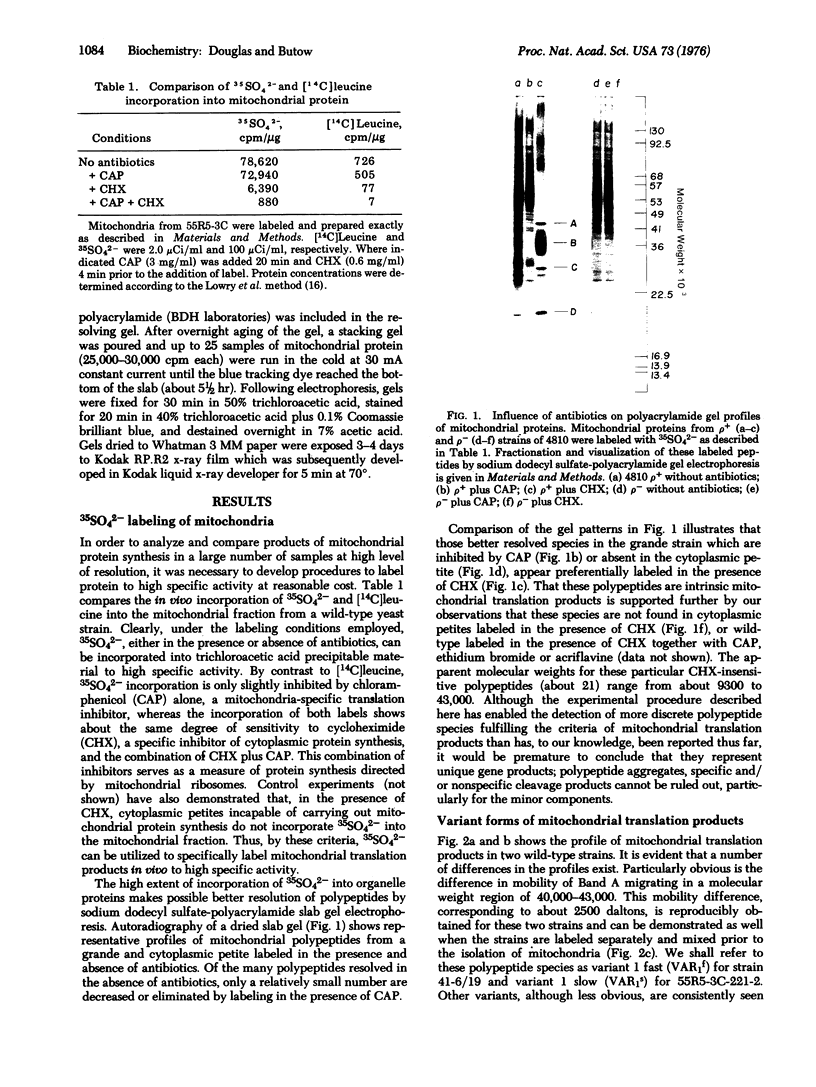
Abstract
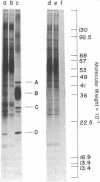
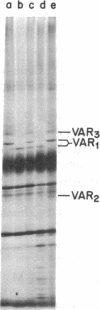
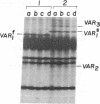
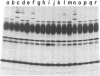
Products of mitochondrial protein synthesis in yeast have been labeled in vivo with 35SO42-. More than 20 polypeptide species fulfilling the criteria of mitochondrial translation products have been detected by analysis on sodium dodecyl sulfate-exponential polyacrylamide slab gels. A comparison of mitochondrial translation products in two wild-type strains has revealed variant forms of some polypeptide species which show genetic behavior consistent with the location of their structural genes on mtDNA. Our results demonstrate the feasibility of performing genetic analysis on putative gene products of mtDNA in wild-type yeast by direct examination of the segregation and recombination behavior of specific polypeptide species.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casey J. W., Hsu H. J., Rabinowitz M., Getz G. S., Fukuhara H. Transfer RNA genes in the mitochondrial DNA of cytoplasmic petite mutants of Saccharomyces cerevisiae. J Mol Biol. 1974 Oct 5;88(4):717–733. doi: 10.1016/0022-2836(74)90395-7. [DOI] [PubMed] [Google Scholar]
- Faye G., Kujawa C., Fukuhara H. Physical and genetic organization of petite and grande yeast mitochondrial DNA. IV. In vivo transcription products of mitochondrial DNA and localization of 23 S ribosomal RNA in petite mutants of saccharomyces cerevisiae. J Mol Biol. 1974 Sep 5;88(1):185–203. doi: 10.1016/0022-2836(74)90304-0. [DOI] [PubMed] [Google Scholar]
- Flury U., Mahler H. R., Feldman F. A novel respiration-deficient mutant of Saccharomyces cerevisiae. I. Preliminary characterization of phenotype and mitochondrial inheritance. J Biol Chem. 1974 Oct 10;249(19):6130–6137. [PubMed] [Google Scholar]
- GALZY P., SLONIMSKI P. P. Variations physiologiques de la levure au cours de la croissance sur l'acide lactique comme seule source de carbone. C R Hebd Seances Acad Sci. 1957 Dec 16;245(25):2423–2426. [PubMed] [Google Scholar]
- Grossman L. I., Goldring E. S., Marmur J. Preferential synthesis of yeast mitochondrial DNA in the absence of protein synthesis. J Mol Biol. 1969 Dec 28;46(3):367–376. doi: 10.1016/0022-2836(69)90182-x. [DOI] [PubMed] [Google Scholar]
- Halbreich A., Franco A. D., Groudinsky O., Cosson J., Slonimski P. P. Translation of mitochondrial RNA from yeast cytoplasmic petite mutants in an E. coli cell-free system. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1286–1292. doi: 10.1016/0006-291x(75)90832-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Saunders G. W., Gingold E. B., Lukins H. B. The biogenesis of mitochondria. V. Cytoplasmic inheritance of erythromycin resistance in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1968 Mar;59(3):903–910. doi: 10.1073/pnas.59.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. L., Schatz G. Cytochrome c oxidase from bakers' yeast. II. Site of translation of the protein components. J Biol Chem. 1973 Feb 25;248(4):1355–1360. [PubMed] [Google Scholar]
- Michaelis G., Douglass S., Tsai M. J., Burchiel K., Criddle R. S. In vitro transcription of mitochondrial deoxyribonucleic acid from yeast. Biochemistry. 1972 May 23;11(11):2026–2036. doi: 10.1021/bi00761a006. [DOI] [PubMed] [Google Scholar]
- Needleman R. B., Tzagoloff A. Breakage of yeast: a method for processing multiple samples. Anal Biochem. 1975 Apr;64(2):545–549. doi: 10.1016/0003-2697(75)90466-2. [DOI] [PubMed] [Google Scholar]
- Scragg A. H., Thomas D. Y. Synthesis of mitochondrial proteins in an Escherichia coli cell-free system directed by yeast mitochondrial DNA. Eur J Biochem. 1975 Aug 1;56(1):183–192. doi: 10.1111/j.1432-1033.1975.tb02221.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B., Zulch G. Assembly of the mitochondrial membrane system. Cytoplasmic mutants of Saccharomyces cerevisiae with lesions in enzymes of the respiratory chain and in the mitochondrial ATPase. J Biol Chem. 1975 Oct 25;250(20):8236–8242. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assesmbly of the mitochondrial membrane system. VI. Mitochondrial synthesis of subunit proteins of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Jan 25;247(2):594–603. [PubMed] [Google Scholar]