Abstract
Background
The most common inherited cardiac arrhythmia, LQT1, is due to IKs potassium channel mutations and is linked to high risk of adrenergic-triggered cardiac events. We recently showed that although exercise triggered events are very well treated by β-blockers for these patients, acute arousal triggered event rate were not significantly reduced after beta-blocker treatment, suggesting that the mechanisms underlying arousal-triggered arrhythmias may be different from those during exercise. IKs is strongly regulated by β-adrenergic receptor (β-AR) signaling, but little is known about the role of α1-AR-mediated regulation.
Methods and Results
Here we show, using a combination of cellular electrophysiology and computational modeling, that IKs phosphorylation and α1-AR regulation via activation of calcium-dependent PKC isoforms (cPKC) may be a key mechanism to control channel voltage-dependent activation and consequently action potential duration (APD) in response to adrenergic-stimulus. We show that simulated mutation-specific combined adrenergic effects (β+α) on APD were strongly correlated to acute stress-triggered cardiac event rate for patients while β-AR effects alone were not.
Conclusion
We were able to show that calcium dependent PKC signaling is key to normal QT shortening during acute arousal and when impaired, correlates with increased rate of sudden arousal triggered cardiac events. Our study suggests that acute α1-AR-cPKC regulation of IKs is important for QT shortening in “fight-or-flight” response and is linked to decreased risk of sudden emotion/arousal triggered cardiac events in LQT1 patients.
Keywords: KCNQ1, LQT1, arrhythmias, sudden cardiac death, K+, KvLQT1, MinK, KCNE1
INTRODUCTION
Sudden cardiac death, presumably due to fatal arrhythmias, is responsible for approximately 300,000 deaths annually in the US 1. Both exercise and strong emotion have been shown to be independently associated with cardiac arrhythmias in the general population 2, 3. Long-QT syndrome causes torsades de pointes ventricular fibrillation, and sudden cardiac death 4. Long QT syndrome type 1 (LQT1) is the most common form of LQTS and is caused by loss-of-function mutations in the KCNQ1-gene encoding the IKs channel alpha subunit 5. Exercise or emotion/sudden noise are known to precipitate arrhythmias associated with LQT1. Normally, β-adrenergic receptor (β-AR) stimulation of IKs suppresses β-adrenergic-induced early afterdepolazations (EADs) and arrhythmogenic premature beats. β-blockers are the treatment of choice for patients with LQT1, for whom IKs function is impaired 6. However, our recent data indicate that although β-blocker therapy is very effective in preventing sudden death for the highest risk patients with mutations in the C-loop region of KCNQ1, the benefit of β-blocker therapy is not as pronounced for the other LQT1 patients, who remain at considerable risk for sudden cardiac death despite therapy 6. Our recent work also showed in a study of 221 LQT1 patients that 55% of cardiac events were associated with exercise and 14% associated with acute emotion/noise 7. We showed that although exercise triggered events are very well treated by β-blockers, rate of acute arousal triggered events were not significantly reduced after beta-blocker treatment, suggesting that the mechanisms underlying arousal-triggered arrhythmias may be different from those during exercise 8. In addition to β-ARs, α1-ARs are also activated upon adrenergic stimulation in the heart [for review 9]. α1-AR activation leads to activation of the downstream kinase, protein kinase C (PKC). PKCα is the main PKC isoform expressed in the human heart, belonging to the Ca2+-dependent PKCs (cPKCs)10, 11. Another cPKC isoform, PKCβII, is poorly expressed in healthy ventricular tissue, but becomes up-regulated during heart failure12. Here we show that α1-AR-cPKC signaling has a strong additional contribution to β-ARs-mediated KCNQ1/KCNE1 activation via phosphorylation of the auxiliary KCNE1 subunit. The KCNE1 subunit shows relatively low homology among species, suggesting this may be a human-specific effect. We introduced cPKC-mediated adrenergic regulation of LQT1 associated mutant channels in a cardiomyocyte computer model to investigate the contribution of α1-AR-cPKC signaling to action potential regulation. We hypothesized here that 1) α1-AR may contribute to the shortening of action potential duration under acute high adrenergic stress condition in human cardiomyocytes and 2) the impairment of α1-AR-mediated channel regulation would increase the cardiac risk during acute emotion/noise stress in LQT1 patients. Our data suggests that when APD changes mediated by cPKC stimulation are taken into account APD correlates better with cumulative rate of acute emotion/noise-triggered events in LQT1 patients than APD prolongation mediated by β-AR stimulation alone. Our data suggest that cPKC activation contributes to shortening APD at high adrenergic states and that Ca2+-dependent PKC activation of IKs may be a novel therapeutic target for treatment of β-blocker-resistant LQT1.
RESULTS
α1-AR stimulation activates human KCNQ1/KCNE1 channel through an independent mechanism from β-AR-PKA signaling
To explore whether α1-adrenergic receptor (α1-AR) signaling can modulate human IKs current, we co-expressed IKs subunits (KCNQ1 and KCNE1) with α1A-AR in HEK293T cells. Cells were stimulated by α1-AR agonist phenylephrine (Phe) (30 μmol/L). Ten-min application of Phe showed two phases of current regulation, first an inhibition of the current (suggested to be mediated by phospholipid depletion 13, 14) followed by the current activation (Fig. 1A&B). Phe applications strongly facilitated voltage-dependent activation of KCNQ1/KCNE1 channels (≅−20 mV in V1/2) and increased the maximal conductance over 2-fold compared to the current with no Phe application (Fig. 1B&C). This current activation by Phe was observed over 10 μmol/L Phe, while significant shifts in V1/2 were observed for concentrations over 30 μmol/L Phe (Supplementary Fig. S2 and S3). Current increase was abolished by a specific α1-AR receptor antagonist prazosin (1 μmol/L) (Supplementary Fig. S4), and the PKC inhibitor BIS-I (1 μmol/L) (Fig. 2A), indicating that this effect was mediated through α1-AR-PKC signaling. The effect of α1-AR on KCNQ1/KCNE1 channel properties was in contrast to that of β-AR signaling activation (by a PKA activator, 10 μmol/L forskolin), which strongly activates Gmax, but showed a small (and not significant) effect on V1/2 (Fig. 1C) consistent with the previous reports15, 16. This concentration of forskolin was shown to produce a maximal β-AR activation in HEK-cells. 16 β-AR-PKA signaling activates the IKs channel via phosphorylation of Ser 27 in the KCNQ1 subunit. 15 To further investigate whether the Phe effects were related to β-AR-PKA signaling, we measured the current activation by Phe for the PKA-insensitive mutant KCNQ1(S27A) 15. The current regulation by Phe was not significantly changed for this mutant (supplementary Fig. S5), suggesting the effect of Phe on the current is not mediated through KCNQ1-S27 phosphorylation. In addition, we also tested whether Phe applications increased phosphorylation of the KCNQ1 at S27. S27 phosphorylation is mildly increased by the Phe applications, but to a much lesser extent than seen with forskolin treatment (supplementary Fig. S6). Our data suggest that the major effect of Phe is independent of S27 phosphorylation and the increase in S27 phosphorylation observed may reflect a crosstalk between these signaling pathways, which contributes to a minor portion of the Gmax increase observed under Phe stimulation.
Figure 1. Effect of α1-adrenoceptor (α1-AR) stimulation on KCNQ1/KCNE1 current.
A. Typical time course of KCNQ1/KCNE1 current response to +20 mV depolarization from −80 mV during phenylephrine (Phe) stimulation (30 μmol/L) followed by a step to −20 mV and return to −80mV holding potential. IKs traces before (black) and after Phe stimulation (red) are also shown as inset. B. Typical series of IKs currents (upper panel) elicited by increasing depolarizing voltages before (black) and after stimulation by Phe (red) together with normalized I–V relationships of tail currents (lower panel). C. Summary data of changes in Gmax (left) and V1/2 (right) after α1-AR stimulation (30 μmol/L Phe) for 10 min (red). The data from the cells stimulated by 10 μmol/L forskolin for 10 min is also shown to compare the effect of α1-AR with that of β-AR signaling stimulation (yellow). The changes in Gmax and V1/2 by Phe were normalized by the changes in those observed in the cells with no Phe application for 10 min (control). D. The effect of Phe stimulation on IKs currents during forskolin treatment. Cells were pretreated by 10 μmol/L forskolin for 5 min and 30 μmol/L Phe stimulation was added in the presence of forskolin for 10 min. E. Summary data of changes in V1/2 and Gmax by the combination of forskolin and Phe (red bars). The effect of 10 μmol/L forskolin for 15 min are also shown as comparison (black bars). *P<0.05.
Figure 2. Negative voltage shift in V1/2 by α1-AR stimulation is mediated by Ca2+-dependent PKC (cPKC).
A. Typical IKs current before (black) and after stimulation by Phe (red) in the absence (left) and in the presence of general PKC inhibitor (1 μmol/L BIS-I) (middle) or Ca2+-dependent PKC (cPKC) inhibitory peptide (right). Normalized I–V relationships for tail currents were also shown (lower panels). B. Summary data of changes in Gmax (left) and V1/2 (right) with and without Phe application (30 μmol/L) in each condition. *P<0.05.
To test whether the effect of α1-AR stimulation on KCNQ1/KCNE1 was additive to that of β-AR stimulation, we examined KCNQ1/KCNE1 regulation by α1-AR in the presence of PKA activation. Cells were pretreated by forskolin for 5min and Phe stimulation was added in the presence of forskolin for 10 min. Currents were increased in response to Phe applications due to a strong shift in the voltage dependence of channel activation in the presence of forskolin, but no additional increase in the maximal conductance was observed when compared to the current by 15-min application of forskolin alone as control (Fig. 1D). Hence, we confirmed that α1-AR stimulation still produces a similar shift in the voltage dependence of the KCNQ1/KCNE1 activation (>20 mV) in the presence of β-AR-PKA signaling activation to that observed during α1-AR stimulation alone (compare Fig. 1C and E). This observation suggested that the IKS activation by α1-AR is mediated through distinct signaling pathways and shows different post-translational channel modification from β-AR signaling, highlighting the importance of the α-AR stimulation for KCNQ1/KCNE1 activation under AR stimulation, in particular the shift in the voltage-dependence of activation.
α1-AR stimulation activates KCNQ1/KCNE1 by a negative shift in the voltage dependence of activation through Ca2+-dependent PKC (cPKC) isoforms
Next, we examined the detailed molecular mechanism underling KCNQ1/KCNE1 activation by α1-AR signaling. PKC is a serine/threonine protein kinase which is one of the downstream pathways of α1-AR signaling and plays important roles in cardiovascular functions including the regulation of ion channels. To test whether PKC was involved in the α1-AR-mediated KCNQ1/KCNE1 regulation, we measured Phe regulation of the channel in the presence of a synthetic PKC inhibitor, BIS-I (1 μmol/L). Both the negative shift in the voltage-dependence of activation and increase in Gmax were inhibited by BIS-I application (Fig. 2), confirming that PKC involvement. α1-AR stimulation can activate a variety of PKC isoforms 17–19. Specific examples include Ca2+-dependent PKCs or classical PKCs (cPKC) (including the cardiac isoforms PKCα, βI and βII) and Ca2+-independent PKCs or novel PKCs (nPKC) (including the cardiac isoforms PKCδ and ε). Therefore, we next investigated which PKC isoforms were involved in the α1-AR regulation of KCNQ1/KCNE1 activation using TAT-conjugated cell-permeable PKC-isoform inhibitory peptides20. Interestingly, cPKC inhibitor peptide (1 μmol/L) selectively abolished α1-AR-mediated shifts in the voltage dependence of channel activation, but did not inhibit the increase in Gmax, providing strong evidence of the unique α1-AR signaling effect of the shift in the voltage-dependence of KCNQ1/KCNE1 activation is mediated specifically through cPKC isoforms. This effect was also confirmed using a cell-permeable cPKC activator peptide20. The activator peptide significantly activated channel current in 5 min with a negative shift in the voltage dependence of activation (≅ −25 mV) without changing Gmax. On the contrary, a cell-permeable Ca2+-independent PKC activator peptide (PKCδ activator peptide)20 only showed increase in Gmax but did not show any shift in voltage dependence of activation, suggesting the possibility that the cPKCs and nPKCs might target different phosphorylation sites inside the channel and show different biophysical modulation (Supplementary Fig. S7).
Direct phosphorylation at KCNE1(Ser102) mediates α1-AR-cPKC negative shift in voltage dependence of KCNQ1/KCNE1 activation
We previously reported that phosphorylation of human KCNE1 subunit at Ser102 contributes to human KCNQ1/KCNE1 regulation by PKC when channels were expressed in Xenopus oocyte 21. Ser102 of human KCNE1 lies within a PKC consensus motif. To test whether α1-AR-PKC signaling could phosphorylate KCNE1 at S102, we directly measured KCNE1 phosphorylation levels at Ser102 before and after Phe stimulation using a custom-made antibody (see also online Material and Methods). α1A-AR and KCNQ1/KCNE1 channels were co-expressed in HEK293T cells and cells were stimulated by 30 μmol/L Phe for 10 min. Phosphorylation of KCNE1-Ser102 was significantly increased after 10-min Phe stimulation. In contrast, expression of the mutant mimicking the non-phosphorylated form of KCNE1 [KCNE1-Ser102Ala mutant: KCNE1(S102A)] abolished the α1-AR-mediated phosphorylation. Next, to investigate whether the negative shift in voltage dependence of channel activation during α1-AR stimulation was mediated through KCNE1 phosphorylation at Ser 102, we measured the effect of α1-AR stimulation on KCNQ1/KCNE1(S102A) mutant channels. The negative shift on the voltage dependence of channel activation was selectively abolished when the KCNE1(S102A) mutant was expressed, while the increase in maximal conductance (Gmax) was still present (Fig. 3). Thus, the mutant channel current was activated in the mutant channel in response to α1A-AR stimulation due to an increase in Gmax, but not through the shift in voltage dependence of activation. To further demonstrate that the α1-AR-mediated effect in voltage dependence of KCNQ1/KCNE1 activation was due to cPKC phosphorylation of KCNE1(S102), wild-type and mutant KCNQ1/KCNE1(S102A) channels were stimulated by a cell-permeable cPKC activator peptide. In contrast to the wild-type channel (Fig. 2), for the mutant channel the cPKC activator peptide treatment did not change channel voltage dependence of activation. In addition, the effect of a Ca2+-independent PKC activator peptide (PKCδ activator peptide) on Gmax still remained (Supplementary Fig. S7). Cells expressing the phosphomimic mutant KCNE1(S102E) also showed a negative shift in voltage dependence of activation when compared to the KCNE1(S102A) mutant (Supplementary Fig. S8 and S9). Our results show that phosphorylation of KCNE1(S102) by α1-AR-cPKC signaling underlies the negative shift in voltage dependence of KCNQ1/KCNE1 channel activation. Consistent with these results, for guinea pig IKs currents, where the KCNE1(S102) residue is absent, α1-AR activation does not change channel voltage dependence of activation 22, suggesting that KCNE1(S102)-cPKC regulation we observe may be specific to humans.
Figure 3. KCNE1(S102A) abolishes cPKC-mediated current activation.
A. KCNE1 phosphorylation levels at Ser102 before and after Phe stimulation using a custom-made antibody (see also online Material and Methods). α1A-AR and IKs channels were co-expressed in HEK293T cells and cells were stimulated by 30 μmol/L Phe for 10 min. Phosphorylation of KCNE1-Ser102 was significantly increased after 10-min Phe stimulation, but not for cells expressing the mutant KCNE1(S102A). B. (left) Typical KCNQ1KCNE1 current before (black) and after stimulation by Phe (red) for Q1/E1(S102A) channels. (right) normalized I–V relationships for tail currents. C. Summary data of changes in Gmax (left) and V1/2 with and without Phe stimulation. *P<0.05 compared to control (0 μM).
Simulation of APD prolongation mediated by overall (alpha+beta) adrenergic stimulation in LQT1
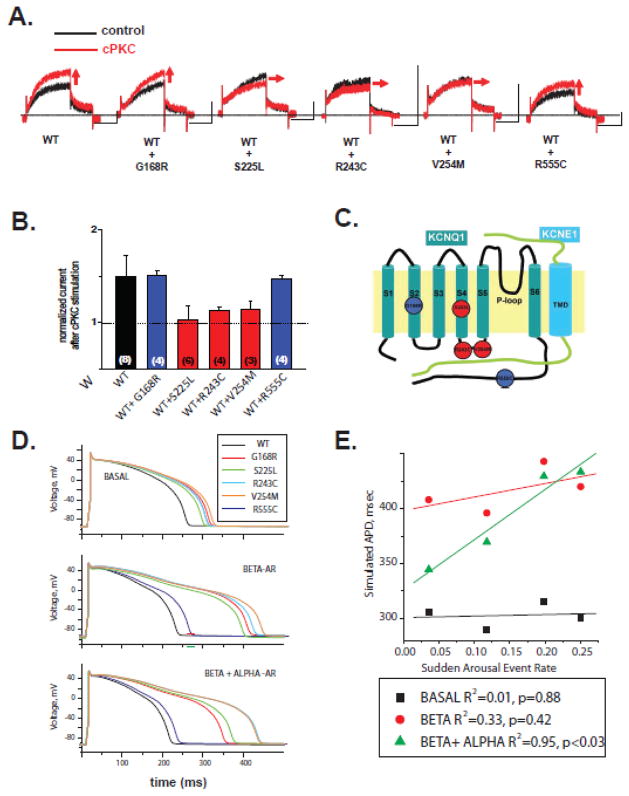
Because our results suggest cPKC-mediated activation has an additional contribution to β-AR activation in the channel function, we next investigated the effect of cPKC peptide applications on five mutant channels associated with LQT1 (G168R, S225L, R243C, V254M and R555C). Mutant channel subunits were co-expressed with wild-type subunits to mimic the dominant nature of the disease. The auxiliary KCNE1 subunits were also co-expressed in all experiments. Location of the mutant are depicted in Fig. 4C. S225L is located in the S4 voltage sensor domain, R243C and V254M in the S4–S5 linker, G168R in the S2 domain and R555C in the cytoplasmic C-terminus of the KCNQ1 submit. cPKC peptides were still able to activated mutant channel currents formed with WT, G168R or R555C, while the channel formed with WT- and the voltage-activation domain mutant subunits (S225L, R243C or V254M) showed impaired cPKC activation (Fig. 4A&B). Thus, consistent with the effect of cPKC on voltage-dependent channel activation, mutations that impaired cPKC activation are located in the voltage-activation domain of the KCNQ1 (Fig. 4A). Consistently with the specificity of the effect, the Ca2+-independent PKC activator peptide (PKCδ activator peptide) increase in Gmax was still present for the KCNQ1(R243C) mutant tested (Supplementary Fig. S10).
Figure 4. Effect of PKC activation on mutant KCNQ1/KCNE1 channels associated with LQT1.
A. Wild-type KCNQ1/KCNE1 current (left) and mutant IKs (n=4) current changes induced by classic PKC (cPKC) activation peptide (n=4). Holding potential was set at −80 mV and 4-s depolarization pulse to +40 mV was applied followed by a step to −20mV. The current was measured every 10sec. Horizontal scale bars indicate 2sec. Vertical scale bars indicate 0.2nA. B. Summary data for effects of 5 min application of cPKC activating peptides for the LQT1 mutant channels indicated (wild type subunit was co-expressed with the mutant subunit at a ratio 1:1) measured in perforated patches at +40 mV. Current activation was normalized to control peptide effect. *P<0.05 C. Scheme depicting the location of the mutant channels studied. D. Cardiac action potential model with wild-type IKs channels, IKs channels with slower activation as indicated E: Cardiac action potential model with wild-type IKs channels, and mutant IKs channels after either β-AR and (β-AR and cPKC) stimulation as indicated. F. Correlation of APD measured in E with rate of acute triggered cardiac events for all patients with channels with each specific mutation by age 40 (from table 1).
The mutations studied affected both channel function and β-AR activation, to different extents, as we previously shown 6. To study possible consequences of impaired cPKC-mediated IKs activation additional to other regulatory and functional impairments in a whole cell context, we introduced detailed cPKC-mediated effects of wild-type and mutant IKs channels (maximal conductance and voltage dependence of activation parameters) into a single-cell mathematical cardiomyocyte model in combination with mutant-specific functional impairment and β-AR regulation 6 to model the acute high adrenergic stimulation (α1- and β-AR stimulation) on the IKs current. The cardiomyocyte model is similar to what we used earlier 23, and have shown that this particular cell model and IKs formulation are able to predict of cardiac risk for LQT1 patients. The model included modifications to the Ca-handling mechanisms to simulate AR stimulation (see Methods). For the model we made a simplifying assumption that β-AR and cPKC regulation were independent for the mutant channels as we observed for the wild-type channel and did not include other channel contribution to α1-AR regulation. Thus, we simulated two cases, β-AR stimulation alone and the combination of α1- and β-AR stimulation (see Supplementary Materials for details) based on the corresponding in vitro data collected by expression of the same mutants in HEK-293 cells, with current measured after 10 min treatment with forskolin alone 24 and the added effects of forskolin and cPKC stimulation. Note that the LQT1 mutations studied impaired to different extents cPKC regulation, but did not abolished regulation, as seen for the channel formed with the KCNE1(S102A) mutant. The data used in the model corresponds to the detailed cPKC regulation response measured for each mutant. For β-AR stimulation, we made additional changes to the Ca-handling mechanisms based on published data (see materials and methods section for detail) because they are thought to contribute to cardiac risk in LQT115. These changes have a AP prolonging effect in the model. Regulation of other ion channels were not added to the model because their contribution to cardiac risk for LQT1 patients is not well established, and because they are expected to contribute similarly to all mutants tested.
For WT-IKs channels, β-AR stimulation alone mildly shortened action potential duration (APD) (Fig. 4D) and the cPKC regulation also contributed to shortening of APD in our adrenergic activation model (cPKC + β-AR stimulation) (Fig. 4D), suggesting that stimulation of cPKC has a beneficial effect to control APD duration during strong adrenergic stimulation. For the cells with mutant IKs channels, β-AR stimulation contributes to APD prolongation (Table 3, Fig. 4D), consistent with β-adrenergic-mediated triggering of events during exercise 6, 25. We simulated cardiomyocyte response to acute strong adrenergic activation for mutant IKs channels by combining measured β-AR activation and cPKC activation (Fig. 4D). Interestingly, cells with KCNQ1(G168R) mutation which is located at the outside of the voltage sensor region, cPKC activation shortened APD after β-AR stimulation (Fig. 4D). In contrast, for one of the voltage sensor region mutation (R243C), combined β-AR/cPKC activation produced an additional prolongation of APD when compare to β-AR alone (Fig. 4D, Table 3). For the other mutant channels tested, there was a range in shortening in APD (7–44ms) (Table 3), suggesting that stimulation of cPKC has generally a beneficial effect, aiding repolarization for LQT1 mutant channels.
Table 3.
Mutation specific simulated APD and mutation specific mean QTc in patients.
| Mutation | APD basal Δ(WT-mut), msec | APD Beta Δ(WT-mut), msec | APD alpha + beta Δ(WT-mut), msec | Mean Patient QTc msec (mean±SDM) |
|---|---|---|---|---|
| G168R | 306 (56) | 408 (177) | 345 (133) | 480±40 |
| S225L | 290 (40) | 396 (165) | 370 (158) | 490±50 |
| R243C | 300 (50) | 420 (189) | 434 (222) | 490±60 |
| V254M | 315 (65) | 443 (212) | 430 (218) | 500±50 |
| R555C | 300 (50) | 263 (32) | 230 (18) | 490±20 |
Mutation-Specific cardiac risk
In a multivariate Cox regression model, the specific mutations included in the study were associated with significantly different risk of cardiac events independent from other clinical variables and after adjustment for the patient’s individual QTc (Table 2). For patients with G168R mutations, cardiac risk was lower than for patients with V254M (8-fold, p<0.001) and R243C (6-fold, p<0.02) mutations and not significantly different than for patients with S225L (p=0.16).
Table 2.
Cox model mutation-specific hazard ratio for the specified triggered events compared to all other mutations combined.
| Mutation | Hazard Ratio acute arousal triggered events n=28 | Hazard Ratio exercise triggered events n=57 |
|---|---|---|
| G168R | 0.434 | 0.54 |
| S225L | 1.04 | 1.00 |
| R243C | 2.67 | 0.67 |
| V254M | 3.40 | 3.11 |
| R555C | 0 | 0 |
adjusted for QTc≥500ms, beta-blocker usage, gender and age
Correlation of simulated APD prolongation mediated by overall (alpha+beta) adrenergic stimulation and risk for sudden arousal triggered arrhythmias in LQT1
We correlated simulated APD after combined β-AR/cPKC activation and β-AR activation alone with risk for cardiac events in LQT1 patients. We investigated the correlation of the β-AR/cPKC APD and exercise and sudden arousal triggered cardiac events as defined by our recent publication 25. Prolonged APD associated with β-AR/cPKC activation was strongly correlated to an increased rate of sudden arousal triggered cardiac events for these patients, while APD prolongation associated with β-AR alone showed a less pronounced correlation (Fig. 4E). R555C mutant was excluded from this correlation because no events were observed for patients in the Long QT Registry, similar results were observed when R555C data was included (R2=0.83, p=0.03). Similarly, simulated APD prolongation also showed strong correlation to mutant specific adjusted cardiac risk. Correlation between simulated APD (Table 3) and Hazard Ratio obtained from the Cox model (Table 2) was as follows: αβ-R2=0.95 (p=0.03), β-R2=0.36 (p=0.40) and basal R2=0.03 (p=0.84). The correlation between cardiac events and β-AR/cPKC regulated APD was not observed for exercised triggered events (R2=0.18, p=0.58), suggesting it is specific to sudden arousal triggered events.
DISCUSSION
Our data shows that α1-AR- cPKC signaling activates the KCNQ1/KCNE1 channel by shifting the voltage dependence of channel activation via phosphorylation of ancillary subunit KCNE1 at Ser102. To investigate relevance of this regulation in shortening of APD at high adrenergic states we study the mutation-specific effects of cPKC activation in LQT1 associated channels. The simulated effect of mutant-specific combined α1- and β-AR-mediated APD showed stronger correlation to the cumulative rate of acute emotion/arousal triggered cardiac events in LQT1 patients than β-AR alone. Our results suggest that α1-AR-cPKC-KCNE1(S102) signaling is important for shortening of APD at high adrenergic stimulation in human cardiomyocytes and activation of this signaling pathway may be explain the lower arrhythmic risk associated with acute emotion/arousal stress when compared to exercise triggers for LQT1 patients. Our data also implicates impairment of cPKC-signaling to increase in acute/emotion/arousal stress triggered events. These results suggest that exercise and acute emotion/arousal may stimulate different signaling pathways and suggest novel targets for anti-arrhythmic drug development.
IKs regulation by β1-AR-PKA signaling has been extensively studied, showing that channel activation is mediated by the direct channel phosphorylation by PKA upon stimulation of the receptor 15, 26. Gq-protein coupling receptors (GqPCRs) have also been suggested to modulate IKs current 27–30 through channel phosphorylation by PKC26, 27, 31, 32, but the overall outcome of channel modulation by GqPCR/PKC signaling differ in experimental systems and species. Consistent with our data, guinea pig IKs currents, where the KCNE1(S102) residue is absent, have been suggested to be activated by PKCε isoforms by an increase in Gmax without changes in channel voltage dependence of activation 22. Non-physiologic activation of PKC by phorbol esters such as PMA which directly stimulates both Ca2+-dependent and independent PKC isoforms, has been widely used for the investigation of PKC regulation of IKs, showing to underlie IKs inhibition via KCNE1 phosphorylation at the S102 residue 27, 33. However this inhibitory effect was not confirmed using PKC activation by physiological receptor signaling and human IKs channel. KCNE1 subunit has relatively low homology among species, suggesting the KCNE1(S102)-cPKC regulation we observe may be specific to humans. We have shown in a previous report that GqPCR stimulation activates the human IKs current through PKC activation34. Using PKC signaling activation by both receptor stimulation and PKC-isoform specific activator, we showed in this study more detailed mechanism that α1-AR-cPKC signaling can activate human IKs channel by shifting the voltage dependence of channel activation via phosphorylation of KCNE1 at Ser102, which contributes to maintain APD during acute high adrenergic state. One limitation of this study is the need to co-transfect a number of cDNAs into cells, because of the inherent variability of transient transfection. To limit problems we used transfection protocols that yielded high transfection efficiency and confirmed co-transfection of at least 80% of the cells for the constructs (see supplementary methods for details).
Our results shows that in one of the major repolarizing channels in human cardiomyocytes, IKs, is strongly regulated by cPKC activation and has profound implication to regulation of cardiac rhythm for inherited LQT1 patients where the repolarizing currents in the heart are decreased. In particular for acute emotion/arousal, norepinephrine which is released from sympathetic neurons innervating the heart and may show high local concentration at the cardiomyocytes, is expected to strongly activate cardiac α1-ARs. On the other hand, during exercise relatively lower concentrations of circulating epinephrine may produce mainly β-AR stimulation at the heart, but a relatively smaller contribution of α1-ARs activation. LQT1 patients are particularly well treated by β-blockers 6, 35, especially adrenergic-triggered events 25, but recent evidence has made increasingly clear that patients have events despite β-blocker treatment. Our results are the first to suggest an additional signaling pathway (α1-AR-cPKC-KCNE1 signaling) that may be compromised in LQT1 and that may be linked to acute arousal events in these patients (see scheme in supplementary figure S11).
Despite the limitations of the correlational analysis of the simulation and clinical data, due to the limited data available for the patients with this rare disease and the inherent low rate of events in LQT, there are a number of clinical observations that are consistent with our data. First, LQT1 patients have a lower rate of acute arousal/emotional triggered events when compared to exercise triggered events 25, 36. For mutations with intact cPKC-mediated signaling, this additional adrenergic regulation may explain this decrease in risk. One example highlighted here is the common LQT1 mutation G168R, from the 97 patients in the registry, 11 had exercise triggered events, but only 2 acute arousal events. Second, demonstrating the robustness of our simulation data, β-AR results presented here are consistent with our previous results showing that mutations with impaired β-AR activation contributed to cardiac risk in patients6, 37. For instance, simulations show that the β-AR-impaired mutation V254M produces a stronger prolongation of APD than G168R, a mutation that is strongly activated by β-AR signaling despite similar basal effects in the current. Thirdly, our simulation results are also consistent with the very low rate of cardiac events observed for patients with the R555C mutation (no cardiac events are observed in the Registry database despite strong QTc prolongation in the patients), and with our previous data suggesting protein kinase activation rescues putative PIP2-interacting residues 38.
Our results imply that patients that have mutations with impaired cPKC activation will be at higher risk for acute arousal cardiac events and stimulation of this pathway may shorten QTc and prevent arrhythmias in LQT1 patients. Long QT syndrome type 2 (LQT2) is due to mutations in the KCNH2 gene, and are due to decrease in function of the IKr potassium channel. In contrast to LQT1, the main trigger for cardiac events in LQT2 are acute arousal events 39. This may be explained by the inbibitory effect of IKr currents suggested to occur by PKC stimulation 40, 41, including via α-AR 42, 43, cPKC-mediated activation of IKs may be particularly important to compensate for IKr inhibition at these high adrenergic states. This was not added to our simulation model, making our results conservative. cPKC activation of IKs or drugs that mimic its action on the IKs channel may provide a new target to help promote effective cardiac repolarization for LQT and drug-induced LQT patients. This may be particularly important for the large number of mutations affecting voltage dependence of activation of the channel 23,44 and mutations associated with increased risk of cardiac events without significant prolongation of QTc in patients 6, 44, 45.
MATERIALS and METHODS
Population
The population involved patients with either genetically confirmed LQT1 mutations or patients that died suddenly at a young age of suspected LQTS and were from a family with a genetically confirmed mutation as previously described 46. Details are provided in supplementary data.
Electrophysiology
The electrophysiological parameters were obtained from expression of wild-type and mutant human KCNQ1 channel subunits at a ratio 1:1 in HEK293Tcells. The auxiliary KCNE1subunit was also co-expressed in all experiments. The procedure is explained in detail in the Supplementary Material and Methods and Supplementary Figure I.
Supplementary Material
Table 1.
Mutation specific triggered events
| Mutation | # patients acute arousal triggered events (%) | # patients with exercise triggered events (%) | Total # patients |
|---|---|---|---|
| G168R | 3 (3) | 10 (11) | 95 |
| S225L | 2 (10) | 5 (24) | 21 |
| R243C | 2 (25) | 1 (13) | 8 |
| V254M | 21 (19) | 41 (37) | 112 |
| R555C | 0 (0) | 0 (0) | 8 |
Highlights.
α1-AR-cPKC activates KCNQ1/KCNE1 independently of β-AR mediated activation.
α1-AR-cPKC-E1(S102) activates KCNQ1/KCNE1 by shifting V1/2 of channel activation.
Simulated mutant PKC+PKA APD effects correlates to acute-arousal events in LQT1.
α1-AR-cPKC-E1(S102) is important for shortening of APD at high adrenergic states.
Data may explain lower rate of acute-arousal than exercise triggers for LQT1.
Acknowledgments
The authors thank Dr. Elena Fujiwara, Ms. Nobiru Suzuki, Ms. Mehreen Butt and Mr. Michael Cypress for their technical assistance. This work was partly supported by AHA grants (09POST2310079 and 14BGIA18830032 to J.O.-U.), Foreign Study Grant Award of Kanae Foundation (to J.O.-U.), Irisawa Memorial Promotion Award for Young Physiologists from the Physiological Society of Japan (to J.O.-U.) and NIH R01HL114792 (to C.M.B.L.).
Footnotes
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 2.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106:1800–1805. doi: 10.1161/01.cir.0000031733.51374.c1. [DOI] [PubMed] [Google Scholar]
- 3.Lampert R. Anger and ventricular arrhythmias. Curr Opin Cardiol. 2010;25(1):46–52. doi: 10.1097/HCO.0b013e32833358e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss AJ. Long QT syndrome. JAMA. 2003;289(16):2041–2044. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 5.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–850. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 6.Barsheshet A, Goldenberg I, Uchi J, Moss AJ, Jons C, Shimizu W, Wilde AA, McNitt S, Peterson DR, Zareba W, Robinson JL, Ackerman MJ, Cypress M, Gray DA, Hofman N, Kanters JK, Kaufman ES, Platonov PG, Qi M, Towbin JA, Vincent GM, Lopes CM. Mutations in Cytoplasmic Loops of the KCNQ1 Channel and the Risk of Life-Threatening Events: Implications for Mutation-Specific Response to Beta-Blocker Therapy in Type-1 Long QT Syndrome. Circulation. 2012;125:1988–1996. doi: 10.1161/CIRCULATIONAHA.111.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldenberg I, Thottathil P, Lopes CM, Moss AJ, McNitt S, Uchi J, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Towbin JA, Vincent M, Barsheshet A. Trigger-specific ion-channel mechanisms, risk factors, and response to therapy in type 1 long QT syndrome. Heart Rhythm. 2012;9:49–56. doi: 10.1016/j.hrthm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg I, Thottathil P, Lopes CM, Moss AJ, McNitt S, Uchi J, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Towbin JA, Vincent M, Barsheshet A. Trigger-specific ion-channel mechanisms, risk factors, and response to therapy in type 1 long QT syndrome. Heart Rhythm. 2012;9:49–56. doi: 10.1016/j.hrthm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Jensen BC, O’Connell TD, Simpson PC. Alpha-1-adrenergic receptors: Targets for agonist drugs to treat heart failure. J Mol Cell Cardiol. 2011;51:518–528. doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 11.Rybin VO, Steinberg SF. Protein-Kinase-C Isoform Expression and Regulation in the Developing Rat-Heart. Circ Res. 1994;74:299–309. doi: 10.1161/01.res.74.2.299. [DOI] [PubMed] [Google Scholar]
- 12.Bowling N, Walsh RA, Song GJ, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 13.Lopes CMB, Remon JI, Matavel A, Sui JL, Keselman I, Medei E, Shen YM, Rosenhouse-Dantsker A, Rohacs T, Logothetis DE. Protein kinase A modulates PLC-dependent regulation and PIP2-sensitivity of K+ channels. Channels. 2007;1:124–134. doi: 10.4161/chan.4322. [DOI] [PubMed] [Google Scholar]
- 14.Matavel A, Lopes CMB. PKC activation and PIP2 depletion underlie biphasic regulation of IKs by Gq-coupled receptors. J Mol Cell Cardiol. 2009;46:704–712. doi: 10.1016/j.yjmcc.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 16.Imredy JP, Penniman JR, Dech SJ, Irving WD, Salata JJ. Modeling of the adrenergic response of the human I-Ks current (hKCNQ1/hKCNE1) stably expressed in HEK-293 cells. Am J Physiol Heart Circ Physiol. 2008;295:H1867–H1881. doi: 10.1152/ajpheart.433.2008. [DOI] [PubMed] [Google Scholar]
- 17.Deng XF, Sculptoreanu A, Mulay S, Peri KG, Li JF, Zheng WH, Chemtob S, Varma DR. Crosstalk between alpha-1A and alpha-1B adrenoceptors in neonatal rat myocardium: Implications in cardiac hypertrophy. J Pharmacol Exp Ther. 1998;286:489–496. [PubMed] [Google Scholar]
- 18.Varma DR, Deng XF. Cardiovascular alpha(1)-adrenoceptor subtypes: functions and signaling. Can J Physiol Pharmacol. 2000;78:267–292. [PubMed] [Google Scholar]
- 19.Korzick DH, Holiman DA, Boluyt MO, Laughlin MH, Lakatta EG. Diminished alpha(1)-adrenergic-mediated contraction and translocation of PKC in senescent rat heart. Am J Physiol Heart Circ Physiol. 2001;281:H581–H589. doi: 10.1152/ajpheart.2001.281.2.H581. [DOI] [PubMed] [Google Scholar]
- 20.Budas GR, Koyanagi T, Churchill EN, Mochly-Rosen D. Competitive inhibitors and allosteric activators of protein kinase C isoenzymes: a personal account and progress report on transferring academic discoveries to the clinic. Biochem Soc Trans. 2007;35:1021–1026. doi: 10.1042/BST0351021. [DOI] [PubMed] [Google Scholar]
- 21.Matavel A, Lopes CM. PKC activation and PIP(2) depletion underlie biphasic regulation of IKs by Gq-coupled receptors. J Mol Cell Cardiol. 2009;46:704–12. doi: 10.1016/j.yjmcc.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toda H, Ding WG, Yasuda Y, Toyoda F, Ito M, Matsuura H, Horie M. Stimulatory action of protein kinase C epsilon isoform on the slow component of delayed rectifier K+ current in guinea-pig atrial myocytes. Br J Pharmacol. 2007;150:1011–1021. doi: 10.1038/sj.bjp.0707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jons C, Uchi J, Moss AJ, Reumann M, Rice JJ, Goldenberg I, Zareba W, Wilde AA, Shimizu W, Kanters JK, McNitt S, Hofman N, Robinson JL, Lopes CM. Use of mutant-specific ion channel characteristics for risk stratification of long QT syndrome patients. Sci Transl Med. 3:76ra28. doi: 10.1126/scitranslmed.3001551. [DOI] [PubMed] [Google Scholar]
- 24.Barsheshet A, Moss AJ, McNitt S, Polonsky S, Lopes CM, Zareba W, Robinson JL, Ackerman MJ, Benhorin J, Kaufman ES, Towbin JA, Vincent GM, Qi M, Goldenberg I. Risk of syncope in family members who are genotype-negative for a family-associated long-QT syndrome mutation. Circ Cardiovasc Genet. 2011 Oct;4(5):491–499. doi: 10.1161/CIRCGENETICS.111.960179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldenberg I, Thottathil P, Lopes CM, Moss AJ, McNitt S, Uchi J, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Towbin JA, Vincent M, Barsheshet A. Trigger-specific ion-channel mechanisms, risk factors, and response to therapy in type 1 long QT syndrome. Heart Rhythm. 2012;9:49–56. doi: 10.1016/j.hrthm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242:67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- 27.Honore E, Attali B, Romey G, Heurteaux C, Ricard P, Lesage F, Lazdunski M, Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO J. 1991;10:2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto Y, Ogura T, Uemura H, Saito T, Masuda Y, Nakaya H. Histamine H1-receptor-mediated modulation of the delayed rectifier K+ current in guinea-pig atrial cells: opposite effects on IKs and IKr. Br J Pharmacol. 1999;128:1545–1553. doi: 10.1038/sj.bjp.0702918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daleau P, Turgeon J. Angiotensin II modulates the delayed rectifier potassium current of guinea pig ventricular myocytes. Pflugers Arch. 1994;427:553–555. doi: 10.1007/BF00374275. [DOI] [PubMed] [Google Scholar]
- 30.Selyanko AA, Hadley JK, Wood IC, Abogadie FC, Jentsch TJ, Brown DA. Inhibition of KCNQ1-4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors. J Physiol. 2000;522:349–355. doi: 10.1111/j.1469-7793.2000.t01-2-00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo CF, Numann R. Independent and exclusive modulation of cardiac delayed rectifying K+ current by protein kinase C and protein kinase A. Circ Res. 1998;83:995–1002. doi: 10.1161/01.res.83.10.995. [DOI] [PubMed] [Google Scholar]
- 32.Xiao GQ, Mochly-Rosen D, Boutjdir M. PKC isozyme selective regulation of cloned human cardiac delayed slow rectifier K current. Biochem Biophys Res Commun. 2003;306:1019–1025. doi: 10.1016/s0006-291x(03)01095-7. [DOI] [PubMed] [Google Scholar]
- 33.Busch AE, Varnum MD, North RA, Adelman JP. An amino acid mutation in a potassium channel that prevents inhibition by protein kinase C. Science. 1992;255:1705–1707. doi: 10.1126/science.1553557. [DOI] [PubMed] [Google Scholar]
- 34.Matavel A, Lopes CMB. PKC activation and PIP2 depletion underlie biphasic regulation of IKs by Gq-coupled receptors. J Mol Cell Cardiol. 2009;46:704–712. doi: 10.1016/j.yjmcc.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldenberg I, Thottathil P, Lopes CM, Moss AJ, McNitt S, Uchi J, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Towbin JA, Vincent M, Barsheshet A. Trigger-specific ion-channel mechanisms, risk factors, and response to therapy in type 1 long QT syndrome. Heart Rhythm. 2012;9:49–56. doi: 10.1016/j.hrthm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 37.Costa J, Lopes CM, Barsheshet A, Moss AJ, Migdalovich D, Ouellet G, McNitt S, Polonsky S, Robinson JL, Zareba W, Ackerman MJ, Benhorin J, Kaufman ES, Platonov PG, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Goldenberg I. Combined assessment of sex- and mutation-specific information for risk stratification in type 1 long QT syndrome. Heart Rhythm. 2012;9:892–898. doi: 10.1016/j.hrthm.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matavel A, Medei E, Lopes CM. PKA and PKC partially rescue long QT type 1 phenotype by restoring channel-PIP2 interactions. Channels (Austin) 2010;4:3–11. doi: 10.4161/chan.4.1.10227. [DOI] [PubMed] [Google Scholar]
- 39.Kim JA, Lopes CM, Moss AJ, McNitt S, Barsheshet A, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Towbin JA, Vincent M, Goldenberg I. Trigger-specific risk factors and response to therapy in long QT syndrome type 2. Heart Rhythm. 2010;7:1797–1805. doi: 10.1016/j.hrthm.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barros F, Gomez-Varela D, Viloria CG, Palomero T, Giraldez T, de la Pena P. Modulation of human erg K+ channel gating by activation of a G protein-coupled receptor and protein kinase C. J Physiol. 1998;511:333–46. doi: 10.1111/j.1469-7793.1998.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas D, Wu K, Wimmer AB, Zitron E, Hammerling BC, Kathofer S, Lueck S, Bloehs R, Kreye VA, Kiehn J, Katus HA, Schoels W, Karle CA. Activation of cardiac human ether-a-go-go related gene potassium currents is regulated by alpha(1A)-adrenoceptors. J Mol Med (Berl) 2004;82:826–837. doi: 10.1007/s00109-004-0582-8. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Xu DJ, Cai JB, Huang YZ, Zou JG, Cao KJ. Rapid component I(Kr) of cardiac delayed rectifier potassium currents in guinea-pig is inhibited by alpha(1)-adrenoreceptor activation via protein kinase A and protein kinase C-dependent pathways. Eur J Pharmacol. 2009;608:1–6. doi: 10.1016/j.ejphar.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Thomas D, Wu K, Wimmer AB, Zitron E, Hammerling BC, Kathofer S, Lueck S, Bloehs R, Kreye VA, Kiehn J, Katus HA, Schoels W, Karle CA. Activation of cardiac human ether-a-go-go related gene potassium currents is regulated by alpha(1A)-adrenoceptors. J Mol Med (Berl) 2004;82:826–837. doi: 10.1007/s00109-004-0582-8. [DOI] [PubMed] [Google Scholar]
- 44.Goldenberg I, Horr S, Moss AJ, Lopes CM, Barsheshet A, McNitt S, Zareba W, Andrews ML, Robinson JL, Locati EH, Ackerman MJ, Benhorin J, Kaufman ES, Napolitano C, Platonov PG, Priori SG, Qi M, Schwartz PJ, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Zhang L. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J Am Coll Cardiol. 2011;57:51–9. doi: 10.1016/j.jacc.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horr S, Goldenberg I, Moss AJ, Uchi J, Barsheshet A, Connelly H, Gray DA, Zareba W, Lopes CM. Ion channel mechanisms related to sudden cardiac death in phenotype-negative long-QT syndrome genotype-phenotype correlations of the KCNQ1(S349W) mutation. J Cardiovasc Electrophysiol. 2011;22:193–200. doi: 10.1111/j.1540-8167.2010.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jons C, Uchi J, Moss AJ, Reumann M, Rice JJ, Goldenberg I, Zareba W, Wilde AA, Shimizu W, Kanters JK, McNitt S, Hofman N, Robinson JL, Lopes CM. Use of Mutant-Specific Ion Channel Characteristics for Risk Stratification of Long QT Syndrome Patients. Sci Transl Med. 2011;3:76ra28. doi: 10.1126/scitranslmed.3001551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.