Abstract
Purpose
Activation of the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway has been implicated in anti-estrogen resistance in breast cancer. We tested the therapeutic potential of the novel PI3K/mTOR dual inhibitor P7170 in a panel of anti-estrogen-sensitive and -resistant models of ER+ breast cancer.
Methods
Estrogen receptor-positive (ER+) breast cancer cells were treated +/− P7170. Fresh cores from primary ER+/HER2− tumors from two patients were treated +/− P7170 ex vivo. Mice bearing breast cancer xenografts were randomized to treatment with vehicle, fulvestrant, P7170, or combinations, and tumor volumes were measured. Tissues and cells were analyzed for markers of pathway activity, cell viability, and apoptosis.
Results
In cell lines, P7170 exhibited IC50 values in the range of 0.9-7 nM and induced apoptosis. P7170 potently inhibited mTOR activity (≤25 nM), and inhibited PI3K at higher concentrations (≥200 nM). P7170 completely inhibited MCF-7 tumor growth, significantly inhibited growth of fulvestrant-resistant T47D tumors, and suppressed tumor cell proliferation but did not induce apoptosis.
Conclusions
While P7170 inhibits PI3K and mTOR in ER+/HER2− human breast cancer cells and tumors ex vivo, in vivo data indicate that the primary mechanism of P7170 anti-tumor action is inhibition of mTOR and cell proliferation. P7170 is a novel agent worthy of further investigation for the treatment of ER+ breast cancer.
Keywords: mTOR, PI3K, breast, estrogen, anti-estrogen
Introduction
Each year, an estimated 900,000 women worldwide are diagnosed with invasive breast cancer that expresses estrogen receptor α (ER) and/or progesterone receptor (PR) (herein collectively referred to as ER+), but does not overexpress the HER2 (ERBB2) protooncogene [1]; this subtype constitutes approximately 65% of breast cancer cases. Due to the high frequency of ER+/HER2-disease, this subtype accounts for more recurrences and deaths than all other breast cancer subtypes combined [2-4]. Patients with stage I-III ER+/HER2− disease at the time of diagnosis are typically treated with five years of adjuvant anti-estrogen therapy to neutralize ER signaling. This therapy may be in the form of a selective ER modulator (e.g., tamoxifen) or an aromatase inhibitor (e.g., letrozole, anastrozole, exemestane). However, nearly 20% of such patients are expected to develop advanced/metastatic disease within 10 years following surgery [5]. Thus, ~180,000 women diagnosed this year will ultimately develop anti-estrogen-resistant advanced breast cancer that is almost uniformly fatal.
We and others have demonstrated that activation of Class IA phosphatidylinositol 3-kinases (PI3Ks) and downstream signaling pathways, including AKT and mechanistic target of rapamycin (mTOR), promotes resistance to anti-estrogens in ER+ breast cancer. The PI3K/AKT/mTOR pathway is the most frequently aberrantly activated pathway in human cancer, and ~80% of primary breast tumors harbor a genetic lesion that can promote pathway activation (extracted from refs. [6,7]). Anti-estrogen-sensitive and -resistant breast cancer cells and tumors are typically sensitive to PI3K/AKT/mTOR pathway inhibition [8-15], and these findings have prompted clinical development of PI3K pathway-targeted agents.
We sought to determine whether dual targeting of PI3K and mTOR is beneficial for the treatment of ER+ breast cancer. Herein, we evaluated the efficacy the novel ATP-competitive PI3K/mTOR dual inhibitor P7170 [16,17] as a single agent and in combination with the anti-estrogen fulvestrant in preclinical models of ER+ breast cancer.
Methods
Cell lines and growth assay
All parental cell lines were obtained from ATCC. MCF-7/fulvestrant-resistant (MCF-7/FR) and T47D/fulvestrant-resistant (T47D/FR) cells were gifts from Matthew Ellis (Washington University). Parental and fulvestrant-resistant cells were maintained in DMEM/10% FBS (Hyclone) in the absence or presence of 1 μM fulvestrant (Tocris and Abmole), respectively; this concentration of fulvestrant is used to ensure complete inhibition of ER transcriptional activity. Long-term estrogen-deprived (LTED) cells were previously described in ref. [8] and maintained in hormone-depleted medium [phenol red-free DMEM with 10% dextran/charcoal-treated FBS (DCC-FBS; Hyclone)]. For growth assays, cells were plated in triplicate in respective growth media at 5×103 cells/well in 96-well plates. The next day, cells were treated with 0-30 nM P7170. Five to eight days later, relative quantities of adherent cells were determined by sulforhodamine B (SRB) assay [18].
Apoptosis assay
Cells seeded in triplicate in 6-well plates at 0.6-1×106 cells/well were treated for 3-4 days as indicated in Fig. 2B. Floating and adherent cells (dislodged by trypsinization) were processed using ApoScreen Annexin Apoptosis kit (Southern Biotech), then analyzed by flow cytometry. Cells staining positively for Annexin-V and/or propidium iodide were considered apoptotic.
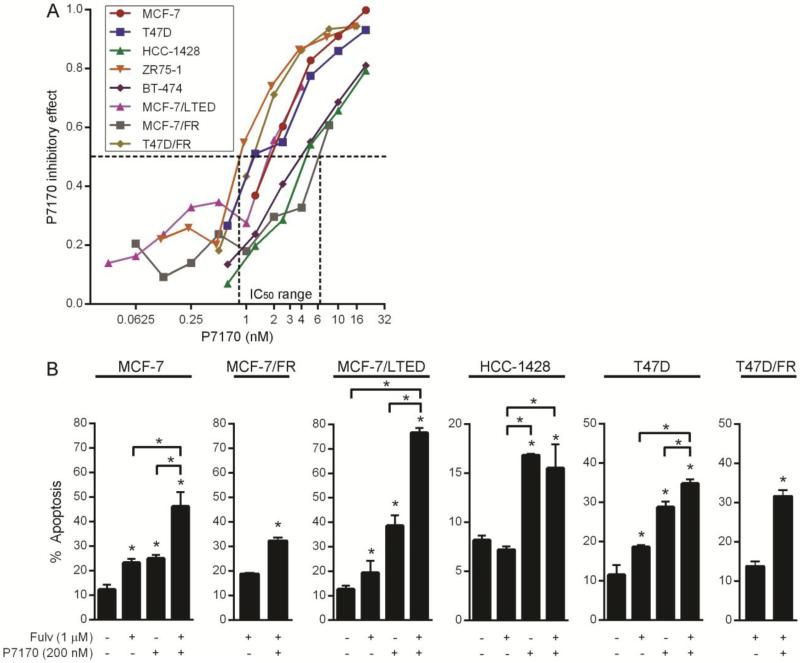
Fig. 2. P7170 decreases proliferation and viability of ER+ breast cancer cells.
A) Cells were treated in triplicate with 0-30 nM P7170 for 5-8 days, then analyzed by SRB assay to quantify relative numbers of adherent cells. FR cells were treated in the presence of 1 μM fulvestrant. LTED cells were treated in hormone-depleted medium. Relative cell numbers were used to calculate inhibitory effect. IC50 values are indicated by dotted lines. B) Cells were treated in triplicate ± 1 μM fulvestrant and 200 nM P7170 for 3-4 days. Fractions of apoptotic cells were determined by Annexin-V/propidium iodide labeling followed by flow cytometry. Mean + SEM is shown. *p≤0.05 by Bonferroni post-hoc test vs. control, unless otherwise indicated by brackets.
Human tumor analysis
Breast tumor samples were obtained under an IRB-approved protocol, and patients provided written informed consent for study participation. Untreated primary tumors from two patients with early-stage ER+/HER2-breast cancer (confirmed by diagnostic biopsy; no neoadjuvant therapy) were surgically resected. Within 1 hour post-resection, 1-mm punch core biopsies (tool from Miltex) were taken from the tumor specimen and put into serum-free DMEM ± 200 nM P7170. After 6 h of ex vivo culture, tissue cores were snap-frozen in liquid nitrogen and stored at −80°C.
Mouse studies
All animal studies were approved by the Dartmouth IACUC. Female NOD-scid IL2Rγ−/− (NSG; NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice (5-6 wks old; obtained from the Norris Cotton Cancer Center Transgenics & Genetic Constructs Shared Resource) were subcutaneously injected with 5-10×106 MCF-7 cells suspended in 50% growth factor-reduced matrigel (BD Biosciences) and a 17β-estradiol pellet (0.72 mg, 60-day-release, Innovative Research of America). A second group of mice was injected with T47D/FR cells in 50% matrigel without 17β-estradiol supplementation, and subcutaneously injected weekly with 5 mg fulvestrant. Tumor dimensions were measured twice weekly using calipers, and volumes were calculated using the formula: volume = length x width2/2 (width is the shorter dimension). Mice bearing MCF-7 tumors ~200 mm3 were randomized to treatment with vehicle, fulvestrant (5 mg/wk s.c. in 100 μL), P7170 (5 or 15 mg/kg/d p.o. in 100 μL), or fulvestrant plus 5 mg/kg/d P7170. P7170 was suspended in 0.5% methylcellulose. Fulvestrant was either obtained in the clinical formulation (Astrazeneca), or in powder form (Abmole), dissolved in ethanol, then diluted 10-fold with castor oil (both formulations contained 50 mg/mL fulvestrant). Tumors were harvested after 3 days of treatment, or at the end of the study (4-6 wks), and cut in pieces for snap-freezing or formalin fixation followed by paraffin-embedding (FFPE).
Immunoblotting
Cells were treated as indicated in figures, then lysed in RIPA buffer [50 mM Tris pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 5 mM NaPPi, 50 mM NaF, 10 mM β-glycerophosphate (Sigma), 1 mM Na3VO4 (New England Biolabs), protease inhibitor cocktail (Pierce)] on ice. Frozen patient-derived tumor samples and xenografts were also homogenized in RIPA buffer. Lysates were sonicated for 10 sec. and centrifuged at 18,000 × g for 10 min. Protein concentrations of supernatants were determined by BCA assay (Pierce). Samples were reduced and denatured by addition of 1.25% β-mercaptoethanol in NuPage sample buffer (Invitrogen). Samples were heated for 1 min. at 95°C before SDS-PAGE. Proteins were transferred to nitrocellulose membranes, which were blocked with 5% BSA/TBS-T and probed using antibodies against P-AKTT308, P-AKTS473, Actin, P-S6S240/244, PARP, cleaved caspase-3, PR (Cell Signaling), and ER (Santa Cruz). Antibody binding was detected using HRP-conjugated secondary antibodies against mouse or rabbit Ig (GE Healthcare), and ECL substrate (Pierce).
Immunohistochemistry (IHC) and TUNEL
Five-micron sections of FFPE tumor tissue were used for H&E staining, IHC with antibodies against Ki67 (Biocare Medical) or P-PRAS40T246 (Cell Signaling), or TUNEL (Promega). For Ki67 IHC and TUNEL, 4-5 high-power (400x magnification) microscopic fields were used to count the numbers of positively-stained and total cells. Percentages of positively stained cells/field were used to calculate a single score for each tumor. In P-PRAS40 IHC, the majority of staining occurred in the tumor periphery, while tumor cores showed little/no staining. We scored P-PRAS40 signal in tumor periphery using the formula: Histoscore = (% cells with weak staining × 1) + (% cells with moderate staining × 2) + (% cells with strong staining × 3).
Statistical analyses
Numbers of apoptotic cultured cells, Ki67- and TUNEL-positive tumor cells, and P-PRAS40 Histoscores were compared between treatment groups by ANOVA with Bonferroni post-hoc test (for MCF-7 tumors), or t-test (for T47D/FR tumors). Tumor volumes were expressed as percentage relative to baseline for each mouse, and analyzed by mixed modeling using JMP software, with Standard Least Squares personality, Restricted Maximum Likelihood method. Relative tumor volumes at individual time points were compared between groups by t-test with Sidak-Bonferroni multiple testing correction using Graphpad Prism software. p≤0.05 was considered significant.
Results
P7170 inhibits PI3K and mTOR in ER+ breast cancer cells
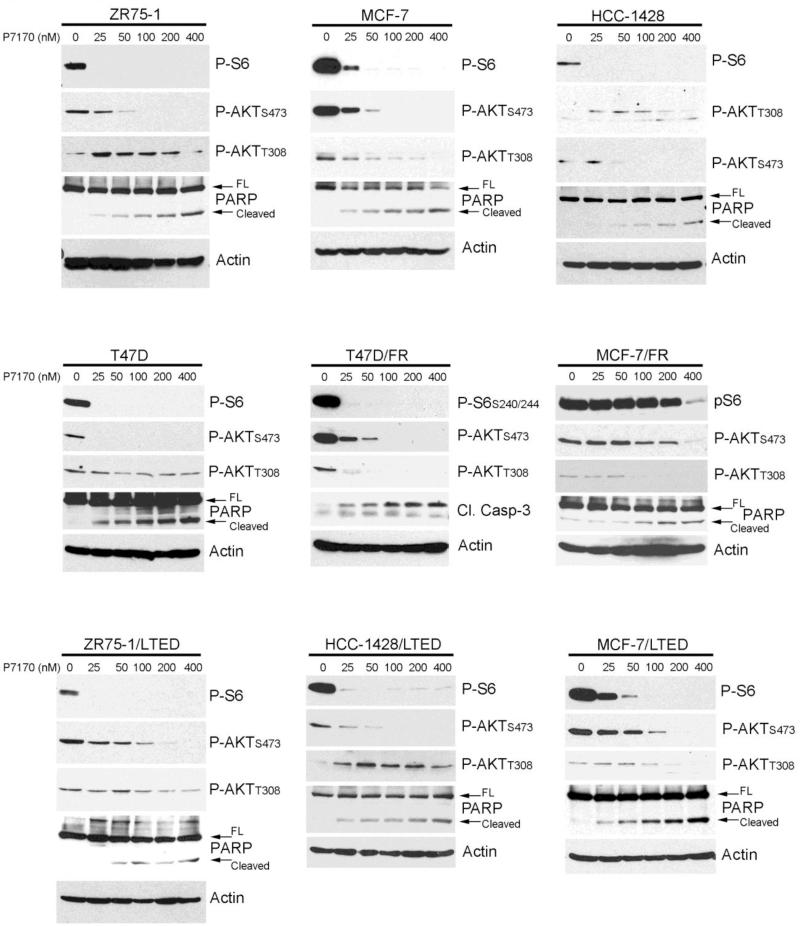
P7170 inhibits the in vitro enzymatic activity of the p110 isoforms of Class IA PI3K and mTOR with IC50 values of 2.2-203 nM and 4.4 nM, respectively [16]. We tested the effects of treatment with P7170 for 16-24 h on PI3K/AKT/mTOR pathway activation over a range of concentrations in a panel of anti-estrogen-sensitive ER+ breast cancer cell lines. Lower concentrations of P7170 (25-50 nM) potently inhibited mTORC1 signaling as indicated by reduced levels of phosphorylation of the downstream effector S6 (Fig. 1). Similar results were observed in ER+ cells adapted to growth in the presence of 1 μM fulvestrant (MCF-7/FR, T47D/FR) or long-term estrogen deprivation (LTED; mimics resistance to aromatase inhibition [8]). Inhibition of mTORC1 relieves negative feedback on activators upstream of PI3K (e.g., insulin-like growth factor-1 receptor (IGF-1R), insulin receptor substrate-1 (IRS-1), HER3 [19-21]). In line with these findings, we observed that low concentrations of P7170 induce PI3K activation as evidenced by increased P-AKTT308 (readout of PDK1 activity) in ZR75-1, HCC-1428, HCC-1428/LTED, and MCF-7/LTED cells. Thus, inhibition of PI3K may require higher concentrations of P7170 in some contexts. Indeed, treatment with 200-400 nM P7170 partially or completely decreased P-AKTT308.
Fig. 1. P7170 inhibits mTORC1 and mTORC2 kinases in anti-estrogen-sensitive and - resistant ER+ breast cancer cells.
Lysates from cells treated with 0-400 nM P7170 for 16-24 h were analyzed by immunoblot using the indicated antibodies. Full-length (FL) and cleaved PARP are indicated by arrows. FR cells were treated in the presence of 1 μM fulvestrant. LTED cells were treated in hormone-depleted medium.
As P7170 is an ATP-competitive inhibitor of mTOR, it can block both mTORC1 and mTORC2. We observed that P7170 decreases AKT phosphorylation at the mTORC2 site Serine-473. However, higher drug concentrations were sometimes required to decrease P-AKTS473 compared to P-S6 (Fig. 1). These observations are supported by evidence suggesting that PI3K activation and AKT phosphorylation on Threonine-308 can promote mTORC2-mediated phosphorylation on Serine-473 (reviewed in ref. [22]). Phosphorylation on both Threonine-308 and Serine-473 is required for full AKT activation [23]. Thus, P7170 likely induces activation of pathways upstream of PI3K, which antagonizes P7170 effects on AKT phosphorylation.
P7170-induced apoptosis was assessed by cleavage of PARP or caspase-3/7. We generally observed P7170 dose-dependent increases in apoptosis (Fig. 1). Given the finding that low doses of P7170 do not effectively inhibit PI3K signaling, these data suggest that mTORC1/mTORC2 inhibition induces only a modest degree of apoptosis, while inhibition of PI3K, mTORC1, and mTORC2 is more effective.
P7170 inhibits ER+ breast cancer cell growth and induces apoptosis
Cells grown in monolayer culture were treated with a dose range of P7170, and relative numbers of viable cells remaining after 5-8 days were quantified. Cell lines exhibited IC50 values for P7170 in the range of 0.9-7 nM (Fig. 2A). We then assessed the apoptotic responses of parental, LTED, and fulvestrant-resistant cells to P7170 with or without fulvestrant. Three days of treatment with 200 nM P7170 significantly increased apoptosis in all cell lines examined (Fig. 2B). In MCF-7, MCF-7/LTED, T47D, and T47D/FR cells, the combination of P7170 and fulvestrant significantly increased apoptosis compared to either drug alone.
Treatment with P7170 suppresses growth of ER+ breast tumors
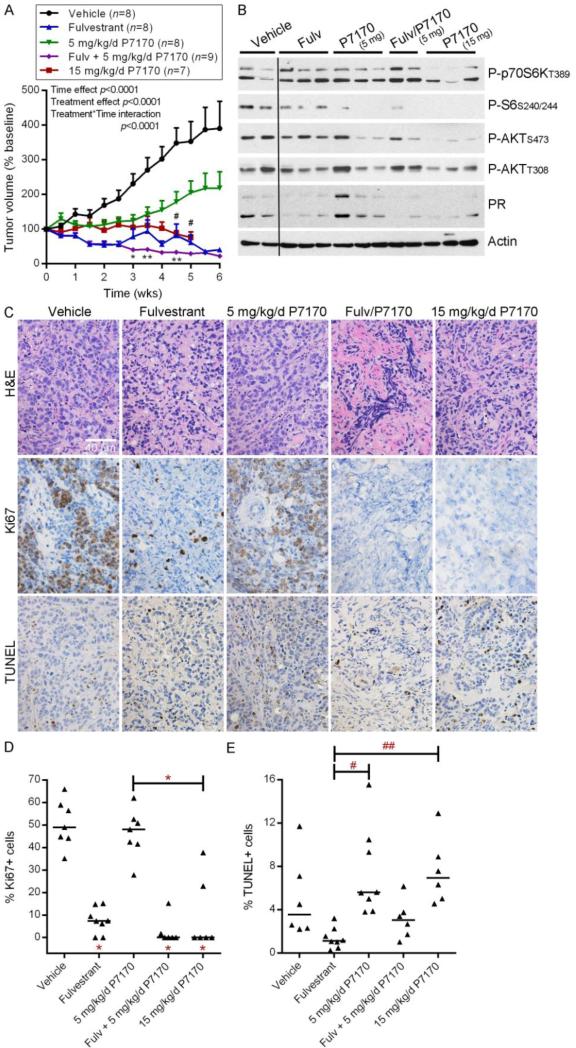
Since P7170 inhibited the growth and viability of ER+ breast cancer cells in vitro, we tested anti-tumor efficacy in vivo. Mice bearing 17β-estradiol-stimulated MCF-7 tumors were randomized to treatment with fulvestrant, P7170 (5 or 15 mg/kg/d), or the combination of fulvestrant plus 5 mg/kg/d P7170. Fulvestrant treatment induced tumor regression, while vehicle-treated tumors continued to grow (Fig. 3A). The addition of 5 mg/kg/d P7170 to fulvestrant significantly decreased tumor volumes compared to fulvestrant alone after 3-4.5 wks of treatment (p<0.05). Single-agent treatment with 5 mg/kg/d P7170 significantly slowed tumor growth compared to vehicle control (p<0.05), while treatment with 15 mg/kg/d P7170 completely blocked growth and was significantly more effective than low-dose P7170 at 4.5-5 weeks (p<0.05).
Fig. 3. Treatment with P7170 inhibits growth of anti-estrogen-sensitive MCF-7 tumors.
A) Mice bearing MCF-7 xenografts ~200 mm3 were randomized to the indicated treatments. Tumor volumes were calculated relative to baseline volume of individual mice, and mean % baseline volumes + SEM are shown. Data were analyzed by mixed modeling, which revealed that tumor volumes significantly changed over time and due to treatment, and the effects of treatments varied over time (all p<0.0001). All treatment groups exhibited significantly (p≤0.05) reduced tumor volume compared to vehicle control at the 3-wk time point or earlier by t-test with Sidak-Bonferroni multiple testing correction. *p<0.05, **p<0.01 when comparing fulvestrant vs. fulvestrant/P7170 at each time point. #p<0.05 when comparing low-vs. high-dose P7170 at each time point. B) Lysates from tumors harvested after 3 days of treatment were analyzed by immunoblot using the indicated antibodies. All lanes were taken from same film and are exposure-matched. C) Tumors harvested after 6 weeks of treatment were analyzed by H&E staining, Ki67 IHC, and TUNEL. Representative images are shown. Scale bar = 40 μm. D-E) Quantification of fractions of Ki67+ and TUNEL+ tumor cells from (C). Horizontal bars indicate median values within each treatment group. *p≤0.05, #p=0.07, ##p=0.1 by Bonferroni post-hoc test vs. vehicle control, unless otherwise indicated by brackets.
Analysis of tumors harvested at 4 h after the last dose of a 3-day treatment regimen with P7170 revealed that high-dose P7170 more effectively inhibited mTORC1 (assessed by P-p70S6K and P-S6 levels), mTORC2 (assessed by P-AKTS473), PI3K (assessed by P-AKTT308), and AKT [assessed by immunohistochemical (IHC) analysis of P-PRAS40] than low-dose P7170 (Figs. 3B, S2). Both doses of P7170 decreased mTORC1 and mTORC2 activities compared to vehicle control. High-dose P7170 decreased P-AKTT308 and P-PRAS40 compared to vehicle control, while low-dose P7170 had only modest effects (Figs. 3B, S2), in agreement with our in vitro observation that higher doses are needed to inhibit PI3K signaling (Fig. 1). Fulvestrant inhibited ER activity as shown by decreased PR levels (PR is encoded by an ER-inducible gene).
IHC analysis of tumors harvested from mice after 6 weeks of treatment revealed that fulvestrant with or without 5 mg/kg/d P7170, or 15 mg/kg/d P7170 alone significantly suppressed tumor cell proliferation as measured by Ki67 positivity (Fig. 3C-D). However, fulvestrant and P7170 alone or in combination did not significantly increase apoptosis compared to vehicle as assessed by TUNEL (Fig. 3C,E). Single-agent P7170 (5 and 15 mg/kg/d) showed a trend toward increased apoptosis compared to fulvestrant alone that did not reach statistical significance (Fig. 3E).
P7170 also inhibits the in vitro enzymatic activity of transforming growth factor-β (TGF-β) type I receptor activin receptor-like kinase (ALK1) with an IC50 value of 47 nM [16]. ALK1 can drive oncogenic phenotypes (reviewed in ref. [24]). While we observed that treatment with 400 nM P7170 partially decreased phosphorylation of Smad1/5 (readout of ALK1 signaling) in cultured MCF-7 cells (Fig. S1), we did not observe drug-induced changes in P-Smad1/5/8 in MCF-7 tumors by immunoblot or IHC (data not shown). Given the order-of-magnitude difference in P7170 IC50 values between PI3K/mTOR and ALK1 [16], and the finding that P7170 only modestly inhibits PI3K in vivo at the doses tested (Fig. 3B), we speculate that the concentrations of P7170 achieved in vivo did not adequately inhibit ALK1 to affect P-Smad1/5/8 levels.
Treatment with P7170 abrogates fulvestrant-resistant growth of ER+ breast tumors
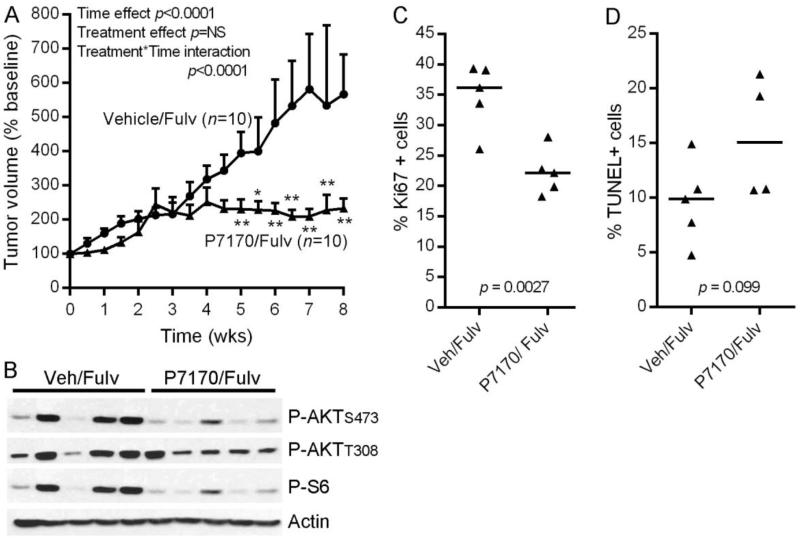
Regression of MCF-7 tumors in response to fulvestrant hindered our ability to assess benefit from P7170 (Fig. 3A). Thus, we tested P7170 in a fulvestrant-resistant model. Mice bearing T47D/FR tumors that had been treated with fulvestrant since the time of cell implantation were randomized to treatment with vehicle or 15 mg/kg/d P7170, each in the context of a fulvestrant backbone. While vehicle/fulvestrant-treated tumors continued to grow, treatment with P7170/fulvestrant significantly inhibited tumor growth (Fig. 4A).
Fig. 4. P7170 suppresses growth of fulvestrant-resistant T47D/FR tumors.
A) Mice bearing T47D/FR xenografts were treated with fulvestrant since the time of cell implantation. When tumors reached ~200 mm3, were randomized to vehicle or 15 mg/kg/d P7170 (both with continued fulvestrant). Tumor volumes were calculated relative to baseline volume of individual mice, and mean % baseline volumes + SEM are shown. Tumor volumes were analyzed as in Fig. 3A. Mixed modeling revealed that tumor volumes significantly changed over time (p<0.0001). While treatment did not have an independent effect, significant interaction between treatment and time indicated that the effects of P7170 treatment varied over time (p<0.0001). *p<0.05, **p<0.01 compared to vehicle control at each time point. B) Lysates from tumors harvested after 3 days of treatment were analyzed by immunoblot using the indicated antibodies. C-D) Quantification of fractions of Ki67+ and TUNEL+ cells in tumors harvested after 6 weeks of treatment. Horizontal bars indicate median values within each treatment group. Ki67 and TUNEL scores were compared by t-test between groups.
Analysis of tumors harvested after 3 days of treatment revealed that P7170/fulvestrant decreased mTORC1 and mTORC2 activities (assessed by P-S6 and P-AKTS473 levels, respectively), and partially decreased PI3K activity (P-AKTT308 levels) compared to vehicle/fulvestrant (Fig. 4B). ER and PR levels continued to be suppressed by fulvestrant treatment in all cases (Fig. S3). In tumors harvested after 6 weeks of treatment, the P7170/fulvestrant group showed significantly decreased Ki67 scores compared to vehicle/fulvestrant (Figs. 4C, S4), and a trend suggesting that addition of P7170 increased TUNEL positivity (Figs. 4D, S4). Thus, P7170 suppressed T47D/FR tumor growth primarily by inhibiting proliferation.
P7170 inhibits PI3K and mTOR activation in ER+/HER2-human breast tumors
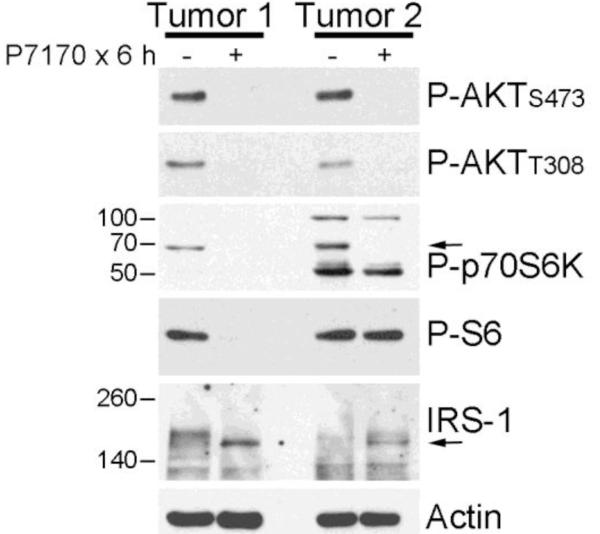
In order to validate observations from model systems, we tested the effects of P7170 on fragments of fresh patient-derived ER+/HER2− breast tumors. P7170 treatment effectively inhibited PI3K (assessed by P-AKTT308), mTORC2 (assessed by P-AKTS473), and mTORC1 (assessed by P-p70S6K) (Fig. 5). P7170 induced upregulation of IRS-1 levels, which occurs as a result of inhibition of p70S6K-induced phosphorylation of IRS-1 that, in turn, slows IRS-1 degradation (reviewed in ref. [25]). Interestingly, P7170 decreased P-S6 levels in one tumor but not the other, suggesting that A) some ER+/HER2-breast cancers engage a non-mTORC1/p70S6K mechanism to activate S6, and/or B) the kinetics of S6 dephosphorylation vary between tumors.
Fig. 5. P7170 inhibits PI3K and mTOR signaling in human breast tumors ex vivo.
Fresh ER+/HER2-breast tumor samples from patients were treated +/− 200 nM P7170 in serum-free medium for 6 h, then frozen. Lysates were analyzed by immunoblot using the indicated antibodies.
Discussion
Herein, we present evidence suggesting that the PI3K/mTOR inhibitor P7170 effectively abrogates growth of anti-estrogen-sensitive and -resistant ER+ breast tumors. The primary mechanism of P7170 anti-tumor action appears to involve inhibition of mTOR and tumor cell proliferation. These findings collectively suggest that P7170 is a candidate therapeutic for the treatment of patients with ER+ breast cancer.
Histological analysis of MCF-7 tumors revealed that single-agent treatment with fulvestrant or 15 mg/kg/d P7170, but not with 5 mg/kg/d P7170 significantly decreased cell proliferation (Fig. 3D). Surprisingly, high-dose P7170 treatment did not significantly increase tumor cell death (Fig. 3E), suggesting that the primary mechanism of anti-tumor action of P7170 involves inhibition of proliferation but not viability. Similarly, P7170 (in the context of a fulvestrant backbone) significantly decreased cell proliferation in T47D/FR tumors, but did not significantly increase apoptosis (Fig. 4C-D). In contrast, treatment with the PI3K inhibitor BKM120 (buparlisib) induces apoptosis in MCF-7 tumors [14]. However, BKM120 robustly inhibits PI3K, which in turn decreases mTORC1 activation in this model [14], while P7170 primarily targets mTORC1/mTORC2 in tumors [indicated by drastic decreases in tumor P-AKTS473 and P-S6, but not P-AKTT308 (Figs. 3B & 4B); decreased P-PRAS40 also indicates AKT inhibition (Fig. S2)]. These data suggest that inhibition of mTORC1/mTORC2 suppresses tumor cell proliferation, while inhibition of PI3K is required to induce apoptosis.
PI3K and AKT inhibition were shown to be critical for induction of apoptosis by the PI3K/mTOR inhibitor BEZ235 [26]. We previously reported that treatment with BEZ235, but not the mTORC1 inhibitor everolimus (RAD001), frequently induces apoptosis in anti-estrogen-sensitive and -resistant ER+ breast cancer cells [8]. However, Brachmann et al. inferred that mTORC2 (but not mTORC1) is involved in such apoptosis, which conflicts with our present findings because P7170 effectively inhibited both mTOR complexes in vivo without inducing apoptosis (Figs. 3B,E & 4B,D). Alternatively, the different kinetics and binding affinities of P7170 vs. BEZ235 may partially account for differences in apoptotic effects in vivo. The lack of P7170-induced apoptosis in T47D/FR tumors may also be attributable to the apoptosis-resistant phenotype of T47D cells; this cell line expresses low levels of the pro-apoptotic protein Bim, and has loss of heterozygosity at the BIM locus [27,28]. Inhibition of PI3K/mTOR and mTORC1 have also been shown to decrease tumor angiogenesis [29,30]. However, we did not observe P7170 effects on tumor blood vessel size or density as assessed by MECA-32 and CD31 IHC (data not shown).
Inhibitors of the PI3K/AKT/mTOR pathway are in clinical development for the treatment of ER+ breast cancer (reviewed in ref. [31]). The allosteric mTORC1 inhibitor everolimus was recently approved for the treatment of advanced ER+ breast cancer in combination with exemestane. Since allosteric mTORC1 inhibition induces feedback upregulation of PI3K/AKT [19,20] and is insufficient to block all mTORC1 activities [32-34], an ATP-competitive mTOR inhibitor that targets both mTORC1 and mTORC2 (e.g., MLN0128, CC-223, and AZD2014) may be more effective. Furthermore, acquired mutations in mTOR that confer resistance to allosteric mTORC1 inhibitors but retain sensitivity to ATP-competitive mTOR inhibitors [35,36] provide support for development of the latter. MLN0128 has been shown to suppress growth of orthotopic MCF-7 xenografts in mice [37]. BEZ235 and GDC-0980 are PI3K/mTOR dual inhibitors undergoing clinical evaluation. GDC-0980 downregulates mTORC1 and mTORC2 activities upon short-term (4 h) treatment at ~100 nM in cultured cells. However, prolonged (24 h) treatment revealed that higher concentrations are required maintain suppression of mTORC1 and mTORC2 [38], likely due in part to drug-induced feedback activation of PI3K. BEZ235 inhibits mTORC1 in the low nanomolar range, and requires doses of 100-500 nM to downregulate P-AKT in vitro [39]. Signaling responses to P7170 (Fig. 1) are akin to those of BEZ235 and GDC-0980, and suggest that the primary mode of anti-tumor action is inhibition of mTOR. BEZ235, GDC-0980, MLN0128, and AZD2014 have shown efficacy in early-phase clinical trials, and it remains to be determined whether mTOR inhibitor-induced feedback activation of PI3K is clinically important. Indeed, Gokmen-Polar et al. found that MLN0128 and rapamycin similarly inhibited the growth of VEGF-expressing MCF-7 xenografts [37].
Activating mutations in PIK3CA (encodes the p110α catalytic subunit of PI3K) and loss-of-function mutations or decreased expression of PTEN (encodes the lipid phosphatase that antagonizes PI3K signaling) are associated with increases sensitivity to PI3K inhibitors in preclinical models [40,41]. In agreement with our prior observations [8], PIK3CA-mutant (MCF-7, T47D) and PTEN-deficient (ZR75-1) ER+ breast cancer cells exhibit increased PI3K signaling (assessed by P-AKT levels) and are more sensitive to PI3K/mTOR inhibition (with P7170) compared to PIK3CA/PTEN-wild-type (HCC-1428) cells (Figs. 1-2). Such lesions have also been linked with increased likelihood of response to PI3K/AKT/mTOR inhibitors in patients with advanced, treatment-refractory cancers [42]; however, 76% of patients in this pooled analysis were treated with a regimen containing the mTORC1 inhibitor everolimus, and only 4.9% of patients had breast cancer. In a Phase I study with the PI3K inhibitor BKM120 (buparlisib) in patients with advanced solid tumors, and in a Phase Ib study of BKM120 with letrozole in patients with advanced ER+ breast cancer, PIK3CA mutations and/or PTEN loss (by IHC) were not associated with clinical benefit [43,44]. Thus, it remains to be determined whether PIK3CA/PTEN status is a useful biomarker to predict response to PI3K inhibitors in patients.
Conclusions
These findings collectively support further exploration of the therapeutic potential of P7170 for the treatment of ER+ breast cancer, both as a single agent and in combination with endocrine therapy, particularly in cases of anti-estrogen resistance. P7170 is currently being tested in dose-finding phase I studies, and if a safe dose/schedule is identified, a phase Ib study in patients with advanced ER+ disease is warranted.
Supplementary Material
Acknowledgements
We thank the Norris Cotton Cancer Center Transgenics & Genetic Constructs, Pathology Translational Research, and Immunoassays & Flow Cytometry Shared Resources for their support. This work was supported by NIH: R00CA142899 (TWM), Dartmouth College Norris Cotton Cancer Center Support Grant P30CA023108; a research grant from Piramal Enterprises, Ltd. (TWM); and the American Cancer Society RSG-13-292-01-TBE (TWM).
Footnotes
Competing interests: Veena R. Agarwal is an employee of Piramal Enterprises, Ltd.
References
- 1.Ferlay JS, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Internat J Cancer. 2010:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31:3083–3090. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentzon N, During M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer. 2008;122:1089–1094. doi: 10.1002/ijc.22892. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF, investigators AL Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, Higham C, Garcia-Echeverria C, Shyr Y, Arteaga CL. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 10.Miller TW, Perez-Torres M, Narasanna A, Guix M, Stal O, Perez-Tenorio G, Gonzalez-Angulo AM, Hennessy BT, Mills GB, Kennedy JP, Lindsley CW, Arteaga CL. Loss of Phosphatase and Tensin Homologue Deleted on Chromosome 10 Engages ErbB3 and Insulin-Like Growth Factor-I Receptor Signaling to Promote Antiestrogen Resistance in Breast Cancer. Cancer Research. 2009;69:4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 12.Sabnis G, Goloubeva O, Jelovac D, Schayowitz A, Brodie A. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway improves response of long-term estrogen-deprived breast cancer xenografts to antiestrogens. Clin Cancer Res. 2007;13:2751–2757. doi: 10.1158/1078-0432.CCR-06-2466. [DOI] [PubMed] [Google Scholar]
- 13.Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM, Parker JS, Miller MA, Huntsman DG, Lin L, Snider J, Davies SR, Olson JA, Jr., Watson MA, Saporita A, Weber JD, Ellis MJ. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69:3955–3962. doi: 10.1158/0008-5472.CAN-08-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller TW, Balko JM, Fox EM, Ghazoui Z, Dunbier A, Anderson H, Dowsett M, Jiang A, Smith RA, Maira SM, Manning HC, Gonzalez-Angulo AM, Mills GB, Higham C, Chanthaphaychith S, Kuba MG, Miller WR, Shyr Y, Arteaga CL. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338–351. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, Brachmann S, Fritsch C, Dorsch M, Chene P, Shoemaker K, De Pover A, Menezes D, Martiny-Baron G, Fabbro D, Wilson CJ, Schlegel R, Hofmann F, Garcia-Echeverria C, Sellers WR, Voliva CF. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal VR, Joshi A, Venkataraman M, Desai N, Bhatia D, Chaudhari S, Bose J, Kolla LS, Deore V, Yewalkar N, Kumar S, Sharma R, Damre A, More A, Gill P, Sharma S. P7170: A potent and oral phosphoinositide 3-kinase (PI3K)-mammalian target of rapamycin (mTOR) and activin receptor-like kinase 1 (ALK1) inhibitor with anti-tumor and anti-angiogenic activity. Cancer Res. 2012;72 Abstract 3742. [Google Scholar]
- 17.Agarwal VR, Joshi A, Venkataraman M, Bhatia D, Bose J, Kolla LS, Gill P, Sharma S. P7170, a novel inhibitor of phosphoinositide 3-kinase (PI3K)-mammalian target of Rapamycin (mTOR) and activin receptor-like kinase 1 (ALK1) as a new therapeutic option for Kras mutated non small cell lung cancer (NSCLC) Cancer Res. 2012;72 Abstract 3759. [Google Scholar]
- 18.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 19.Miller TW, Forbes JT, Shah C, Wyatt SK, Manning HC, Olivares MG, Sanchez V, Dugger TC, de Matos Granja N, Narasanna A, Cook RS, Kennedy JP, Lindsley CW, Arteaga CL. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res. 2009;15:7266–7276. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drabsch Y, ten Dijke P. TGF-beta signaling in breast cancer cell invasion and bone metastasis. Journal of mammary gland biology and neoplasia. 2011;16:97–108. doi: 10.1007/s10911-011-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Destefano MA, Jacinto E. Regulation of insulin receptor substrate-1 by mTORC2 (mammalian target of rapamycin complex 2) Biochemical Society transactions. 2013;41:896–901. doi: 10.1042/BST20130018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, Wang S, Garcia-Echeverria C, Maira SM. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faber AC, Corcoran RB, Ebi H, Sequist LV, Waltman BA, Chung E, Incio J, Digumarthy SR, Pollack SF, Song Y, Muzikansky A, Lifshits E, Roberge S, Coffman EJ, Benes CH, Gomez HL, Baselga J, Arteaga CL, Rivera MN, Dias-Santagata D, Jain RK, Engelman JA. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 2011;1:352–365. doi: 10.1158/2159-8290.CD-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, Wooster R. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnell CR, Stauffer F, Allegrini PR, O’Reilly T, McSheehy PM, Dartois C, Stumm M, Cozens R, Littlewood-Evans A, Garcia-Echeverria C, Maira SM. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res. 2008;68:6598–6607. doi: 10.1158/0008-5472.CAN-08-1044. [DOI] [PubMed] [Google Scholar]
- 30.Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, Hensley HH, Hamilton TC, Testa JR. RAD001 (everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Research. 2007;67:2408–2413. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 31.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 Phosphorylation Sites Encode Their Sensitivity to Starvation and Rapamycin. Science. 2013;341:364–+. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-Site Inhibitors of mTOR Target Rapamycin-Resistant Outputs of mTORC1 and mTORC2. PLoS biology. 2009;7:371–383. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. P Natl Acad Sci USA. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan B, Akcakanat A, Sangai T, Evans KW, Adkins F, Eterovic AK, Zhao H, Chen K, Chen H, Do KA, Xie SM, Holder AM, Naing A, Mills GB, Meric-Bernstam F. Catalytic mTOR inhibitors can overcome intrinsic and acquired resistance to allosteric mTOR inhibitors. Oncotarget. 2014;5:8544–8557. doi: 10.18632/oncotarget.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, Gray N, Barletta JA, Guo Y, Swanson SJ, Ruan DT, Hanna GJ, Haddad RI, Getz G, Kwiatkowski DJ, Carter SL, Sabatini DM, Janne PA, Garraway LA, Lorch JH. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371:1426–1433. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gokmen-Polar Y, Liu Y, Toroni RA, Sanders KL, Mehta R, Badve S, Rommel C, Sledge GW., Jr. Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat. 2012;136:673–682. doi: 10.1007/s10549-012-2298-8. [DOI] [PubMed] [Google Scholar]
- 38.Wallin JJ, Edgar KA, Guan JE, Berry M, Prior WW, Lee L, Lesnick JD, Lewis C, Nonomiya J, Pang JD, Salphati L, Olivero AG, Sutherlin DP, O’Brien C, Spoerke JM, Patel S, Lensun L, Kassees R, Ross L, Lackner MR, Sampath D, Belvin M, Friedman LS. GDC-0980 Is a Novel Class I PI3K/mTOR Kinase Inhibitor with Robust Activity in Cancer Models Driven by the PI3K Pathway. Molecular Cancer Therapeutics. 2011;10:2426–2436. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 39.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 40.Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, Lesnick JD, Lewis C, Nonomiya J, Pang J, Salphati L, Olivero AG, Sutherlin DP, O’Brien C, Spoerke JM, Patel S, Lensun L, Kassees R, Ross L, Lackner MR, Sampath D, Belvin M, Friedman LS. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–2436. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 41.Spoerke JM, O’Brien C, Huw L, Koeppen H, Fridlyand J, Brachmann RK, Haverty PM, Pandita A, Mohan S, Sampath D, Friedman LS, Ross L, Hampton GM, Amler LC, Shames DS, Lackner MR. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res. 2012;18:6771–6783. doi: 10.1158/1078-0432.CCR-12-2347. [DOI] [PubMed] [Google Scholar]
- 42.Janku F, Hong DS, Fu S, Piha-Paul SA, Naing A, Falchook GS, Tsimberidou AM, Stepanek VM, Moulder SL, Lee JJ, Luthra R, Zinner RG, Broaddus RR, Wheler JJ, Kurzrock R. Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell reports. 2014;6:377–387. doi: 10.1016/j.celrep.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, Goldbrunner M, Baselga J. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 44.Mayer IA, Abramson VG, Isakoff SJ, Forero A, Balko JM, Kuba MG, Sanders ME, Yap JT, Van den Abbeele AD, Li Y, Cantley LC, Winer E, Arteaga CL. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2014;32:1202–1209. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.