Abstract
The finding that neonatal mice are able to regenerate myocardium after apical resection has recently been questioned. We determined if heart regeneration is influenced by the size of cardiac resection and whether surgical retraction of the ventricular apex results in an increase in cardiomyocyte cell cycle activity. We performed moderate or large apical ventricular resections on neonatal mice and quantified scar infiltration into the left ventricular wall at 21 days post-surgery. Moderately resected hearts had 15±2% of the wall infiltrated by a collagen scar; significantly greater scar infiltration (23±4%) was observed in hearts with large resections. Resected hearts had higher levels of cardiomyocyte cell cycle activity relative to sham hearts. Surgically retracting the ventricle often resulted in fibrosis and induced cardiomyocyte cell cycle activity that was comparable to that of resected hearts. We conclude that apical resection in neonatal mice induces cardiomyocyte cell cycle activity and neomyogenesis, although scarring can occur. Surgical technique and definition of approach to assessing the extent of regeneration are both critical when using the neonatal mouse apical resection model.
Keywords: Cardiac Regeneration, Apical Resection, Neomyogenesis, Fibrosis
1. Introduction
The discovery by Porrello et al in 2011 that neonatal mice have the potential to regenerate resected myocardium generated much excitement in cardiovascular biology [1]. A recent study by Andersen et al questioned the utility of the apical resection model [2]. Andersen et al found that extensive scarring occurred in apically resected hearts at 21 days post-surgery and found limited evidence for neomyogenesis [2]. Understandably, the conflicting results produced by this study has generated some confusion and controversy as several laboratories have produced data that the neonatal mouse heart does have regenerative capabilities and is able to undergo neomyogenesis after sustaining myocardial injury [3]. Much speculation has occurred as to why the Porrello and Anderson studies appear to conflict, and it is possible that technical considerations as well as determining how regeneration is defined are important [3–5].
We systematically examined how technical considerations influence this important experimental model. These considerations included the size of apical resection, which was posited to be one cause for the conflicting findings, as well as the mechanical fixation (surgical retraction) of the ventricle during surgery [3]. We aimed to systematically and quantitatively clarify the extent of regeneration, neomyogenesis, and scarring that occur in this model. We show that new myocardial formation clearly occurs after apical resection, but that this regeneration is often accompanied by some scarring at 21 days post-resection (dpr). The extent of scarring is related to resection size. We also show that there is an increase in cardiomyocyte cell cycle activity, but fibrosis and an increase in cardiomyocyte cell cycle activity occur when sham operations include surgical retraction; the surgical retraction effect in control hearts can mask the induction of cardiomyocyte cell cycle activity in resected hearts. These data clarify the neonatal mouse apical resection model.
2. Materials and Methods
2.1. Surgical procedures
Neonatal mice were anesthetized for 4 minutes on ice. We performed thoracotomy followed by resection of either 10 or 20% of the ventricle. Our typical sham operation did not involve mechanically fixing the apex of the left ventricle. For non-retracted sham operations, we performed thoracotomy without resection. For surgical retraction experiments, we gently fixed the left ventricle with a micro needle holder after open thoracotomy.
2.2. Statistical analysis
Data are presented as mean ± SEM. A one-way ANOVA followed by a pairwise t-test with a Bonferroni correction was used to compare three or more groups. One or two-tailed t-tests were used for two groups. For detailed methods, see Online Supplement.
3. Results and Discussion
3.1. Scarring often accompanies new heart muscle formation after apical resection and is related to resection size
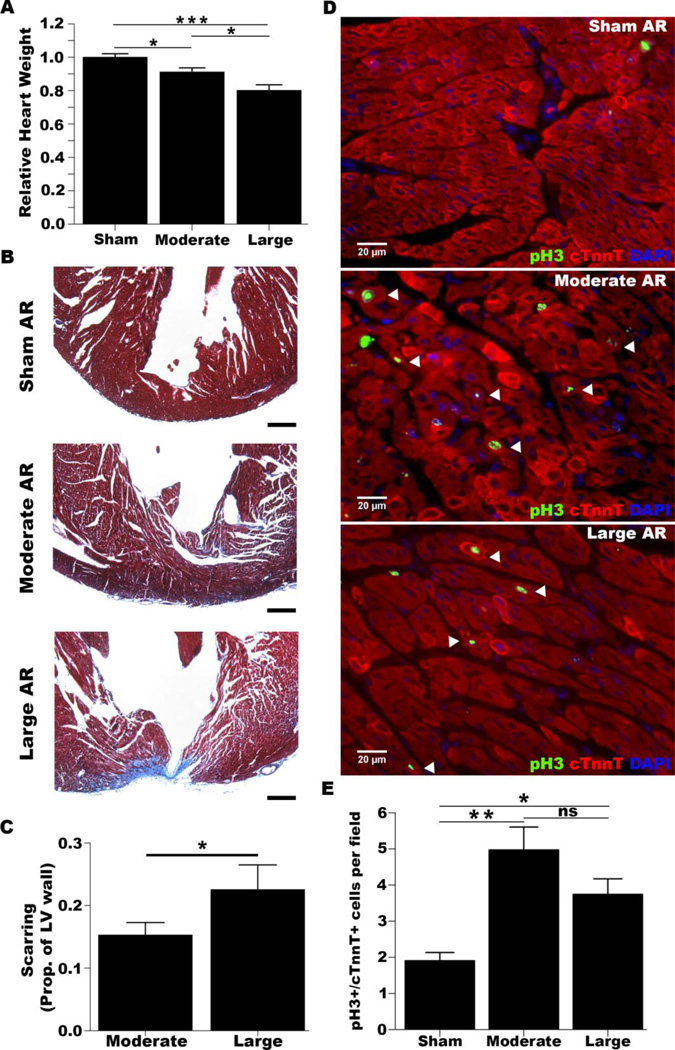
To study the impact of resection size, we measured the relative size of the resected myocardium (Supplemental Figure 1A). As intended, large resections were approximately twice as large as moderate resections (Supplemental Figure 1B). After quantifying the weights of hearts at 3 hours post-surgery, we found that moderate and large resections removed about 10% and 20% of the ventricular myocardium respectively (Figure 1A). Thus, we were able to quantitatively distinguish resection size, and the sizes of our moderate resections were similar to those of Porrello et al and Andersen et al [1, 2].
Figure 1. Regeneration and scarring occur after apical resection.
Hearts were isolated at 3 hours post-resection, 21 dpr and 7 dpr. A. Relative heart weights at 3 hours post-resection. (Sham: n=21, Moderate: n=25, Large: n=9). *p<0.05,***p<0.001. B. Representative trichrome stainings of sections from hearts at 21 dpr. Apical regions of the left ventricle are shown. Scar tissue stains blue. Scale bar: 100 µm. C. Quantification of scar incursion into the left ventricular wall (prop.=proportion). (Sham: n=6, Moderate: n=23, Large: n=10). *p<0.05. See also, Supporting Figure 2. D. Representative immunofluorescent stainings of sections at 7 dpr for phospho-H3 (pH3) and cardiac troponin t (cTnnT). Arrowheads indicate pH3+/cTnnT+ cells. E. Quantification of pH3+/cTnnT+ cells. (Sham: n=6, Moderate: n=8, Large: n=8). *p<0.05,**p<0.01, ns=non-significance.
We isolated hearts at 21 days post-surgery to determine the extent of regeneration. Although we observed morphological differences between sham and resected hearts as previously described, these differences were more pronounced in the large-resected group, and several moderately-resected hearts appeared similar to sham hearts (Supplemental Figure 1C) [2]. In contrast to Andersen et al’s findings, both moderate and large-resected hearts tended to weigh more than sham hearts, but this was not statistically significant (data not shown). While Porrello et al reported minimal scarring, Andersen et al noted extensive scarring in resected hearts that tended to occur more posteriorly and anteriorly [1, 2]. To assess scarring throughout the ventricle, we performed a Masson’s trichrome stain of sections from 5 regions spanning the chamber of the left ventricle (Supplemental Figure 2A). We observed fibrotic tissue in both moderately and large-resected hearts, but scarring was more evident in the latter group (Figure 1B). For each region of the heart, we quantified the extent of scarring as a proportion of the left ventricular wall infiltrated by scar tissue and averaged this proportion across regions (Supplemental Figure 2B). We speculated that hearts that received larger resections would exhibit more scarring than hearts with moderate resections. Indeed, we found that scarring was greater in hearts with large resections (~23% infiltration) relative to hearts with moderate resections (~15% infiltration) (p<0.05, Figure 1C).
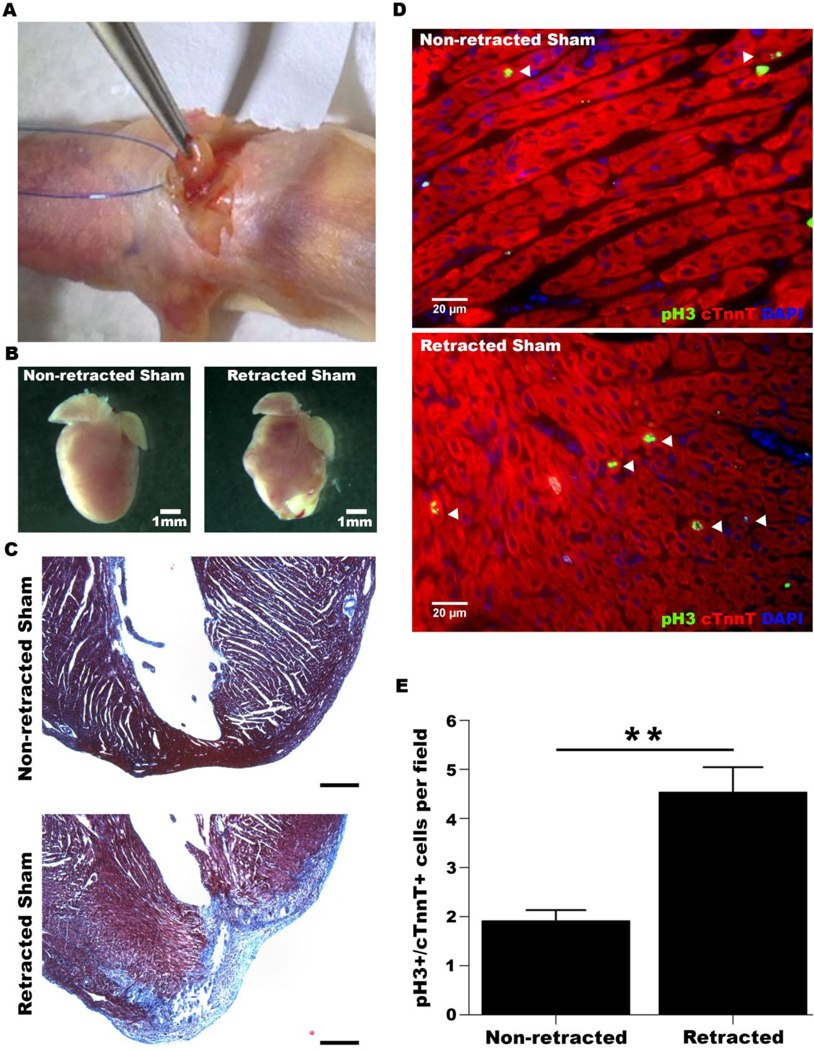
Figure 2. Surgical retraction results in fibrosis and an increase in cardiomyocyte cell cycle activity.
Hearts were isolated and stained at 7 dpr. A. Image depicting the surgical retraction procedure. B. Representative images of non-retracted and retracted sham hearts. C. Trichrome staining of sections from non-retracted and retracted sham hearts. Apical regions of the left ventricle are shown. Scale bar: 100 µm. D. Representative stainings of sections at 7 dpr for phospho-H3 and cardiac troponin t. Arrowheads indicate pH3+/cTnnT+ cells. E. Quantification of pH3+/cTnnT+ cardiomyocytes. (Non-retracted Sham: n=6, Retracted Sham: n=10). **p<0.01.
Because our surgical procedure exposed the chamber of the left ventricle, we reasoned that hearts with more limited regeneration would exhibit a full-thickness scar in at least one of the regions analyzed whereas hearts that had undergone extensive neomyogenesis would not have full-thickness scars. We observed that ~35% (n=8/23) of moderately-resected hearts had a full-thickness scar in at least one region, suggesting that neomyogenesis had occurred in a majority of these hearts. In contrast, a significantly higher percentage (70%; n=7/10) of hearts with large resections had a full-thickness scar (p<0.05).
3.2. Increased cardiomyocyte cell cycle activity occurs after apical resection
One key feature of the neonatal mouse resection model noted by Porrello et al is an increase in cardiomyocyte cell cycle activity after injury [1]. In contrast to Porrello et al, Andersen et al did not observe an increase in cardiomyocyte cell cycle activity in resected hearts [2]. To test whether resection results in an increase in cell cycle activity, we stained cardiac sections for the cardiac troponin T and phospo-Histone H3 at 7 dpr. Similar to Porrello et al‘s findings, we found that resected hearts had more proliferating cardiomyocytes relative to sham-operated hearts (Figure 1D,E). However, there was no significant difference in cardiomyocyte cell cycle activity between the moderate and large groups. These data are consistent with our findings that less neomyogenesis occurs in hearts with large resections as their cardiomyocyte cell cycle response was not greater than moderately-resected hearts despite having sustained twice as much damage.
3.3. Surgical retraction of the ventricle results in fibrosis and an increase in cardiomyocyte cell cycle activity
Andersen et al. reported a surgical resection procedure that involved gently fixing (surgically retracting) the apex of the ventricle [2]. Moreover, they note that their sham operation was performed exactly the same as this procedure without resection of the ventricle [2]. This retraction was not performed by Porrello et al, and it is possible that this procedure could induce a proliferative response [1]. To test this possibility, we surgically retracted the apices of hearts and examined whether cardiomyocyte cell-cycle activity is increased at 7 days post-procedure (Figure 2A). Upon isolation at 7 dpr, we noticed that many retracted hearts were fibrotic (Figure 2B). Trichrome staining of sections from retracted hearts indicated that interstitial fibrosis was present in most (9/10) of these hearts (Figure 2C). Moreover, we noted a significant increase in cardiomyocyte cell cycle activity in retracted hearts relative to non-retracted hearts (Figure 2D,E). Interestingly, the proliferative response in retracted hearts was not significantly different from that of resected hearts (p-val=1; compare Figure 2E to Figure 1E).
3.4. Conclusions
We observed more extensive scarring than Porrello et al, but our quantitative analyses of resected hearts revealed that these hearts often replaced much of the myocardial wall that was resected, and that the extent of scarring was greater in hearts that received large resections. If our criterion for assessing regeneration was the presence and not extent of fibrosis, then we would have concluded that most hearts did not regenerate. However, this definition would result in underestimating the degree of myogenesis and regeneration. Although we reasoned that the presence of a full-thickness scar in any region would indicate more limited regeneration, this does not exclude the possibility that neomyogenesis still occurs. For example, if a full-thickness scar is found in the anterior-most region, then it is possible that regeneration occurred near the center of the chamber, which was exposed upon resection. Moreover, resected hearts have increased cell cycle activity relative to sham hearts at 7 dpr, and our data suggest that the less extensive regeneration observed in hearts with large resections may be due to an inability to mount a proliferative response that is proportional to resection size. Future studies aimed at characterizing the damage in the myocardium bordering the plane of resection will advance our understanding of this model. Moreover, these studies will be especially important for determining why larger myocardial injuries do not result in a proportionally greater regenerative response.
Lastly, our data indicate that retracting the ventricle leads to interstitial fibrosis and an increase in cardiomyocyte cell cycle activity comparable to that of resected hearts. Using surgically retracted hearts as controls could therefore mask the response seen in resected hearts. If the sham procedure was conducted in this manner, then this could account for why Andersen et al did not observe an increase in cardiomyocyte cell cycle activity after apical resection.
Supplementary Material
Highlights.
Regeneration occurs after apical resection in neonatal mice
The extensiveness of scarring after apical resection is related to resection size
Apical resection results in increased cardiomyocyte cell cycle activity
Surgical retraction of the ventricle increases cardiomyocyte cell cycle activity
Acknowledgments
Funding Sources
This work was supported by an HHMI Gilliam Fellowship (D.M.B.), an NIH National Research Service Award (C.C.O.), and grants from the NIH (AG040019 and HL117986 to R.T.L.).
Non-standard Abbreviations
- AR
Apical Resection
- CM
Cardiomyocyte
- DPR
Days post-resection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Porrello E, Mahmoud A, Simpson E, Hill J, Richardson J, Olson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen D, Ganesalingam S, Jensen C, Sheikh S. Do neonatal mouse hearts regenerate following heart apex resection? Stem Cell Reports. 2014;2:406–413. doi: 10.1016/j.stemcr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadek H, Martin J, Takeuchi J, Leor J, Nei Y, Giacca M, et al. Multi-Investigator letter on the reproducibility of neonatal mouse heart regeneration following apical resection. Stem Cell Reports. 2014;3:1. doi: 10.1016/j.stemcr.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotlikoff M, Hesse M, Fleischmann B. Comment on “Do neonatal mouse hearts regenerate following heart apex regeneration?”. Stem Cell Reports. 2014;3:2. doi: 10.1016/j.stemcr.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen D, Jensen C, Sheikh S. Response to Sadek et al. and Kotlikoff et al. Stem Cell Reports. 2014;3:3–4. doi: 10.1016/j.stemcr.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.