetics, University of Maryland, USA for kindly providing antibodies for these studies. We acknowledge the assistance of Ms. Vicki Braunstein for cell culture and parasite maintenance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors state that they have conflict of interest.
REFERENCES
- 1.Hidron AI, Gilman RH, Justiniano J, Blackstock AJ, Lafuente C, Selum W, et al. Chagas cardiomyopathy in the context of the chronic disease transition. PLoS Negl Trop Dis. 2010;4:e688. doi: 10.1371/journal.pntd.0000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machado FS, Jelicks LA, Kirchhoff LV, Shirani J, Nagajyothi F, Mukherjee S, et al. Chagas heart disease: report on recent developments. Cardiol Rev. 2012;20:53–65. doi: 10.1097/CRD.0b013e31823efde2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima FM, Oliveira P, Mortara RA, Silveira JF, Bahia D. >The challenge of Chagas' disease: has the human pathogen, Trypanosoma cruzi, learned how to modulate signaling events to subvert host cells? N Biotechnol. 2010;27:837–84. doi: 10.1016/j.nbt.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Andrews NW, Hong KS, Robbins ES, Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987;64:474–84. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 5.Scharfstein J, Morrot A. A role for extracellular amastigotes in the immunopathology of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):51–6. doi: 10.1590/s0074-02761999000700005. [DOI] [PubMed] [Google Scholar]
- 6.Hudson L, Sprent J. Specific adsorption of IgM antibody onto H-2-activated mouse T lymphocytes. J Exp Med. 1976;143:444–49. doi: 10.1084/jem.143.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 8.Simons M, Raposo G. Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–81. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–83. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 10.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 11.Delage E, Zurzolo C. Exploring the role of lipids in intercellular conduits: breakthroughs in the pipeline. Front Plant Sci. 2013;4:504. doi: 10.3389/fpls.2013.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–10. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–43. doi: 10.1038/nri2020. 2007. [DOI] [PubMed] [Google Scholar]
- 14.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 15.Vanherberghen B, Andersson K, Carlin LM, Nolte-’t Hoen EN, Williams GS, Höglund P, et al. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci USA. 2004;101:16873–78. doi: 10.1073/pnas.0406240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cone RE, Sprent J, Marchalonis JJ. Antigen-binding specificity of isolated cell-surface immunoglobulin from thymus cells activated to histocompatibility antigens. Proc Natl Acad Sci USA. 1972;69:2556–60. doi: 10.1073/pnas.69.9.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorber MI, Loken MR, Stall AM, Fitch FW. I-A antigens on cloned alloreactive murine T lymphocytes are acquired passively. J Immunol. 1982;128:2798–03. [PubMed] [Google Scholar]
- 18.Brezinschek RI, Oppenheimer-Marks N, Lipsky PE. Activated T cells acquire endothelial cell surface determinants during transendothelial migration. J Immunol. 1999;162:1677–84. [PubMed] [Google Scholar]
- 19.Russo V, Zhou D, Sartirana C, Rovere P, Villa A, Rossini S, Traversari C, Bordignon C. Acquisition of intact allogeneic human leukocyte antigen molecules by human dendritic cells. Blood. 2000;95:3473–77. [PubMed] [Google Scholar]
- 20.Sprent J. Swapping molecules during cell–cell interactions. Sci STKE. 2005;2005:pe8. doi: 10.1126/stke.2732005pe8. [DOI] [PubMed] [Google Scholar]
- 21.Ralston KS, Solga MD, Mackey-Lawrence NM, Bhattacharya S, Petri WA., Jr. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature. 2014;508:526–30. doi: 10.1038/nature13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews NW, Robbins ES, Ley V, Hong KS, Nussenzweig V. Developmentally regulated, phospholipase C-mediated release of the major surface glycoprotein of amastigotes of Trypanosoma cruzi. J Exp Med. 1988;167:300–14. doi: 10.1084/jem.167.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowin KS, Tanowitz HB, Wittner M, Nyguen HT, Nadal-Ginard B. Inhibition of muscle differentiation by Trypanosoma cruzi. Proc Natl Acad Sci USA. 1983;80:6390–94. doi: 10.1073/pnas.80.20.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanowitz HB, Huang H, Jelicks LA, Chandra M, Loredo ML, Weiss LM, et al. Role of endothelin-1 in the pathogenesis of chronic chagasic heart disease. Infect Immun. 2005;73:2496–03. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida N. Molecular basis of mammalian cell invasion by Trypanosoma cruzi. An Acad Bras Cienc. 2006;78:87–111. doi: 10.1590/s0001-37652006000100010. [DOI] [PubMed] [Google Scholar]
- 26.Villalta F, Scharfstein J, Ashton AW, Tyler KM, Guan F, Mukherjee S, et al. Perspectives on the Trypanosoma cruzi-host cell receptor interactions. Parasitol Res. 2009;104:1251–60. doi: 10.1007/s00436-009-1383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klueg KM, Muskavitch MA. Ligand–receptor interactions and trans-endocytosis of Delta, Serrate and Notch: members of the Notch signaling pathway in Drosophila. J Cell Sci. 1999;112:3289–97. doi: 10.1242/jcs.112.19.3289. [DOI] [PubMed] [Google Scholar]
- 28.Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–85. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 29.Cagan RL, Kramer H, Hart AC, Zipursky SL. The bride of sevenless and sevenless interaction: internalization of a transmembrane ligand. Cell. 1992;69:393–9. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- 30.Marston DJ, Dickinson S, Nobes CD. Rac-dependent transendocytosis of ephrinBs regulates Eph–ephrin contact repulsion. Nat Cell Biol. 2003;5:879–88. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 31.Barraud-Lange V, Naud-Barriant N, Bomsel M, Wolf JP, Ziyyat A. Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J. 2007;21:3446–49. doi: 10.1096/fj.06-8035hyp. [DOI] [PubMed] [Google Scholar]
- 32.Anderson SM, Yu G, Giattina M, Giattina M, Miller JL. Intercellular transfer of a glycosylphosphatidylinositol (GPI)-linked protein: release and uptake of CD4-GPI from recombinant adeno-associated virus-transduced HeLa cells. Proc Natl Acad Sci USA. 1996;93:5894–98. doi: 10.1073/pnas.93.12.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeMaoult J, Caumartin J, Carosella ED. Exchanges of membrane patches (trogocytosis) split theoretical and actual functions of immune cells. Hum. Immunol. 2007;68:240–43. doi: 10.1016/j.humimm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Pardigon N, Takeda K, Saunier B, Hornung F, Gibbs J, Weisberg A, et al. CD8 alpha alpha-mediated intraepithelial lymphocyte snatching of thymic leukemia MHC class Ib molecules in vitro and in vivo. J Immunol. 2006;177:1590–98. doi: 10.4049/jimmunol.177.3.1590. [DOI] [PubMed] [Google Scholar]
- 35.He T, Tang C, Liu Y, Ye Z, Wu X, Wei Y, Moyana T, Xiang J. Bidirectional membrane molecule transfer between dendritic and T cells. Biochem Biophys Res Commun. 2007;359:202–08. doi: 10.1016/j.bbrc.2007.05.099. [DOI] [PubMed] [Google Scholar]
- 36.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, et al. TCR-mediated internalization of peptide–MHC complexes acquired by T cells. Science. 1999;286:952–54. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 37.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 15:751–61. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 38.Tabiasco J, Espinosa E, Hudrisier D, Joly E, Fournie JJ, Vercellone A. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur J Immunol. 2002;32:1502–08. doi: 10.1002/1521-4141(200205)32:5<1502::AID-IMMU1502>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 39.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–61. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 40.Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111:5621–28. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beum PV, Mack DA, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J Immunol. 2008;181:8120–32. doi: 10.4049/jimmunol.181.11.8120. 2008. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee S, Sadekar N, Ashton AW, Huang H, Spray DC, Lisanti MP, et al. Identification of a functional prostanoid-like receptor in the protozoan parasite, Trypanosoma cruzi. Parasitol Res. 2013;112:1417–25. doi: 10.1007/s00436-012-3271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machado FS, Mukherjee S, Weiss LM, Tanowitz HB, Ashton AW. Bioactive lipids in Trypanosoma cruzi infection. Adv Parasitol. 2011;76:1–31. doi: 10.1016/B978-0-12-385895-5.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]