Abstract
Ischemic myalgia is a unique type of muscle pain in the patient population. The role that discrete muscle afferent subpopulations play in the generation of pain during ischemic events, however, has yet to be determined. Using two brachial artery occlusion models to compare prolonged ischemia or transient ischemia with reperfusion of the muscles, we found that both injuries caused behavioral decrements in grip strength, as well as increased spontaneous pain behaviors. Using our ex vivo forepaw muscles, median and ulnar nerves, dorsal root ganglion (DRG), and spinal cord recording preparation, we found after both prolonged and transient ischemia, that there was a significant increase in the number of afferents that responded to both noxious and non-noxious36 chemical (lactate, ATP, varying pH) stimulation of the muscles compared to uninjured controls. However, we found an increase in firing to heat stimuli specifically in muscle afferents during prolonged ischemia, but a distinct increase in afferent firing to non-noxious chemicals and decreased mechanical thresholds after transient ischemia. The unique changes in afferent function observed also corresponded with distinct patterns of gene expression in the DRGs. Thus the development of ischemic myalgia may be generated by unique afferent based mechanisms during prolonged and transient ischemia.
Perspective
This study analyzes the response properties of thinly myelinated group III and unmyelinated group IV muscle afferents during prolonged and transient ischemia in addition to pain behaviors and alterations in DRG gene expression in mouse. Results suggest that mechanisms of pain generation during prolonged ischemia may be different from ischemia/reperfusion.
Keywords: ischemic myalgia, electrophysiology, gene expression, artery occlusion, plasticity
Introduction
Myalgia is one of the most common complaints of patients seeking treatment for pain; however, multiple disorders can cause muscle pain42. One vexing, but quite prevalent5, set of muscle pain disorders in the population are those that originate from issues of peripheral circulation. Patients with peripheral vascular disease (PVD) for example, have small occlusions of the supplying vessels in the limb muscles which causes them to experience intermittent claudication2. In addition, many patients with sickle cell anemia have chronic pain as a result of successive ischemia/reperfusion events during and after a crisis64. Recently, complex regional pain syndrome (CRPS) has also been linked to issues of peripheral perfusion where both hyper- and hypo-perfusion have been documented, and this has thought to be a major underlying cause of subsequent muscle pain9. A unique feature of disorders of peripheral perfusion is that transient ischemia with reperfusion causes muscle atrophy and microvasculature changes distinct from prolonged occlusion7, which may suggest that muscle pain in these distinct injury states may be generated through different mechanisms.
It has been well-documented that primary muscle afferents are crucial in generating muscle pain after inflammation16,55 or incision61; however the contribution of specific populations of muscle afferents to ischemic myalgia is not understood to the same degree. In uninjured animals, it is known that the thinly myelinated group III and unmyelinated group IV muscle afferents respond to mechanical and thermal stimuli similar to cutaneous afferent subpopulations; however, roughly half of group III and group IV muscle afferents are chemosensitive23. The chemical responsiveness of these afferents can be subdivided into two physiologically relevant classes based on their sensitivity to distinct metabolite mixtures6,23,36. Metaboreceptors, which are thought to regulate sympathetic reflexes and possibly the sensations of fatigue, have recently been defined as cells that respond to a non-noxious metabolite mixture of lactate and ATP (pH 7.0) that is typically found to be produced in the muscles during moderate exercise23,27,28,50,53. Conversely, metabo-nociceptors, which are thought to regulate pain responses, are stimulated by higher concentrations of these same metabolites (pH 6.6, increased lactic acid and ATP); a mixture known to be produced in the muscles during ischemic contractions1,23,29,35,36.
Since ischemic conditions produce these mixtures in injured muscles, it is reasonable to suggest that the afferents that respond to these metabolites and modifications of gene expression in the dorsal root ganglia (DRGs) are crucial in the generation of ischemic myalgia14,20,37,40. Therefore, in this study, we investigated whether muscle afferent response characteristics uniquely changed in response to prolonged or transient peripheral ischemia using an ex vivo forepaw muscle/median and ulnar nerves/DRG/spinal cord recording preparation and compared the results to behavioral changes after injury to determine how the observed changes in afferents could lead to muscle nociception. Prolonged ischemia was induced by a total brachial artery occlusion (BAO) in one forelimb while transient ischemia with reperfusion was established by removing the BAO after several hours. Finally, we surveyed DRG receptor expression and muscle-derived signaling pathways to explore potential mechanisms involved in the hypothesized differential muscle afferent plasticity.
Methods
Animals
Adult male Swiss Webster mice (Charles River), between 3-6 weeks of age were used in all experimental analyses. Mice were held in climate-controlled barrier facility with 12-hr light/dark housing and ad libitum access to food and water. All procedures were approved by the Cincinnati Children's Hospital Research Foundation Institutional Animal Care and Use Committee and adhered to NIH Standards of Animal Care and Use under AAALAC approved practices.
Induction of prolonged and transient peripheral ischemia
One day prior to all analyses, mice were anesthetized with 3% isofluorane. The right brachial artery was exposed proximal to the ulnar artery/radial artery split. The vessels were gently loosened from adjacent connective tissue and then the brachial artery was occluded with a 7-0 silk suture. Incisions were closed with 6-0 silk sutures. For the prolonged ischemia condition (brachial artery occlusion: BAO), the occlusion was left intact for 24hrs or up to three days for behavioral analyses. For transient ischemia with reperfusion (I/R), a modified version of Coderre et al. (2004) was employed. Briefly, animals were again anesthetized with 3% isofluorane six hours post-occlusion for removal of the brachial artery suture. I/R mice were allowed to recover for 18 hours after the second surgery to induce reperfusion injury. Again for behavioral analyses only, a three day time point post initial occlusion was also analyzed. To account for possible effects of sutures around the nerves for ex vivo or molecular experiments (below), a sham surgery was also utilized in which a suture was placed around the artery, but was not tied such that the artery was not occluded. In addition, another group of mice received a 6hr BAO only to aid in the determination of how the occlusion aspect of these injuries may be different in addition to determination of how prolonged ischemia may differ from the reperfusion phase.
Nerve crush injuries
Mice were anesthetized as described using 3% isofluorane. For grip strength positive control experiments (see below), the right median and ulnar nerves were exposed just above the elbow and crushed with #5 fine tip forceps for approximately 3-4sec. For ATF3 immunostaining positive control experiments (see below), only the right ulnar nerve was exposed just above the elbow and crushed with the forceps. The nerve(s) was then visually inspected to confirm the injury and qualitatively verify a translucent appearance of the nerve that is prevalent after these types of peripheral injuries25. The wounds were then closed using 7.0 silk sutures. One day after unilateral median and/or ulnar nerve crush, animals (n=8) underwent grip strength testing (below) for comparisons to mice that received BAO or I/R. In other experiments, C8 and T1 DRGs were isolated and processed immunocytochemically as described below one day after ulnar crush injury.
Behavioral Assays
Separate groups of sham, I/R and BAO mice were used for behavioral analysis (n=7-9 per group). Mice were first tested at baseline, approximately two hours prior to injury (described above), then again on days one and three post-injury. All behavioral testing was performed in the morning during light hours, and each testing day included three behavioral tasks: forelimb guarding, von Frey filament stimulation of the plantar surface of the forepaws, and forepaw grip strength in this order. Mice were placed in a raised plexiglass chamber with a steel mesh bottom, and allowed to habituate for at least 30 minutes. To evaluate guarding behavior, mice were assigned a score of 0-2 (0=mouse places foot firmly on mesh, 1=mouse does not bear full weight on foot, 2=mouse holds foot completely above mesh) every 5 minutes for 12 total observations61. The average score for the 12 trials was determined for each mouse per behavioral day. This was then averaged across all mice within the group.
Since ischemia has been shown to induce cutaneous mechanical hypersensitivity, we then confirmed successful artery occlusion in both BAO and I/R models52 by determining the mechanical paw withdrawal thresholds of mice via stimulating the plantar surface of the forepaws with an increasing series of von Frey filaments (0.07g to 4g). Threshold to withdrawal was recorded for at least three trials with five minute intervals in between stimulations, and the average of the three trials was used for analysis52. Lastly, mice were assayed for forepaw muscle strength using a grip strength meter (BioSeb, France). Animals were held by the tail over a metal grid until they firmly held it with both forepaws, but were not allowed to grip the grid with their hindpaws. Then they were quickly pulled back horizontally (along the axis of the force sensor) until they could not retain their grip. Grip strength was measured (in g) in three rounds of three trials each, with 5 minutes between each round in their home cage. Mice were assessed for mean grip strength by taking the average of the 9 total trials per testing day. Experimenters for behavioral studies were blinded to all conditions with the exception of the median/ulnar nerve crush experiments designed for grip strength positive control testing, since these latter tests were performed after these other experiments. However this was not an issue as these injuries prevent adequate blinding since one limb becomes essentially non-functional due to the nerve crush.
Hematoxylin/Eosin Staining of Muscle Tissue
To examine the extent of muscle damage after both types of injury, a cohort of adult naïve, sham, BAO, and I/R mice were perfused with 4% paraformaldehyde 24 hours post injury for forepaw muscle excision. Muscles were rinsed in phosphate buffer, and then embedded in 10% gelatin. 45μm sections were cut on a sliding microtome and mounted on gelatin-coated slides. Slides were hydrated by dipping in serial dilutions of EtOH, and stained with hematoxylin and lithium carbonate, before dehydrating in EtOH and staining with eosin. Slides were coverslipped with xylene and sealed with Permount, then imaged on a Leica fluorescence microscope under brightfield optics at 20× magnification.
Ex vivo recording and analysis of muscle sensory neurons
The mouse forepaw muscle ex vivo preparation has been described in detail previously24. Dissection and recording was performed exactly as described therein. Briefly, mice were deeply anesthetized with 90 mg/kg ketamine and 10 mg/kg xylazine and transcardially perfused with ice cold, oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF; in mM: 127.0 NaCl, 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 D-Glucose). Then, the vertebral column (from caudal brainstem to approximately thoracic segment 8) and right forearm were separated and transferred to a bath of the oxygenated aCSF solution. The forepaw muscles, median and ulnar nerves (with cutaneous branches bisected well proximal to the muscles), C6-T2 DRGs, and corresponding spinal cord segments were dissected in continuity. The intact preparation was then transferred to a recording chamber filled with fresh oxygenated aCSF, where the forepaw was segregated from the DRGs by elevation in a distinct inner chamber. The ice cold aCSF recording solutions were then slowly warmed to 32°C for intracellular recordings. Performing ex vivo recordings at 32°C helps maintain the integrity of the preparation during intracellular recording and repetitive stimulation of the muscles. However, the muscles are still able to contract normally to electrical stimulation of the nerves, which also helps to verify preparation viability prior to recording. This temperature has also been determined to minimally affect conduction velocity (CV). Group III muscle afferents are defined as those conducting at CVs 10-1.2m/s and Group IV fibers are those that conduct < 1.2m/s as reported previously23,31 and this can be clearly determined with these preparations.
Median and ulnar afferents were recorded from their somata in the C7, C8, and T1 DRGs via sharp intracellular quartz microelectrodes (impedance >150Ω) containing 5% neurobiotin (Vector Laboratories, Burlingame, CA) in 1M potassium acetate. To locate afferents supplying the forepaw muscles, a suction electrode was placed onto the sides of the median and ulnar nerves, and orthograde search stimuli applied (0.4-2mA, 1ms duration at 0.5Hz). To localize the receptive fields (RF) of electrically driven fibers to the muscle, a concentric bipolar electrode was then used to ensure that the driven cells innervated the muscles (6-8mA, 1ms duration). Once a RF was determined to be in the muscle, the RFs were exposed to a series of mechanical, thermal and chemical stimuli with adequate recovery times (∼20-30 s) between each stimulus. An increasing series of calibrated von Frey filaments from 0.07g to 8g were used to test mechanical sensitivity. Cold (0°C) and hot (52°C) physiological saline (0.9 % NaCl) was then applied to the RF to test thermal responsiveness. The bath was slightly lowered and the hot or cold saline was applied to the receptive fields as close as possible using a syringe with a needle without manually manipulating the RF with the needle ending. While this type of stimulus did not permit us to determine thermal thresholds, it did allow us to determine general responsiveness of individual afferents to these different thermal stimuli. Although it is possible that repetitive but brief applications of hot saline could produce sensitization of muscle afferents like that observed in some other studies analyzing cutaneous fibers, we have determined that no differences in firing or responsiveness to the initial stimuli being delivered are detected between cells obtained at the beginning of an experiment compared to the cells acquired at the end of recording (not shown). Thus it is unlikely that brief but repeated application of hot saline is causing significant sensitization of muscle afferents in these particular preparations. After mechanical and thermal testing, forepaw muscles were exposed to 10mL of a “low” (15 mM lactate, 1 mM ATP, pH 7.0) and then a “high” (50 mM lactate, 5 mM ATP, pH 6.6) metabolite mixture to determine chemosensitivity23,36. Metabolite mixtures were oxygenated and delivered to the muscles via a valve controller with an in-line heater to sustain bath temperature. The appropriate amount of ATP was added to the oxygenated lactic acid mixtures immediately prior to exposure. After chemical stimulation, mechanical and thermal stimuli were repeated to uncover any changes that may have occurred as a result of chemical application to individual cells.
In a few instances, some fibers were unable to be fully characterized by all of the mechanical, thermal and chemical stimuli, but were partially characterized by some of these. In these few cases, cells were also included for determination of percent responders for the stimulus applied and for the properties in which we obtained physiological data. Responses to stimuli were recorded and analyzed offline using Spike2 software (Cambridge Electronic Design). The distance between the positioning of the suction electrode and the DRG were used to calculate conduction velocity (CV) along with recorded spike latency from the fibers. Overall, we recorded and characterized a total of 245 cells. We obtained an average of 4-5 cells from each preparation. This was obtained from 15 naïve mice, 10 mice with sham surgeries and 12 mice from the BAO condition and 13 mice from the I/R condition (naïve: n=71 total cells recorded, sham: n=48 total cells; BAO: n=57 total cells and I/R: n=69 total cells). After recording of response properties, select cells were iontophoretically injected with neurobiotin (1-2 cells per DRG) for subsequent immunocytochemical analysis, with a total of 28 cells undergoing neurochemical phenotyping after recording.
Immunohistochemistry
DRGs containing stained and characterized cells were removed and immersion fixed in 3% paraformaldehyde in 0.1M phosphate buffer (PB) for 30 minutes at room temperature. Fixed DRGs were embedded in 10% gelatin, incubated overnight in 3% paraformaldehyde, and then cryoprotected in 20% sucrose. Embedded DRG sections (50 μm) were blocked and incubated overnight with two of the following primary antibodies depending on response characteristics: transient receptor potential vanilloid type 1 (rabbit anti-TRPV1, Alomone; 1:3000), acid-sensing ion channel 3 (guinea pig anti-ASIC3, Millipore; 1:2000), or P2X3 (rabbit anti-P2X3, ThermoScientific; 1:2000). In general, cells were mostly processed with combinations of ASIC3 and P2X3 antibodies since muscle afferents have been found to often contain one of these markers43. However, when cells were found to have vigorous heat responses, cells were immunolabeled with combinations of TRPV1 and ASIC3. Sections were then fluorescently labeled with corresponding secondary antibodies (Jackson ImmunoResearch anti-guinea pig AlexaFluor647; 1:400 or Jackson ImmunoResearch anti-rabbit AlexaFluor594; 1:400), as well as FITC-Avidin (Vector Laboratories; 1:750) for visualization of neurobiotin. Sections were mounted on gelatin-coated permafrost slides with Fluoro-Gel (Electron Microscopy Sciences) and stored in the dark at room temperature. Labeling was characterized and documented using a Leica confocal microscope with sequential scanning to avoid bleed-through of the different fluorophores. Images were compiled and prepared for publication using Photoshop (Adobe).
A separate cohort of mice was used to evaluate neuronal injury after sham surgery, prolonged ischemia, or transient ischemia with reperfusion injury to confirm that axonal damage during surgery was not contributing to the observed phenotypes. To determine injury scope, DRGs were taken from animals with sham surgery, BAO or I/R and compared to DRGs of an animal with a complete ulnar nerve crush injury for labeling with an antibody against activating transcription factor 3 (ATF3), a marker of injured neurons57. For this cohort, animals were injured one day prior to transcardial perfusion with 3% paraformaldehyde, and C8 and T1 DRGs excised. As stated above, DRGs were post-fixed, embedded, cryoprotected, and sectioned for staining. Sections were blocked and incubated overnight in primary antibody for ATF3 (Millipore rabbit anti-ATF3; 1:1000), then fluorescently labeled with secondary antibody (Jackson ImmunoResearch anti-rabbit AlexaFluor594; 1:400), and characterized as described above. Images were acquired on a Leica fluorescence microscope at 20× magnification and compiled as described using Adobe Photoshop.
RNA isolation, reverse transcription, PCR arrays and realtime PCR
DRGs and forepaw muscle tissue were collected from a separate cohort of naïve, sham, BAO, and I/R animals for PCR arrays (forepaw muscles from one naïve and one BAO mouse) or quantitative PCR (n=3-5 from each condition and tissue type). RNA was isolated using the Qiagen RNeasy kit, where DRG RNA was isolated according to the manufacturer's protocol, and muscle RNA was isolated using the manufacturer's protocol for fibrous tissues. For PCR arrays, muscle RNA samples from naïve or BAO mice were converted into cDNA following the RT2 First Strand Kit according to the manufacturer's protocol. Then cDNA samples were mixed with an RT2 Profiler SYBR Green PCR Array, realtime PCR master mix (SA Biosciences) and loaded into either the mouse growth factor (Cat#: PAMM-041) or cytokine (Cat#: PAMM-150) PCR arrays (SA Biosciences). These plates were then run on an Applied Biosystems model 7300 realtime PCR machine.
For standard quantitative realtime PCR, 500ng of total RNA was DNAse I treated (Invitrogen) and reverse transcribed using Superscript II (Invitrogen) reverse transcriptase. 20ng of cDNA were used in SYBR Green realtime PCR reactions that were performed in duplicate and analyzed on a Step-One realtime PCR machine (Applied Biosystems). ASIC1, ASIC3, TRPV1, and GAPDH primer sequences (forward and reverse) were obtained from Elitt et al14 for realtime PCR reactions. Primer sequences used for P2X3, P2Y1, and GFRα1 have been detailed previously23. Primer sequences used for IL-1r1, IL-1β, P2X5, and GDNF are as follows: IL-1r1: forward: 5′-AGG AAT GTG GCT GAA GAG CAC AGA -3′; reverse: 5′- ACT CGT GTG ACC GGA TAT TGC TTC -3′; IL-1β: forward: 5′- TAC AAG GAG AAC CAA GCA AC -3′; reverse: 5′- GGT GTG CCG TCT TTC ATT A -3′; P2X5: forward: 5′- TGG CAA GGC GGG AAA AT -3′; reverse: 5′- CCG GAA CCA ATG TTG ATG ACT -3′; GDNF: forward: 5′- AGC TGC CAG CCC AGA GAA TT -3′; reverse: 5′- GCA CCC CCG ATT TTT GC -3′. Cycle time (Ct) values for all targets were all normalized to a GAPDH internal control. Δ ΔCt values (used to determine fold change after injury) were then obtained by subtracting the normalized target gene's Ct value from naive controls. Then fold change was determined as 2Δ ΔCt (Applied Biosystems). The error of the difference in means is then also calculated for the fold-change. Values were then converted and reported as a percent change where 2-fold change = 100% change.
Statistical analysis
Differences in phenotype frequency between groups were compared with chi-square analysis and Yates correction (or Fisher Exact, if applicable). Firing frequencies and thresholds to different stimuli were compared via Kruskal-Wallis one-way ANOVA on ranks with Dunn's post-hoc, and behavioral assays were tested with two-way repeated measures ANOVA with Holm-Sidak post-hoc. Percent change from realtime PCR data was analyzed via one-way ANOVA with Tukey's post-hoc. Our critical significance level was set at p<0.05.
Results
Prolonged ischemia and transient ischemia with reperfusion injury alter spontaneous pain behaviors and grip strength
To first determine the effects of prolonged (BAO) or transient ischemia (I/R) on pain behaviors, we performed measures to analyze spontaneous/ongoing pain and muscle strength. In order to confirm that our artery occlusion surgeries were successful, we first measured cutaneous mechanical hypersensitivity using von Frey filament stimulation of the plantar surface of the forepaws52. We found that both injury groups had significantly lower average ipsilateral paw withdrawal thresholds at days one and three when compared to their respective baselines (BAO (n=9): BL: 2.52 ± 0.19 g, D1: 0.34 ± 0.07 g, D3: 0.36 ± 0.062 g; I/R (n=8): BL: 3.08 ± 0.18 g, D1: 0.47 ± 0.07 g, D3: 0.39 ± 0.11 g p<0.001, two-way repeated measures (RM) ANOVA; Fig. 1a). Also on days one and three for both injury groups, the paw withdrawal threshold for the injured forepaw was significantly lower than that for the contralateral paw (p<0.001). The contralateral limb thresholds were found to be lower at these time points, but were not found to be significantly decreased from baseline at day 1 in either condition, nor at day 3 in the BAO condition (p>0.1); however, in the I/R condition, the contralateral limb showed a significant decrease from its baseline at day 3 (BAO: BL: 2.1 ± 0.27 g, D1: 1.13 ± 0.12 g, D3: 1.05 ± 0.17 g; I/R: BL: 2.43 ± 0.39 g, D1: 1.68 ± 0.36, D3: 1.26 ± 0.31 g#; #p<0.02 vs contralateral I/R baseline, two-way repeated measures (RM) ANOVA). No significant differences in withdrawal threshold after sham surgery (n=7) were detected (Ipsilateral BL: 2.35 ± 0.56 g, D1: 1.41 ± 0.09 g, D3: 1.38 ± 0.09 g; Contralateral BL: 1.78 ± 0.4 g, D1: 1.38 ± 0.08 g, D3: 1.52 ± 0.17 g).
Figure 1. Prolonged and transient ischemia induce changes in evoked and spontaneous pain behaviors in addition to altering grip strength.
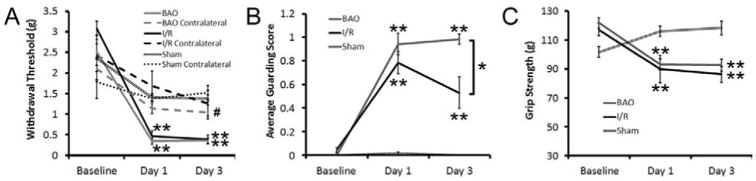
(A) Paw withdrawal thresholds from delivery of an increasing series of von Frey filaments to the plantar surface of the ipsilateral forepaw after brachial artery occlusion (BAO) or transient ischemia with reperfusion (I/R) injury is significantly lower on days one and three when compared to their respective baseline measurements (**p < 0.001, two-way RM ANOVA). The contralateral forepaw withdrawal response is not significantly different from baseline at any time point after BAO; however, after I/R, there was a statistical decrease in the contralateral forepaw withdrawal threshold, but only at the three day time point and not at day one (#p<0.05, Holm-Sidak post-hoc). Sham surgery did not alter mechanical withdrawal thresholds in the ipsilateral or contralateral forepaw. (B) There was also a significant increase in paw guarding after BAO and I/R at both one day and three days post injury compared to baseline (**p < 0.001, two-way RM ANOVA). However, after I/R, the guarding scores were significantly different from animals in the BAO group at the three day time point (*p < 0.02, Holm-Sidak post-hoc). No differences in paw guarding were found after sham surgery. (C) Mice from both injury groups also showed significantly reduced grip strength on day one and day three when compared to their respective baseline measurements (**p < 0.001, two-way RM ANOVA). In addition, no differences in grip strength were found between injury conditions at either time point after injury. Grip strength was not found to be reduced by a sham surgery.
We then wanted to determine if there were any effects of ischemia or reperfusion on spontaneous pain and specifically muscle function in our groups by analyzing paw guarding behaviors and grip strength (Fig. 1b, c). In the guarding assay, we found a significant increase in guarding behaviors one and three days after injury in the ipsilateral forelimb (Main effect of Timepoint, p<0.02, two-way RM ANOVA). However, the mice that underwent transient I/R injury showed a significant improvement in guarding by three days compared to one day (D1: 0.79±0.09 and D3: 0.53±0.14; p<0.003, Holm-Sidak post-hoc test), while the paw guarding in the BAO group remained elevated at both one day and three day time points (D1: 0.91±0.08 and D3: 0.91±0.09; p<0.2, Holm-Sidak post-hoc test). Sham surgery did not alter paw guarding (D1: 0.012±0.012 and D3: 0.0±0.0; p<0.4). Finally, both injury groups showed significantly lower grip strength (Fig. 1c) on day one and day three when compared to baseline measurements (BAO BL: 119.7±3.9 g, D1: 89.52±4.6 g, and D3: 89.83±4.3 g; I/R BL: 117.2±4.4 g, 90.0±9.6 g, 86.2±5.6 g; p<0.001, Holm-Sidak post-hoc test). Reduced grip strength after prolonged ischemia and I/R was surprisingly found to be similar to that observed if one limb virtually loses function via median and ulnar nerve crush (BL: 105.7±1.6g, D1: 84.1±5.3g; p value < 0.05). No differences were found between the two injury groups at either time point for grip strength. Sham surgery did not reduce grip strength over time relative to baseline (BL: 101.9±3.6 g, D1: 116.0±3.6 g, and D3: 118.4±4.8 g) as was detected after BAO or I/R.
Ischemia and/or reperfusion injury alters the response properties of group III and IV muscle afferents
To determine the effects of prolonged or transient ischemia on muscle afferent responsiveness, we then performed ex vivo recording in mice one day after BAO or I/R. We first however, qualitatively determined the extent of ischemic muscle injury between the two injury models, by performing hematoxylin and eosin staining on forepaw muscle tissue one day after BAO or I/R, and compared them to naïve and sham operated forepaw muscles. Both prolonged and transient ischemia induce some muscle tissue damage relative to these uninjured controls, but prolonged ischemia (BAO) appeared to affect the forepaw muscles to a slightly greater degree as evidenced by more extensive muscle fiber degeneration and muscle atrophy compared to the I/R condition. Although both ischemic injuries do not severely alter the muscles, our results are consistent with other reports of muscle tissue integrity during these types of artery occlusions8,19,47,49. To then confirm that our occlusion surgeries were not causing direct injury to the sensory neurons, we also analyzed ATF3 staining (a marker of neuronal injury) in the C8 DRGs in mice that underwent BAO or I/R. Compared to mice that experienced an ulnar crush injury which readily produced ATF3 immunopositive nuclei in DRG neurons, neither BAO or I/R damaged a significant number of primary afferents similar to that observed after sham surgery (Fig. 2). Therefore, in conjunction with the data presented below, it is likely that any effects of ischemia or reperfusion on the sensory neurons are due to alterations in the periphery rather than direct damage at the surgical site.
Figure 2. Prolonged and transient ischemia provoke differing levels of muscle injury without inducing neuronal injury.
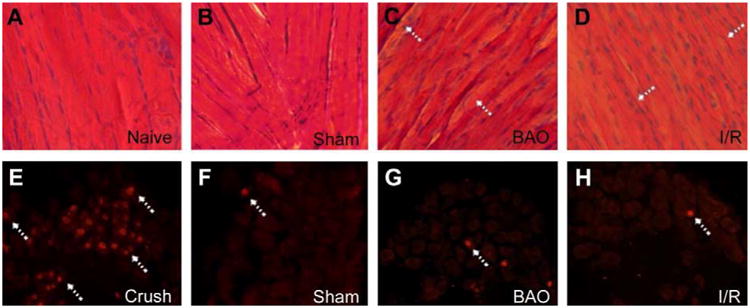
(A-D) One day after brachial artery occlusion (BAO), the forepaw muscles appear to have distinct muscle fiber degeneration and atrophy (arrows) in a hematoxylin and eosin (H&E) stain compared to uninjured animals (naïve and sham). The extent of muscle fiber injury appeared to exceed the amount of tissue damage (arrows) caused by a transient, six hour, occlusion with an 18 hour reperfusion (I/R). (E-H) Nuclear activating transcription factor 3 (ATF3) staining (arrows) in the C8 or T1 DRGs after sham, BAO, I/R, or ulnar crush injury showed that animals undergoing both prolonged and transient ischemia do not suffer extensive axonal damage similar to uninjured sham surgery controls but unlike that observed after crushing the nerve .
Using ex vivo recording, we found that prolonged ischemia and ischemia with reperfusion injury differentially modified the response properties of group III and IV muscle afferents when compared to naïves (Fig. 3). No significant differences were found between group III and IV fibers for any of the response properties analyzed and were thus combined for ease of presentation. In addition, no differences were found between naïve and sham conditions for any of the response properties analyzed (not shown). Specifically, prolonged ischemia caused a significant increase (p<0.05, Kruskal-Wallis one-way ANOVA on ranks with Dunn's post-hoc test) in the mean peak instantaneous frequencies to heat stimuli compared to naïve/uninjured mice (Naïve (n=17): 10.86 ± 4.32Hz; Sham (n=8): 10.1 ± 6.3Hz; BAO (n=13): 34.9 ± 9.62Hz) while I/R did not significantly alter heat responsiveness (n=10, 24.6 ± 8.8Hz, p<0.1) in the group III and IV muscle afferents (Fig. 3a). However, in the I/R condition, we did find that mechanical thresholds were significantly decreased (Naïve (n=11): 3.22 ± 0.67g; Sham (n=13): 2.55 ± 0.52g; I/R (n=13): 1.1 ± 0.29g; p<0.04), and responsiveness to the innocuous (“low”) metabolite mixtures were increased (Fig. 3b, c) compared to naïves (Naïve (n=13): 1.46 ± 0.33Hz; Sham (n=10): 1.87 ± 0.72Hz; I/R (n=19): 6.59 ± 1.90Hz, p<0.05). No changes in these response properties were detected after BAO (Mechanical threshold: 1.68 ± 0.51g, p<0.33; Low IF: 2.52 ± 0.7Hz p<0.6). No changes in mean peak instantaneous frequencies to cold or mechanical stimuli, or the high metabolite mixtures were found in either injury condition relative to uninjured controls (Figs. 3d-f, respectively).
Figure 3. Prolonged ischemia and transient ischemia with reperfusion injury differentially affect response properties of Group III and IV muscle afferents.
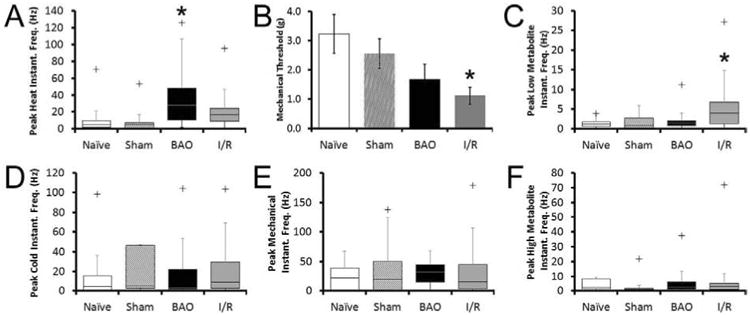
Brachial artery occlusion (BAO) uniquely increased the mean peak instantaneous frequencies to heat stimuli (A) in muscle afferents compared to naïve conditions, while transient ischemia with reperfusion injury (I/R) decreased mechanical thresholds (B). I/R also increased responsiveness of group III and IV muscle afferents to the low (innocuous) metabolite solutions (C) 24 hours post-injury when compared to naïves. No changes in responsiveness to cold stimuli (D), mean peak instantaneous frequencies to mechanical deformation of the muscles (E) or responsiveness to the high (noxious) metabolite mixtures (F) were found between any of the conditions. No differences are detected between naïve and sham conditions for any of these six response properties presented. Kruskal-Wallis one-way ANOVA on ranks with Dunn's post-hoc was performed for each parameter. * indicates p < 0.05 compared to naïve. + indicates the maximal outlier (more than two standard deviations from the upper quartile) of a dataset. All data points in each condition however, including outliers, were utilized for statistical analyses.
Prolonged and transient ischemia injury modify group III and IV muscle afferent prevalence
In addition to differential changes in afferent response properties, we also observed common changes in the prevalence of specific muscle afferent types after BAO and I/R (Fig. 4a). Specifically, both types of injury significantly increased the number of cells responding to both low and high metabolite solutions compared to naïve mice (Naïve: 5.4%, n=3/56; Sham: 5.3%, n=2/38; BAO: 20.0%, n=9/45; I/R: 30.8%, n=16/52; p<0.05, χ2). While we did not observe a compensatory change in the number of cells that responded only to low (Naïve: 19.6%%, n=11/56; Sham: 21.1%, n=8/38; BAO: 11.1%, n=5/45 P=0.32) or high (Naïve: 19.6%, n=11/56; Sham: 26.3%, n=10/38; BAO: 17.8%, n=8/45; p<0.8) metabolite mixtures after BAO, we did observe significantly fewer cells responding to low metabolites after I/R (1.92%, n=/52, p<0.013 vs naive). However, we did not detect changes in the numbers of cells responding to high metabolites (13.0%, n=6/52) in this condition (p<0.4 vs. naïve). Interestingly, the numbers of cells that responded to mechanical deformation of the muscles showed a trend towards a significant difference between BAO and I/R(BAO: 19.3%, n=11/57; I/R: 36.2%, n=25/69, p=0.07), but no changes in mechanical responders were detected after I/R or BAO compared to naives (28.2%, n=20/71, p<0.4). No changes in the numbers of cells recorded that responded to the administration of cold saline (Naïve: 22.1%, n=15/68; Sham: 15.2%, n=7/46; BAO: 21.1%, n=12/57; I/R: 22.2%, n=14/63; p<0.9) or hot saline (Naïve: 25.0%, n=17/68; Sham: 17.4%, n=8/46; BAO: 24.1%, n=14/57; I/R: 16.4%, n=10/61; p<=0.7) were found in either injury condition (not shown).
Figure 4. Prolonged ischemia and transient ischemia with reperfusion injury differentially alter the percentage of specific subtypes of group III and IV muscle afferents recorded.
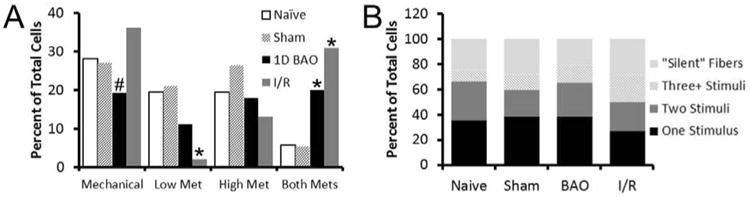
(A)Transient ischemia with reperfusion (I/R) decreased the number of cells responding to the low metabolite solution. Both injury models however were found to increase the number of cells responsive to both metabolite mixtures without altering the numbers of cells that responded only to the high metabolite solutions. Although BAO was found to decrease the average number of cells that responded to mechanical deformation of the muscles during recording, this was not found to be statistically different from naives; however a trend towards statistical differences in mechanically sensitive fiber prevalence was found between BAO and I/R conditions. (B) Analysis of the percentage of cells that responded to the five different stimuli (mechanical, cold, hot, low metabolites, and high metabolites) delivered to the forepaw muscles reveals no overt differences in the general proportions of afferents with responsiveness to one, two or three or more stimuli after BAO or I/R compared to uninjured controls (naïve and sham). χ2; *p < 0.05 compared to naïve, # indicates p < 0.07 compared to I/R, but p < 0.36 compared to naïve.
In uninjured mice, we also found that most muscle afferents responded to only one of the five stimuli that were delivered to the forepaw muscles (Naïve: 34.6%; n=19/55; Sham: 38.1%, n=16/42). A comparable number of afferents also respond to multiple stimuli (Two stimuli: Naïve: 30.9%; n=17/55; Sham: 21.4%, n=9/42; Three or more stimuli: Naïve: 9.1%; n=5/55; Sham: 11.9%, n=5/42) in uninjured mice similar to previous reports19. When analyzing the general number of stimuli that muscle afferents responded after BAO and I/R, a similar pattern was found. In mice with BAO and I/R, a similar percentage of single stimulus responding afferents were detected during recording (BAO: 38.5%; n=20/52; I/R: 26.8%; n=15/56) as were the numbers of afferents responding to two stimuli (BAO: 26.9%; n=14/52; I/R: 23.2; n=13/56) or three or more stimuli (BAO: 13.5%; n=7/52; I/R: 21.4%; n=12/55). There were more cells that responded to three or more stimuli after I/R overall, but this was not found to be statistically significant (p<0.2). We also did not observe any differences in the numbers of cells that did not respond to any of our five stimuli but were electrically driven from the forepaw muscles (“silent” afferents; Naïve: 25.5%, n=14/55; Sham: 30.9%, n=12/42; BAO: 21.2%, n=11/52; I/R: 28.6%, n=16/56) under any experimental condition (Fig. 4b). Finally, few muscle afferents were found to be spontaneously active in naïve (4.2%; n=3/71) or sham (6.3%; n=3/48) mice, and this did not change after BAO (1.8%; n=1/57) or I/R (4.3%; n=3/69).
Neurochemical identities of group III and IV muscle afferents after prolonged or transient ischemia with reperfusion
To then assess whether there could be corresponding changes in neurochemical phenotype in addition to the changes in response properties observed in the muscle afferents after prolonged ischemia or ischemia/reperfusion, we intracellularly stained 28 cells from the various ex vivo preparations with neurobiotin and processed the DRGs containing single cells for P2X3, TRPV1 or ASIC3 immunoreactivity. These markers were chosen since it has been previously shown that these molecules often mark or are believed to distinguish specific muscle afferent subpopulations22,30,36,59.
In uninjured mice (naïve and sham), we have verified previous reports of muscle afferent phenotypes23 in that cells that only responded to the high metabolite mixtures were often found to be ASIC3 immunopositive (n=3/5) or contain TRPV1 (n=1/2). We also found one high metabolite responding cell (n=1/4) that was immunoreactive for P2X3 in the uninjured controls; this particular cell was also found to contain ASIC3 (Fig. 5; Table 1; not shown). The three recovered cells that responded only to the low metabolite mixture were all found to be negative for both ASIC3 and P2X3 (Fig. 5; Table 1) similar to previous reports23. Mechanically sensitive muscle afferents were found to contain either ASIC3 (n=1/4) or P2X3 (n=2/4) but not TRPV1 (n=0/1) in uninjured mice. The one ASIC3 immunopositive mechano-responsive muscle afferent also contained P2X3 (not shown). Both heat (n=0/4) and cold (n=0/1) sensitive muscle afferents were negative for ASIC3 in uninjured control mice; however, some were found to be immunoreactive for P2X3 (Heat: n=1/2; Cold: n=1/2) or TRPV1 (Heat: n=1/2). None of the thermally sensitive muscle afferents however were found to contain both ASIC3 and P2X3 (not shown). Similar to earlier work23, silent fibers were not ASIC3 positive (n=0/3) but the two of these afferent types that were stained and recovered after recording did contain P2X3 (n=2/2).
Figure 5. Examples of intracellularly stained and physiologically characterized muscle sensory neurons in uninjured (naïve/sham) mice recovered from the ex vivo recording preparation.
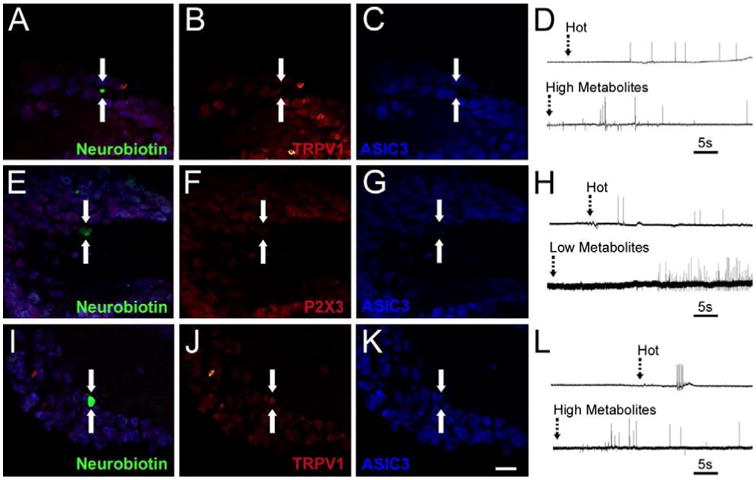
A neurobiotin stained (arrows), uninjured muscle afferent (A, green) that was responsive to hot saline and the high metabolite solution (D) was found to be immunonegative (arrows) for both TRPV1 (B, red) and ASIC3 (C, blue). Another muscle afferent (arrows) recovered from an uninjured ex vivo preparation (E, green) was found to be responsive to hot saline and the low metabolite mixture (H) was negative (arrows) for both P2X3 (F, red) and ASIC3 (G, blue). Another uninjured muscle afferent (arrows) that was intraceullarly filled with neurobiotin (I, green) was responsive to hot saline and the high metabolite mixture (L) was immunopositive (arrows) for both TRPV1 (J, red) and ASIC3 (K, blue). Scale bar for all images, 40μm. Dashed arrows in D, H and L indicate onset of stimulus in the muscles.
Table 1.
Quantification of chemosensitive muscle afferent phenotypes recovered from the ex vivo recording preparations in uninjured (naïve/sham) or combined ischemic (BAO and I/R) mice.
| Response Type | Condition | ASIC3+ | P2X3+ | ASIC3+/P2X3+ |
|---|---|---|---|---|
| High Metabolites | Naïve/Sham | 3/5 | 1/4 | 1/4 |
| Only | Combined Ischemia | 1/4 | 3/3 | 1/3 |
| Low Metabolites | Naïve/Sham | 0/3 | 0/3 | 0/3 |
| Only | Combined Ischemia | nd | nd | nd |
| Low & High | Naïve/Sham | nd | nd | nd |
| Metabolites | Combined Ischemia | 3/6 | 3/6 | 3/6 |
After ischemic injury (BAO and I/R combined), similar results were obtained for most of the functional subtypes of muscle afferents. High metabolite responders contained ASIC3 (n=1/4) and P2X3 (n=3/3), albeit more cells did contain this latter marker. Mechanically sensitive cells were also positive for these markers in similar proportions as uninjured mice (ASIC3: n=2/5; P2X3: n=2/4). In addition, heat sensitive afferents were again found to be immunonegative for ASIC3 (n=0/4), while some contained P2X3 (n=1/2). However, since ischemic injury was found to significantly increase the number of cells that responded to both metabolites (Fig. 4 above), we were able to intracellularly fill six (6) of these afferent subtypes after BAO or I/R. We found that 50% of these cells types contained ASIC3 (n=3/6) and P2X3 (n=3/6; Fig. 6, Table 1). Each of these three cells contained both of these markers (n=3/6). Interestingly, a few cold responsive muscle afferents were also found to contain ASIC3 (n=2/6) or P2X3 (n=2/4) after ischemia, which was not found in uninjured controls (not shown) .
Figure 6. Examples of intracellularly stained and physiologically characterized muscle sensory neurons recovered from the ex vivo recording preparation after ischemic muscle injury (prolonged ischemia or transient ischemia with reperfusion).
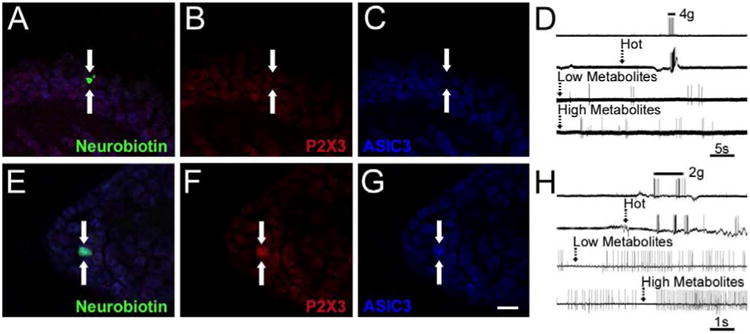
A muscle afferent (arrows) intracellularly filled with neurobiotin (A, green) was recovered from an ex vivo preparation in a mouse after ischemic muscle injury was found to be responsive to mechanical stimuli, hot saline and both low and high metabolite mixtures (D) was found to be immunonegative (arrows) for both P2X3 (B, red) and ASIC3 (C, blue). Another neurobiotin filled (E, green) muscle afferent was found to be responsive to mechanical stimuli, hot saline and both low and high metabolites (H) was found to coexpress (arrows) both P2X3 (F, red) and ASIC3 (G, blue). Some spontaneous activity was present in the latter cell prior to metabolite stimulation. Scale bar for all images, 40μm. Dashed arrows in D and H indicate onset of stimulus in the muscles.
Ischemic injuries upregulate discrete receptors in the DRGs and growth factors or cytokines in the muscles
In order to determine potential mechanisms for the changes in afferent function and subsequent alterations in pain behaviors induced by BAO or I/R, we performed realtime PCR on the T1/C8/C7 DRGs or forepaw muscles one day after injury and compared them to naïve controls. We first performed a PCR array on the muscle tissue from naïve mice and those with BAO injury. This was performed in this manner to serve as a guide for us to determine if particular cytokines or growth factors may be involved in discrete changes in signaling after ischemic injuries. PCR array analysis verified the ischemic nature of the tissue as evidenced by upregulation of previously described factors such as vascular endothelial growth factor (VEGF), transforming growth factor b (TGFb), and fibroblast growth factor 2 (FGF2; not shown)17,21,32,48,51. Although there were no significant changes in expression of NGF, NT3, or artemin, there was a significant upregulation of the neurotrophic factor GDNF (284%) after BAO. In addition, although many inflammatory cytokines were upregulated (not shown), IL1β (450%) was one of the most dramatic changes. GDNF and IL-1β expression in the injured muscle tissue were thus verified by realtime PCR after BAO and I/R. Each were shown to be significantly increased in both injury models in the muscles (BAO: GDNF, 132 ± 82% and IL1β, 200 ± 100%; I/R: GDNF, 1438 ± 59% and IL1β, 1078 ± 82%; p<0.05). Corresponding with the changes in these growth factors and cytokines was an upregulation of their receptors GFRα1 and IL1r1, respectively in the DRGs after both BAO and I/R (BAO: GFRα1, 97 ± 8% and IL1r1, 113 ± 47%; I/R: GFRα1, 139 ± 31% and IL1r1, 261 ± 75%; p<0.05; Table 2).
Table 2.
Percent changes in mRNA in the C7/C8/T1 DRGs or forepaw muscles one day after prolonged ischemia (BAO) or transient ischemia with reperfusion injury (I/R) compared to naïve controls.
| Gene: DRGs | BAO | l/R |
|---|---|---|
| ASIC1 | 14+/-57 | 185+/-57* |
| ASIC2a | nt | 33 +/- 16 |
| ASIC3 | 80 +/- 38* | 151 +/-71* |
| P2X3 | -13+/-37 | 293 +/- 39* |
| P2X4 | 1183+/-111* | 1174+/-106* |
| P2X5 | 1292+/-49* | 491 +/- 53* |
| P2Y1 | 826 +/- 46* | -71 +/- 38* |
| TRPV1 | -34+/-13 | -1 +/- 10 |
| TRPM8 | 29 +/- 22 | 23 +/- 6 |
| TRPA1 | -7 +/- 8 | 6 +/- 39 |
| TRPM3 | 55 +/- 62 | -42+/- 10 |
| Piezo 2 | 58 +/- 94 | -65 +/- 68 |
| IL1r | 113+/-47* | 261 +/- 75* |
| GFRαl | 97 +/- 8* | 139+/-31* |
| GFRα3 | 59 +/- 22 | -1 +/- 32 |
| Gene: Muscle | ||
| IL17β | 200 +/- 100* | 1078+/-82* |
| GDNF | 132+/-82* | 148+/- 59* |
p < 0.05 vs. naïve;
One-way ANOVA with Tukey's post-hoc. Values indicate percent change ± error of the difference in means vs. naïve.
In addition to common changes in receptor expression detected after both BAO and I/R, we also found differential modulation of a number of other channels implicated in sensory transduction (Table 2). BAO specifically caused a significant increase in P2Y1 (826 ± 46%; p<0.05), whereas I/R exhibited significant increases in P2X3 and ASIC1 (P2X3: 293 ± 39% and ASIC1: 185 ± 57%; p<0.05). I/R also displayed a significant decrease in P2Y1 (-71 ± 38%; p<0.05). Both conditions however, showed a significant increases in ASIC3 (BAO: 80 ± 38% and I/R: 151 ± 71%; p<0.05), P2X4 (BAO: 1183 ± 111% and I/R: 1174 ± 106%; p<0.05) and P2X5 (BAO: 1292 ± 49% and I/R: 491 ± 53%; p<0.05) mRNA. No changes in gene expression were found after BAO or I/R for ASIC2a, TRPV1, TRPM8, TRPA1, TRPM3 or Piezo2 (Table 1). We also found that none of the receptors or channels analyzed was upregulated after sham surgeries or after a 6hr brachial artery occlusion (not shown) in the DRGs with the exception of P2X4 which was upregulated only after a 6hr occlusion (272 ± 7%, p<0.05). Thus it is likely that the role that altered gene expression plays between BAO and I/R results from discrete effects between prolonged ischemia and the reperfusion component of our I/R conditions.
Discussion
A subset of common, albeit not well-characterized, causes for muscle pain are those that stem from issues of peripheral perfusion such as peripheral vascular disease, sickle cell anemia and even complex regional pain syndrome3,44,45,63. The current report has shown that prolonged ischemia is distinct from transient ischemia with reperfusion injury in that the group III and IV muscle afferents differentially sensitize in the two unique injury states. The receptors and channels possibly involved in mediating these changes in afferent function were also found to be dissimilar between injury types. Regardless, each condition resulted in significant ongoing/spontaneous pain in addition to reduced grip strength suggesting that the mechanisms of how ischemic myalgia develops may be etiologically specific.
Injury specific sensitization of group III and IV muscle afferents
Although it can be argued that no single current behavioral test is muscle specific in rodents, the combination of multiple behavioral analyses may provide indirect evidence of the animal's deep tissue pain. Therefore, to determine the behavioral effects of prolonged or transient ischemia, we analyzed paw guarding behaviors to assess generalized spontaneous pain and grip strength to determine the overall degree of muscle function. We combined these tests with cutaneous hypersensitivity that is established during ischemia52 to better determine general pain levels and the potential contribution of the muscles to the overall pain (Fig. 1). The latter test (although more specific for cutaneous hypersensitivity) could also provide a small degree of indirect evidence of muscle pain as pressing on the injured muscle tissue through the skin has been used previously in other reports after muscle inflammation or acid injection to determine mechanical hypersensitivity54,58. Nevertheless, we found that both prolonged and transient ischemia with reperfusion increased spontaneous pain behaviors and reduced grip strength to similar degrees (Fig. 1). Ongoing pain however, was the only measure that was different between the injury types as paw guarding returned towards baseline levels in the I/R condition faster. Interestingly, the reduced grip strength observed after these ischemic injuries was similar to that observed when virtually eliminating forepaw function with a nerve crush (see results), suggesting that these injuries are quite debilitating in regards to muscle function.
This may suggest that prolonged ischemia and reperfusion injury may mediate muscle pain via similar means. However, to begin to determine this, we qualitatively analyzed the integrity of the muscle tissue between the two injuries. Prolonged ischemia (BAO) seemed to cause more extensive damage to the muscle tissue compared to transient ischemia with reperfusion (I/R) as the tissue appeared to display more muscle fiber degeneration and atrophy when compared to naïve muscle fibers (Fig. 2). The extent of this damage thus suggested that the primary afferents innervating the injured tissues may in fact respond differently to these distinct insults even though the pain resulting from these injuries is relatively similar in mice (Fig. 1).
Using our ex vivo muscle preparation, we found that BAO and I/R indeed caused differential sensitization of group III and IV muscle afferents. Both prolonged ischemia and transient ischemia with reperfusion did alter the percentage of afferents that responded to both innocuous and noxious36 mixtures of metabolites (Fig. 4), but responses to heat and mechanical stimuli were differentially affected between the two injury models. Transient ischemia also increased the afferent firing to the innocuous metabolites (Fig. 3). Thus the role that the primary afferents may play in the generation of ischemic myalgias may be different depending on the type of ischemic muscle injury that is sustained. In support of this notion, the sensitization that occurs in muscle afferents after other peripheral injuries such as incision, inflammation, acid injection or eccentric exercise is not always similar4,11,16,54,58,60,62.
Our current data and previous reports23,36,46 also support the notion of two separate chemosensitive populations of muscle afferents. These can be classified into those that are metaboreceptors (“low” responders or cells that respond to both metabolite mixtures) thought to be involved in general muscle homeostasis or fatigue sensations, and those that are more nociceptive in nature (“high” responders). After both ischemic injuries however, we observed phenotypic changes in these muscle afferents (Figs. 4-6; Table 1). Mechanistically, this may be a result of alterations in ASIC3 since we have shown in naïve mice, that ASIC3 is isolated to the chemosensitive-nociceptors23 (Fig. 5; Table 1), but after BAO or I/R injuries, ASIC3 is upregulated in the DRGs (Table 2) and in cells that can respond to both metabolite mixtures (Fig. 6; Table 1). Support for this hypothesis comes from studies that have shown that ASIC channels are crucially involved in the exercise pressor reflex18,27,33,38. These reports hypothesized that ASIC channels were present in the metaboreceptors, but since these studies were performed in the context of an injury, alterations in afferent phenotype may actually be the reason for their claim. Alternatively, it has been hypothesized that the increase in ATP caused by ischemia acts on P2X5 (which is also upregulated after both BAO and I/R: Table 2) to increase pH sensitivity in muscles by altering the sensitivity of the ASIC3 channel6. Thus P2X5 upregulation in combination with alterations in ASIC3, could alter muscle afferent chemosensitivity leading to nociceptive behaviors after ischemic muscle injury as similarly described in other pain models16,54.
The mechanisms that may be mediating the distinct changes in afferent function between BAO and I/R is also likely different. Dynamic changes in DRG gene expression after peripheral injuries have been shown in recent studies to be convincingly linked to altered sensory afferent function e.g. 24, 25. Here we have demonstrated that changes in afferent function observed between the two different injury conditions coincided with specific changes in mechanically sensitive, heat responsive, and ATP sensitive receptors in the DRGs (Tables 1, 2; Figs. 4-6). For example, the altered heat responsiveness in muscle afferents after BAO corresponds to the specific upregulation of the purinergic/ADP receptor P2Y1, which is known to regulate heat sensitivity in cutaneous afferents24,43. Thermally sensitive muscle afferents have been attributed to playing a role in muscle nociception in addition to alterations in the exercise pressor reflex after injury10,15,55. Thus specifically in the context of prolonged ischemia (BAO) the upregulation of P2Y1 and potential alterations in heat sensitivity may play a role in pain generation in this state. Conversely, the alterations in mechanical responsiveness after I/R correlate to the upregulation of ASIC1 which has been linked to muscle hypersensitivity in other pain models in rodents58. In a model of thrombus-induced ischemic pain, antagonists for ASIC channels have also been shown to relieve mechanosensitivity52, adding to the evidence that ASICs may be partially responsible for mechanical sensitization after injury and thus also play a role in the development of ischemic myalgia after I/R. In addition, P2X3 has been linked to pressor responses27,39 and the upregulation of this channel could also play a role in altered afferent function and subsequent nociception after this distinct ischemic injury due to potential regulation of the observed changes in firing to innocuous metabolites (Table 2; Fig. 3). Future analysis of afferent function after ischemic injury with distinct receptor inhibition would unquestionably be necessary however to confirm these hypotheses and also determine if certain muscle afferent subpopulations can also play a dual role in regulating both cardiovascular reflexes and nociception.
Conclusions
In this study, we have characterized two models of muscle injury leading to discrete response alterations of the primary afferents that may be mediated by distinct changes in receptor/channel expression in the affected DRGs. However, we have also found upregulation of cytokine and growth factor mRNA within the muscle tissue after these injuries (Table 2). Specifically, we found that there was a significant upregulation of GDNF and IL1β in the forepaw muscles after both BAO and I/R with a subsequent upregulation of their receptors, GFRα1 and IL1r1, respectively, in the DRGs (Table 2). Previous research has documented that target derived factors such as these can have significant control on DRG gene expression and sensory neuron plasticity26. Group IV muscle afferent sensitivity to mechanical stimuli specifically has been shown to be altered by cytokine and neurotrophic factor administration in animal models20,42,56. Thus peripheral actions of IL-1β or GDNF in the two injury states may also play a role in uniquely altering response properties of group III and IV muscle afferents and subsequent muscle pain. Moreover, targeting these types of molecules specifically may lead to more viable pharmacotherapies for ischemic myalgias, thus highlighting the importance of future mechanistic investigation.
Highlights.
Prolonged and transient ischemia both alters spontaneous pain and grip strength.
Prolonged ischemia alters muscle afferents differently than transient ischemia.
Mechanisms of ischemic myalgia may be different depending on the insult sustained.
Acknowledgments
We would like to thank Dr. Jun-Ming Zhang for helpful comments on the manuscript.
This work was supported by grants to MPJ from the Rita Allen Foundation and American Pain Society, the International Association for the Study of Pain Early Career Grant, institutional funds from the Department of Anesthesia at CCHMC and the National Institutes of Health (R01AR064551-01A1).
Footnotes
Disclosures: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alam M, Smirk F. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–83. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali F, Carman T. Medical management for chronic atherosclerotic peripheral arterial disease. Drugs. 2012;72:2073–85. doi: 10.2165/11640810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Allison M, Ho E, Denenberg J, Langer R, Newman A, Fabsitz R, Criqui M. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez F, Bullinger K, Titus H, Nardelli P, Cope T. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injuries. Ann NY Acad Sci. 2010;1198:231–41. doi: 10.1111/j.1749-6632.2010.05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard J. Chronic lower limb ischemia. West J Med. 2000;173:854–7. doi: 10.1136/ewjm.173.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birdsong W, Fierro L, Williams F, Spelta V, Naves L, Knowles M, Josephine MH, Adelman J, Almers W, Elde R, Edwin M. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–49. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaisdell F. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Vascular. 2002;10:620–30. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 8.Carmo-Araújo E, Dal-Pai-Silva M, Dal-Pai V, Cecchini R, Ferrira A. Ischaemia and reperfusion effects on skeletal muscle tissue: morphological and histochemical studies. Int J Exp Path. 2007;88:147–154. doi: 10.1111/j.1365-2613.2007.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coderre T, Bennett G. A hypothesis for the cause of complex regional pain syndrome-type I (reflex sympathetic dystrophy): pain due to deep-tissue microvascular pathology. Pain Med. 2010;11:1224–38. doi: 10.1111/j.1526-4637.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins K. Age-related changes in autonomic control: the use of beta blockers in the treatment of hypertension. Cardiovasc Drugs Ther. 1991;4(Suppl 6):1257–62. doi: 10.1007/BF00114230. [DOI] [PubMed] [Google Scholar]
- 11.Dina O, Green P, Levine J. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neurosci. 2008;152:521–5. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan WR, Lu J, Xie YK. Mechanisms of topical analgesics in relieving pain in an animal model of muscular inflammation. Pain Med. 2013;14:1381–7. doi: 10.1111/pme.12199. [DOI] [PubMed] [Google Scholar]
- 13.Elitt C, Sabrina M, Lawson J, Malin S, Molliver D, Cornuet P, Koerber H, Davis B, Albers K. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–87. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellrich J, Makowska A. Nerve growth factor and ATP excite different neck muscle nociceptors in anaesthetized mice. Cephalalgia. 2007;27:1226–35. doi: 10.1111/j.1468-2982.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Li J, Sinoway L, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol. 2007;102:2288–93. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- 16.Gautam M, Benson C, Sluka K. Increased response of muscle sensory neurons to decreases in pH after muscle inflammation. Neurosci. 2010;170:893–900. doi: 10.1016/j.neuroscience.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafsson T, Kraus WE. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front Biosci. 2001;6:D75–89. doi: 10.2741/gustafss. [DOI] [PubMed] [Google Scholar]
- 18.Hayes S, McCord J, Kaufman M. Role played by P2X and P2Y receptors in evoking the muscle chemoreflex. J Appl Physiol. 2008;17033:538–41. doi: 10.1152/japplphysiol.00929.2007. [DOI] [PubMed] [Google Scholar]
- 19.Henderson PW, Jimenez N, Ruffino J, Sohn AM, Weinstein AL, Krijgh DD, Reiffel AJ, Spector JA. Therapeutic delivery of hydrogen sulfide for salvage of ischemic skeletal muscle after the onset of critical ischemia. J Vasc Surg. 2011;53:785–91. doi: 10.1016/j.jvs.2010.10.094. [DOI] [PubMed] [Google Scholar]
- 20.Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 2005;114:168–76. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Hudlicka O, Brown MD. Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: role of shear stress, nitric oxide and vascular endothelial growth factor. J Vasc Res. 2009;46:504–12. doi: 10.1159/000226127. [DOI] [PubMed] [Google Scholar]
- 22.Jankowski M, Lawson J, Sabrina M, Rau K, Anderson C, Albers K, Koerber H. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009;29:1636–47. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankowski M, Rau K, Ekmann K, Anderson C, Koerber H. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol. 2013;109:2374–81. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jankowski M, Rau K, Soneji D, Ekmann K, Anderson C, Molliver D, Koerber H. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal thresholds and sensory neuron phenotypic switching during peripheral inflammation. Pain. 2012;153:410–9. doi: 10.1016/j.pain.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jankowski M, Sabrina M, Jing X, Cornuet P, Salerno K, Koerber H, Albers K. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowski MP, Koerber HR. Neurotrophic Factors and Nociceptor Sensitization. In: Kruger L, Light A, editors. Transl Pain Res From Mouse to Man. Boca Raton, FL: CRC Press; 2010. [PubMed] [Google Scholar]
- 27.Kaufman M, Hayes S. The exercise pressor reflex. Clin Aut Res. 2002;12:429–39. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman M, Rybicki K. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–I65. [PubMed] [Google Scholar]
- 29.Kniffki K, Mense S, Schmidt R. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978;31:511–22. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- 30.Koerber H, Sabrina M, Lawson J, Malin S, Anderson C, Jankowski M, Davis B. Cutaneous C-polymodal fibers lacking TRPV1 are sensitized to heat following inflammation, but fail to drive heat hyperalgesia in the absence of TPV1 containing C-heat fibers. Mol Pain. 2010;6:58. doi: 10.1186/1744-8069-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68:581–95. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- 32.Lee YS, Jang HS, Kim JM, Lee JS, Lee JY, Kim KL, Shin IS, Suh W, Choi JH, Jeon ES, Byun J, Kim DK. Adenoviral-mediated delivery of early growth response factor-1 gene increases tissue perfusion in a murine model of hindlimb ischemia. Mol Ther. 2005;12:328–36. doi: 10.1016/j.ymthe.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Gao Z, Kehoe V, Xing J, King N, Sinoway L. Interstitial adenosine triphosphate modulates muscle afferent nerve-mediated pressor reflex. Muscle Nerve. 2008;38:972–7. doi: 10.1002/mus.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, King N, Sinoway L. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol. 2003;95:577–83. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- 35.Li J, King N, Sinoway L. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation. 2005;111:2748–51. doi: 10.1161/CIRCULATIONAHA.104.510669. [DOI] [PubMed] [Google Scholar]
- 36.Light A, Hughen R, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling L, Honda T, Shimada Y. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. J Comp Neurol. 2003;150:140–50. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Hear Circ Physiol. 2010;299:H1357–H1364. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCord JL, Tsuchimochi H, Kaufman MP. P2X2/3 and P2X3 receptors contribute to the metaboreceptor component of the exercise pressor reflex. J Appl Physiol. 2010;109:1416–23. doi: 10.1152/japplphysiol.00774.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMahon S, Wall P. The distribution and central termination of single cutaneous and muscle unmyelinated fibres in rat spinal cord. Brain Res. 1985;359:39–48. doi: 10.1016/0006-8993(85)91410-6. [DOI] [PubMed] [Google Scholar]
- 41.Mense S. Algesic agents exciting muscle nociceptors. Exp Brain Res. 2009;196:89–8100. doi: 10.1007/s00221-008-1674-4. [DOI] [PubMed] [Google Scholar]
- 42.Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep. 2003;7:419–25. doi: 10.1007/s11916-003-0057-6. [DOI] [PubMed] [Google Scholar]
- 43.Molliver D, Rau K, Sabrina M, Jankowski M, Koerber H. The ADP receptor P2Y1 is necessary for normal thermal sensitivity in cutaneous polymodal nociceptors. Mol Pain. 2011;7:13. doi: 10.1186/1744-8069-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Mos M. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Muir R. Peripheral arterial disease: Pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs. 2009;27:26–30. doi: 10.1016/j.jvn.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Naves L, McCleskey E. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38:1561–9. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]
- 47.Oklu R, Albadawi H, Jones JE, Yoo HJJ, Watkins MT. Reduced hind limb ischemia-reperfusion injury in Toll-like receptor-4 mutant mice is associated with decreased neutrophil extracellular traps. J Vasc Surg. 2013;58:1627–36. doi: 10.1016/j.jvs.2013.02.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onimaru M, Yonemitsu Y, Tanii M, Nakagawa K, Masaki I, Okano S, Ishibashi H, Shirasuna K, Hasegawa M, Sueishi K. Fibroblast growth factor-2 gene transfer can stimulate hepatocyte growth factor expression irrespective of hypoxia-mediated downregulation in ischemic limbs. Circ Res. 2002;91:923–30. doi: 10.1161/01.res.0000043281.66969.32. [DOI] [PubMed] [Google Scholar]
- 49.Ouma GO, Rodriguez E, Muthumani K, Weiner DB, Wilenski RL, Mohler ER. In vivo electroporation of constitutively expressed HIF-1α plasmid DNA improves neovascularization in a mouse model of limb ischemia. J Vasc Surg. 2014;59:786–93. doi: 10.1016/j.jvs.2013.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollak K, Swenson J, Vanhaitsma T, Hughen R, Jo D, Light K, Schweinhardt P, Amann M, Light A. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol. 2014;99:368–80. doi: 10.1113/expphysiol.2013.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider MD, McLellan WR, Black FM, Parker TG. Growth factors, growth factor response elements, and the cardiac phenotype. Bas Res Cardiol. 1992;87(Suppl 2):33–48. doi: 10.1007/978-3-642-72477-0_4. [DOI] [PubMed] [Google Scholar]
- 52.Seo H, Roh D, Yoon S, Kang S. Peripheral acid-sensing ion channels and P2X receptors contribute to mechanical allodynia in a rodent thrombus-induced ischemic pain model. J Pain. 2010;11:718–27. doi: 10.1016/j.jpain.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol. 1989;66:429–36. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- 54.Sluka K, Price M, Breese N, Stucky C, Wemmie J, Welsh M. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–39. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 55.Sluka K, Radhakrishnan R, Benson C, Eshcol J, Price M, Babinski K, Audette K, Yeomans D, Wilson S. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–12. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svensson P, Cairns B, Wang K, Lars AN. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–7. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 57.Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–82. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- 58.Walder R, Rasmussen L, Rainier J, Light A, Wemmie J, Sluka K. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–8. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2009;296:H1380–H1387. doi: 10.1152/ajpheart.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Brennan T. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain. 2009;144:329–39. doi: 10.1016/j.pain.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Brennan T. Guarding pain and spontaneous activity of nociceptors after skin versus skin plus deep tissue incision. Anesthesiol. 2010;112:153–64. doi: 10.1097/ALN.0b013e3181c2952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu J, Gu H, Brennan T. Increased sensitivity of group III and group IV afferents from incised muscle in vitro. Pain. 2010;151:744–55. doi: 10.1016/j.pain.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y, Shah A, Watson M, Mankad V. Comparison of costs to the health sector of comprehensive and episodic health care for sickle cell disease patients. Public Heal Rep. 1995;110:80–6. [PMC free article] [PubMed] [Google Scholar]
- 64.Zempsky W, Palermo T, Corsi J, Lewandowski A, Zhou C, Casella J. Daily changes in pain, mood and physical function in children hospitalized for sickle cell disease pain. Pain Res Manag. 2013;18:33–8. doi: 10.1155/2013/487060. [DOI] [PMC free article] [PubMed] [Google Scholar]


