Abstract
Background
In genome-wide association studies (GWAS) five putative risk loci are associated with intracranial aneurysm. As brain arteriovenous malformations (AVM) and intracranial aneurysms are both intracranial vascular diseases and AVMs often have associated aneurysms, we investigated whether these loci are also associated with sporadic brain AVM.
Methods
We included 506 patients (168 Dutch and 338 American) and 1548 controls, all Caucasians. Controls for both cohorts had been recruited as part of previous GWAS. Dutch patients were genotyped by KASPar® assay and US patients by Affymetrix SNP 6.0 array. Associations in each cohort were tested by univariable logistic regression modeling, with subgroup analysis in 205 American cases with aneurysm data. Meta-analysis was performed by a Mantel-Haenszel fixed-effect method.
Results
In the Dutch cohort none of the SNPs were associated with AVMs. In the American cohort, genotyped SNPs near SOX-17 (OR 0.74; 95% confidence interval (CI) 0.56–0.98), RBBP8 (OR 0.76; 95% CI 0.62–0.94) and an imputed SNP near CDKN2B-AS1 (OR 0.79; 95% CI 0.64–0.98) were significantly associated with AVM. The association with SNPs near SOX-17 and CDKN2B-AS1 but not RBBP8 were strongest in AVM patients with associated aneurysms. In the meta-analysis we found no significant associations between allele frequencies and AVM occurrence, but rs9298506, near SOX-17 approached statistical significance (OR 0.77; 95% CI 0.57–1.03, p= 0.08).
Conclusions
Our meta-analysis of two Caucasian cohorts did not show an association between the five aneurysm-associated loci and sporadic brain AVM. Possible involvement of SOX-17 and RBBP8, genes involved in cell cycle progression, deserves further investigation.
Keywords: cerebrovascular disease, stroke, genetics, brain arteriovenous malformation
INTRODUCTION
Both brain arteriovenous malformations (AVM) and intracranial aneurysms are vascular diseases that may cause intracranial hemorrhage. In 20–50 percent of patients with AVM, aneurysms are present either on the arteries feeding the AVM or within the nidus, with the proportion increasing with use of super-selective angiography.1–3 In contrast to AVMs caused by disorders such as hereditary hemorrhagic telangiectasia (HHT), an autosomal dominant disease, caused by loss of functional mutations in the transforming growth factor beta (TGFβ) signaling pathway, the precise etiology of sporadic AVM remains unknown. However, evidence is mounting that genetic factors play a role.4–10 Previous studies of common genetic variation and AVM reported significant associations with variants in genes related to angiogenesis (e.g. ACVRL1 and VEGFA),6,8 vascular wall remodeling (e.g. MMP-3)9 and inflammation (e.g. IL-1 and IL6).10 Genome wide association studies (GWAS) for sporadic brain AVM are underway, but have not yet been published.
GWAS studies in intracranial aneurysms have found six independent single nucleotide polymorphisms (SNPs) in five risk loci to be associated at genome-wide significance levels.11 These putative risk genes, SOX-17, CDKN2B-AS1, RBBP8, STARD13-KL and CNNM2, have been suggested to act via pathways involved in cell cycle progression and cell proliferation including endothelial cell formation and maintenance.12–17 Recently, a small case-control study analyzing 78 Italian cases and 103 controls suggested that the SNP in the CDKN2B-AS1 gene was also associated with AVMs,18 with further data from an American cohort suggesting that this association may be due to the presence of brain AVM-associated aneurysms.19 The purpose of our study was to investigate whether the genetic risk loci for aneurysms also play a role in sporadic brain AVMs.
PATIENTS AND METHODS
The study was approved by the institutional ethical committee of the University Medical Center Utrecht (UMCU), The Netherlands, and by the Institutional Review Boards at the University of California, San Francisco (UCSF) and Kaiser Permanente Medical Care Plan of Northern California (KPNC), USA.
Patients and controls
The Dutch cohort consisted of 168 Caucasian patients (mean age 46 years, standard deviation (SD) 14 years; 56% males) with an AVM who had presented to the University Medical Center Utrecht, the Netherlands between 1985 and 2010. In 152 patients (90.5%) the diagnosis had been confirmed by digital subtraction angiography, in 10 (5.9%) by pathology and in 6 (3.6%) by MRA or CTA. Seventy-six patients (45%) presented with an intracerebral hemorrhage. Dutch control subjects (mean age 62 years, SD 10 years; 59% male) were 1038 healthy volunteers recruited for a previous GWAS.20
The American cohort included 338 Caucasian AVM cases (mean age 39 years, SD 18 years; 46% males) who had presented to UCSF between 2000 and 2010 (n=232) or to KPNC before 2005 (n=106) who provided blood or saliva specimens, and consented for genetic studies. American control subjects were 510 Caucasians (mean age 48 years, SD 15 years; 50% males) who were recruited for a previous GWAS.21 For 232 UCSF cases, the diagnosis of AVM was confirmed by digital subtraction angiography (97%), by MRI/CT and pathology (2%), or by MRI/CT and chart review (1%). KPNC cases were identified initially through computerized search of all databases and the definitive diagnosis of AVM was based on two or more sources, including clinical history, neuroimaging (CT, MRI or angiography as a single study or in combination), and pathology. Study neurologists adjudicated any suspect cases; exact percentage breakdowns are not available. A total of 127 patients (38%) presented with an intracerebral hemorrhage.
Patients with a definite clinical diagnosis of HHT were excluded.
For patients in the Dutch cohort, angiograms for assessment of associated aneurysms were accessible for 63 of 168 patients (38%). Eleven of 63 patients harbored a brain AVM-related aneurysms (17.5%; in all flow-related; one of the 11 patients also had a nidal aneurysm) and we found no unrelated aneurysms. In the American cohort presence of aneurysms could be assessed in 205 of 338 patients (61.0%); aneurysm data was not available for KPNC cases. Seventy-four patients (36%) harbored associated flow-related or intranidal aneurysms (73% flow-related aneurysms, 19% intranidal aneurysms, and 8% had both). Additionally, 6 subjects (3%) had aneurysms unrelated to the shunt flow, and 62% had no aneurysms. Angiograms were reviewed by experienced interventional neuroradiologists at both sites.
SNP selection
SNPs were considered for genotyping in AVM patients when shown to be associated with intracranial aneurysm development at genome-wide significance level (p ≤ 5 × 10−8) and successfully replicated in an independent cohort.11,22 Table 1 summarizes the chromosomal position of the SNPs as well as the function of the putative risk genes. The two SNPs in SOX-17 were not in linkage disequilibrium.11
Table 1.
Risk loci for intracranial aneurysm development and their annotated function
| Gene | Chr. | SNP | Functional annotation |
|---|---|---|---|
| SOX-17 | 8 | rs10958409 | Transcription factor involved in vascular development and endothelial formation.13,14 |
| SOX-17 | 8 | rs9298506 | Transcription factor involved in vascular development and endothelial formation.15,14 |
| CDKN2B-AS1 | 9 | rs1333040 | Encodes tumor suppressor proteins: cyclin dependent kinase inhibitor P15INK4b, P16INK4a and ARF, involved in tissue maintenance and repair.17 |
| RBBP8 | 18 | rs11661542 | Influences cell cycle progression and DNA repair through recruitment of BRCA1.15 |
| STARD13-KL | 13 | rs9315204 | Tumor suppressor gene influencing cell proliferation through cytoskeleton reorganization.12 |
| CNNM2 | 10 | rs12413409 | Bivalent metal transporter involved in hypertension.16 |
Chr indicates chromosome; SNP, single nucleotide polymorphism.
Genotyping
In Dutch patients genomic DNA was extracted from peripheral blood lymphocytes using automated nucleic acid isolation procedures (Chemagen, Baesweiler, Germany). Genotyping was performed by KASPar® assays (KBiosciences, Herts, UK) on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, California, USA) according to standard protocol. Controls were genotyped on Illumina 317K and Illumina 370K platforms (Illumina, San Diego, California, USA) as previously described.20
In the American cohort, genomic DNA from cases and controls was extracted from peripheral blood lymphocytes using a salt modification method (Gentra Systems, Minneapolis, Minnesota, USA) or from saliva using Oragene kits (DNA Genotek, Ontario, Canada). Genotyping was performed using the Affymetrix Genome-Wide SNP 6.0 array (Affymetrix, Santa Clara, California, USA) according to the manufacturer’s protocol at the UCSF Genomics Core Facility. Controls were genotyped on the same platform in the same laboratory. Genotypes for both cases and controls were called together using Birdseed version 2 implemented in Affymetrix Genotyping Console.
In the American cohort, three SNPs (rs9298506, rs9315204, and rs12413409) were directly genotyped on the Affymetrix 6.0 array. For rs11661542, we identified a perfect proxy (r2=1) using HapMap data, SNP rs11082043. For the remaining two SNPs (rs10958409 and rs1333040) good proxy SNPs were not available and we therefore obtained genotypes from imputation to the 1000 Genomes reference dataset (EUR population) using MaCH and minimac.23,24
For both cohorts, SNPs with a call-rate >95% were considered successfully genotyped.
Statistical analysis
To compare genotypes between AVM patients and controls, we applied univariable logistic regression analysis implemented in PLINK (v1.07).25 We tested an additive genetic model (0, 1, or 2 copies of the risk allele) and determined odds ratios (ORs) with 95% confidence intervals (CIs). Association testing for the two imputed SNPs was performed using an additive model of genotype dosages implemented in Mach2dat.23,24
Results were corrected for multiple testing of five SNPs using the Bonferroni correction and a value of p≤0.01 was considered statistically significant. Allele frequencies in both cohorts were combined as ORs with 95% CIs by a Mantel-Haenszel fixed effect model. Under the assumption of one cohort of 506 patients and three times as many controls, this study had the power to detect a susceptibility locus with an OR of 1.44–1.54, depending on the SNP under investigation (http://pngu.mgh.harvard.edu/~purcell/gpc/).
To assess whether potential associations with AVMs were affected by presence of brain AVM-related aneurysms, we performed subgroup analyses in the 205 patients in the American cohort for whom information on presence of aneurysm was available. Because of the small number of patients with information on presence of aneurysm, subgroup analysis was not possible in the Dutch cohort.
RESULTS
The two cohorts combined included 506 patients and 1548 controls. Allele frequencies were consistent with 1000Genomes (EUR population) frequencies and in Hardy Weinberg Equilibrium (Supplemental Table). In the Dutch cohort, one SNP (rs9315204) in STARD13-KL was unsuccessfully genotyped with a call-rate of 91%, and excluded from further analysis. In the American cohort, imputation of the two SNPs, rs10958409 and rs1333040 was successful (r2=0.9). Allele frequencies were similar in the Dutch and American controls (Table 2 and 3).
Table 2.
Association analysis of SNP genotype with AVM in Dutch AVM patients and controls.
| Gene | SNP | Number | Risk allele frequencies | OR (95% CI)* | p-value* | |
|---|---|---|---|---|---|---|
|
| ||||||
| cases | cases | control | ||||
| SOX-17 | rs10958409 A1=A A2=G |
164 | 0.152 | 0.140 | 1.08 (0.77 – 1.50) | 0.660 |
| SOX-17 | rs9298506 A1=A A2=G |
163 | 0.837 | 0.807 | 1.23 (0.90 – 1.68) | 0.195 |
| CDKN2B-AS1 | rs1333040 A1=T A2=C |
160 | 0.531 | 0.550 | 0.94 (0.74 – 1.19) | 0.601 |
| RBBP8 | rs11661542 A1=C A2=A |
164 | 0.512 | 0.501 | 1.04 (0.83 – 1.31) | 0.734 |
| STARD13-KL | rs9315204^ A1=T A2=C |
- | - | - | - | - |
| CNNM2 | rs12413409 A1=G A2=A |
164 | 0.915 | 0.922 | 0.90 (0.59 – 1.36) | 0.624 |
Univariable logistic regression analysis (additive model);
Excluded based on unsuccessful call-rate; A1 is the risk allele being tested
Table 3.
Association analysis of SNP genotype with AVM in American AVM patients and controls.
| Gene | SNP | Number | Risk allele frequencies | AVM vs. Controls | 74 AVM with aneurysm vs. controls | 131 AVM without aneurysm vs. controls | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| cases | cases | controls | OR (95% CI) | p-value* | OR (95% CI) | p-value* | OR (95% CI) | p-value | ||
| Genotyped SNPs | ||||||||||
| SOX-17 | rs10958409 A1=A A2=G |
- | - | - | - | - | - | - | - | - |
| SOX-17 | rs9298506 A1=A A2=G |
336 | 0.857 | 0.819 | 1.35 (1.02 – 1.79) | 0.035† | 2.16 (1.20 – 3.88) | 0.010 | 1.12 (0.77 – 1.64) | 0.546 |
| CDKN2B-AS1 | rs1333040 A1=T A2=C |
- | - | - | - | - | - | - | - | - |
| RBBP8 | rs11082043^ A1=C A2=G |
326 | 0.554 | 0.491 | 1.31 (1.07 – 1.61) | 0.010§ | 1.20 (0.84 – 1.71) | 0.319 | 1.19 (0.89 – 1.58) | 0.234 |
| STARD13-KL | rs9315204 A1=T A2=C |
338 | 0.192 | 0.229 | 0.80 (0.62 – 1.02) | 0.066 | 0.82 (0.53 – 1.26) | 0.364 | 0.81 (0.58 – 1.14) | 0.229 |
| CNNM2 | rs12413409 A1=G A2=A |
337 | 0.904 | 0.887 | 1.22 (0.87 – 1.71) | 0.290 | 1.60 (0.84 – 3.08) | 0.156 | 1.37 (0.85 – 2.20) | 0.195 |
| Imputed SNPs | ||||||||||
| SOX-17 | rs10958409 A1=A A2=G |
338 | 0.167 | 0.151 | 1.16 (0.88 – 1.53) | 0.301 | 1.13 (0.70 – 1.85) | 0.618 | 1.10 (0.75 – 1.61) | 0.634 |
| CDKN2B-AS1 | rs1333040 A1=T A2=C |
338 | 0.620 | 0.568 | 1.26 (1.02 – 1.56) | 0.029† | 1.43 (0.98 – 2.08) | 0.064 | 1.01 (0.76 – 1.36) | 0.931 |
Univariable logistic regression analysis (additive model);
Proxy SNP for rs11661542;
significantly associated before correction for multiple testing;
significantly associated after correction for multiple testing; A1 is the risk allele tested.
In the Dutch cohort, none of the SNPs were significantly associated with brain AVM (Table 2). In the American cohort, rs9298506 (OR 0.74; 95% CI 0.56–0.98; near SOX-17), (rs11082043, a proxy for rs1661542, OR 0.76; 95% CI 0.62–0.94, near RBBP8) and rs1333040 (OR 0.79; 95% CI 0.64–0.98; near CDKN2B-AS1) were associated with brain AVM. After correction for multiple testing of five SNPs, the RBBP8 SNP remained significantly associated (p = 0.01; Table 3).
In the meta-analysis, none of the SNPs under investigation showed a significant association with brain AVM. However, rs9298506, located near SOX-17, approached the cutoff value for significance with an OR of 0.77 (95% CI 0.57–1.03, p-value 0.08) (Figure 1). When we added data from the Italian case-control study on rs1333040 near CDKN2B-AS1 18 to the meta-analysis, this SNP remained unassociated with AVM with an OR of 0.98; 95% CI 0.80–1.20 (Figure 2).
Figure 1. Meta-analysis of the association between the five SNPs and AVM in the Dutch and American cohorts.
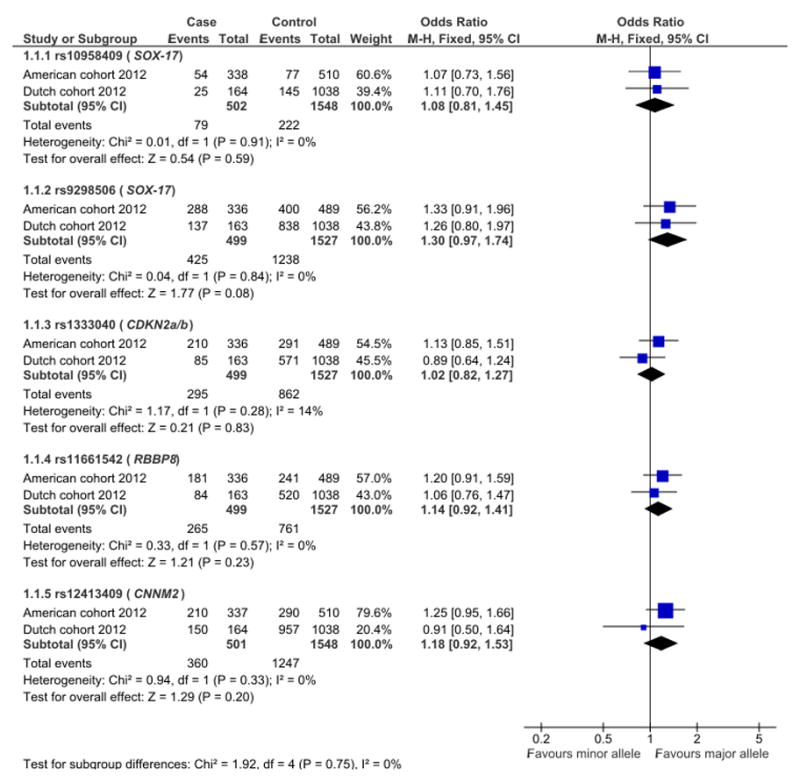
Reference allele (events) compared to total allele number (total) in cases and controls.
Figure 2. Meta-analysis of the association between rs1333040 and AVM in the Dutch, American and Italian cohorts.
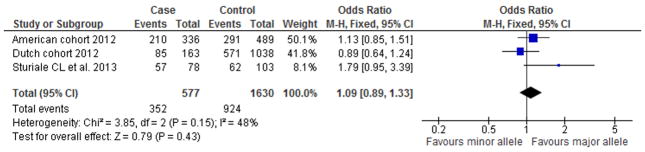
Reference allele (events) compared to total allele number (total) in cases and controls.
Stratified analysis of the 205 American patients with brain AVM-related aneurysm data available, showed that the observed associations with AVM in the American cohort may be explained by the presence of related aneurysms for rs9298506 (near SOX-17) and rs1333040 (near CDKN2B-AS1, as previously reported,19 but not for rs11082043 (near RBBP8, Table 3). Carriers of the SOX-17 rs9298506 A allele had an OR of 2.16 (95% CI: 1.20–3.88, p-value 0.01) for association with AVM aneurysm, whereas no association was observed in the 131 AVMs without aneurysms (OR=1.12, 95% CI: 0.77–1.64, p-value 0.55, Table 3).
DISCUSSION
Our meta-analysis did not find a significant association with sporadic brain AVMs for five SNPs previously found to be associated with intracranial aneurysms. Our data do, however, suggest that SNPs near SOX-17 and RBBP8 may be associated with sporadic brain AVMs.
We consider the evidence for the association of the SNP near SOX-17 suggestive because the point-estimates of the effects, as reflected in the ORs, were of similar magnitude and in the same direction in the Dutch and American cohorts, and the association approached statistical significance in the meta-analysis. Stratified analysis for patients with and without brain AVM associated aneurysms in the American cohort suggests that the AVM association with the SNP near SOX-17 may be explained by the presence of brain AVM-related aneurysms, similar to a recent report showing this pattern for SNPs in the 9p21 region, including the CDKN2B-AS1 SNP.19 The SNP near RBBP8 was associated with AVM after correction for multiple testing in the American cohort but not in the meta-analysis. In contrast with the possible association with the SNP near SOX-17, this putative association was not explained by presence of brain AVM associated aneurysms.
We could not find firm evidence for an association of brain AVM with the SNP near CDKN2B-AS1 in either the American or Dutch cohorts analyzed separately, or in the meta-analysis, even when we included allele frequencies from the Italian cohort that had previously published a positive association.18 The association of the CDKN2B-AS1 SNP with sporadic brain AVM in that study might have been due to a different distribution of AVM cases with related aneurysms. In the Italian study the proportion of patients with a brain AVM with associated aneurysms was not reported but 41% of patients had a relatively large AVM extending beyond four centimeters and aneurysms tend to occur more frequently with larger AVMs. Similarly, the absence of an association for the CDKN2B-AS1 SNP in the Dutch cohort may be a relatively small proportion of patients with aneurysms; in a sample of 63 patients of the Dutch cohort the proportion was indeed small at 17.5%. While in our meta-analysis we could not firmly establish an association, SOX-17 and RBBP8 emerge from our study as promising candidates that deserve further studies in brain AVM. Both genes are involved in cell cycle progression. SOX-17 is expressed in vascular endothelial cells and involved in regulation of tissue differentiation during various developmental processes. It serves as a regulator of cell cycle progression in multiple tissues.26,27 SOX-17−/− mice show vascular abnormalities including defective endothelial sprouting and impaired vascular remodelling.14,15 Another recent study showed that SOX-17 promotes tumor angiogenesis and destabilizes tumor vessels in mice.28 RBBP8 is involved in the repair of double strand breaks in DNA during cell cycle progression and influences this process by interacting with BRCA1.16 It has been related to growth defects, mental retardation and skeletal abnormalities as a result of defects in cell cycle progression.29 In human AVM tissue, endothelial cell proliferation was increased when compared to cortical vessels from patients without AVM.30 SOX-17 and RBBP8 are both expressed in normal artery tissue (http://www.gtexportal.org/home), but not differentially expressed in blood samples from unruptured AVM patients compared to healthy controls31 or in AVM tissue compared to normal artery, although no probe was available for SOX-17 in that study.32 How SOX-17or RBBP8 might affect AVM development remains as yet unclear but deserves further study.
A strength of our study is the relatively large sample size that we achieved by combining two populations. This study also has limitations. SNPs found to be associated with AVM in the American cohort were not replicated in the Dutch, possibly due to the smaller size of the Dutch cohort and different genotyping methods used in the two laboratories. Cases in the Dutch cohort were genotyped by KASPar® assay, while Dutch controls were gathered from multiple sources and genotyped on a different platform. American cases and controls were both genotyped on an Affymetrix Genome-Wide SNP 6.0 array. Genotyping on different platforms might have resulted in varying degrees of systematic error and, therefore, decreased precision. However, for all but one SNP, genotype call rates were >95%, all SNPs were in HWE equilibrium, and allele frequencies in controls were comparable in both cohorts and to control frequencies in public databases. It is also possible that there are population differences between Dutch and other Caucasians. Also, we could not perform the subgroup analysis for the effect of presence of AVM-related aneurysms in the Dutch cohort. Furthermore, the effect estimates of the putative risk loci in intracranial aneurysms are modest and therefore require even larger numbers of AVM patients to confirm a possible association with AVM. Although we were able to include a uniquely large cohort of patients with AVMs, an even larger sample may be needed, especially when choosing an unbiased, genome-wide approach.
In conclusion, in this largest genetic association analysis in sporadic AVM patients published to date, we did not detect an association for SNPs involved in intracranial aneurysms with sporadic brain AVM. Future studies containing larger patient populations are warranted for further identification of genetic risk factors for AVM development.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This study was funded by the Van Leersum Fund, Royal Netherlands Academy of Arts and Sciences, The Netherlands and by a grant from “Running-for-Nona”. C.J. M. Klijn is supported by a clinical established investigator grant from the Dutch Heart Foundation (2012T077).
Assembling of data for controls was supported by the Prinses Beatrix Fonds, VSB fonds, H. Kersten and M. Kersten (Kersten Foundation), The Netherlands ALS Foundation, J.R. van Dijk, and the Adessium Foundation (L.H.v.d.B.), the Netherlands Organization of Scientific Research NWO Investments (175.010.2005.011, 911-03-012), the Research Institute for Diseases in the Elderly (014-93-015; RIDE), and the Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO; 050-060-810). Y.M. Ruigrok was supported by a VENI-grant from NWO (91610016). The American GWAS studies were funded by National Institutes of Health grants: R01 NS034949 and P01 NS044155 for AVM cases, and P50 NS2372 and U19 AI52349 for shared control data.
Footnotes
CONFLICT-OF-INTEREST/DISCLOSURE
None.
CONTRIBUTORS
Drafted the article, analyzed and interpreted the data: PK and SW. Analyzed and interpreted the American data: NB, SW, LP and HK. Supervised the analyses and interpretation of Dutch and meta-analysis data: BK, LP, YR, HK and CK. Contributed to the design and conceptualization of the study: LP, GR, HK, CK. Acquired the data and set up the cohorts: HK, LP, JZ, SS, and CK. Acquired the data for Dutch controls and supervised the study: LB and JV. Approved the final version of the manuscript before submission: HK and CK. All authors acknowledge and agree on the content of the manuscript.
References
- 1.Stapf C, Labovitz DL, Sciacca RR, et al. Incidence of adult brain arteriovenous malformation hemorrhage in a prospective population-based stroke survey. Cerebrovasc Dis. 2002;13:43–46. doi: 10.1159/000047745. [DOI] [PubMed] [Google Scholar]
- 2.Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000;46:793–800. doi: 10.1097/00006123-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wedderburn CJ, van Beijnum J, Bhattacharya JJ, et al. Outcome after interventional or conservative management of unruptured brain arteriovenous malformations: a prospective, population-based cohort study. Lancet Neurol. 7:223–230. doi: 10.1016/S1474-4422(08)70026-7. 200. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Marchuk DA, Pawlikowska L, et al. Genetic considerations relevant to intracranial hemorrhage and brain arteriovenous malformations. Acta Neurochir Suppl. 2008;105:199–206. doi: 10.1007/978-3-211-09469-3_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leblanc GG, Golanov E, Awad IA, et al. Biology of vascular malformations of the brain. Stroke. 2009;40:694–702. doi: 10.1161/STROKEAHA.109.563692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon M, Franke D, Ludwig M, et al. Association of a polymorphism of the ACVRL1 gene with sporadic arteriovenous malformations of the central nervous system. J Neurosurg. 2006;104:945–9. doi: 10.3171/jns.2006.104.6.945. [DOI] [PubMed] [Google Scholar]
- 7.Sturiale CL, Puca A, Sebastiani P, et al. Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: where do we stand? Brain. 2013;136:665–81. doi: 10.1093/brain/aws180. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Gu Y, Wu W, et al. Polymorphisms of the vascular endothelial growth factor A gene and susceptibility to sporadic brain arteriovenous malformation in a Chinese population. J Clin Neurosci. 2011;18:549–553. doi: 10.1016/j.jocn.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Li P, Fan W, et al. The rs522616 polymorphism in the matrix metalloproteinase-3 (MMP-3) gene is associated with sporadic brain arteriovenous malformation in a Chinese population. J Clin Neurosci. 2010;17:1568–1572. doi: 10.1016/j.jocn.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Hysi PG, Pawlikowska L, et al. Common variants in interleukin-1-beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc Dis. 2009;27:176–182. doi: 10.1159/000185609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuno K, Bilguvar K, Bijlenga P, et al. Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat Genet. 2010;42:420–425. doi: 10.1038/ng.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 13.Leung TH, Ching YP, Yam JW, et al. Deleted in liver cancer 2 (DLC2) suppresses cell transformation by means of inhibition of RhoA activity. Proc Natl Acad Sci U S A. 2005;102:15207–15212. doi: 10.1073/pnas.0504501102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui T, Kanai-Azuma M, Hara K, et al. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto Y, Hara K, Kanai-Azuma M, et al. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem Biophys Res Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- 16.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goytain A, Quamme GA. Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol Genomics. 2005;22:382–9. doi: 10.1152/physiolgenomics.00058.2005. [DOI] [PubMed] [Google Scholar]
- 18.Sturiale CL, Gatto I, Puca A, et al. Association between the rs1333040 polymorphism on the chromosomal 9p21 locus and sporadic brain arteriovenous malformations. J Neurol Neurosurg Psychiatry. 2013;84:1059–62. doi: 10.1136/jnnp-2012-304045. [DOI] [PubMed] [Google Scholar]
- 19.Bendjilali N, Nelson J, Weinsheimer S, et al. Common variants on 9p21.3 are associated with brain arteriovenous malformations with accompanying arterial aneurysms. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2013-306461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Es MA, Veldink JH, Saris CG, et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet. 2009;41:1083–1087. doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
- 21.Kornum BR, Kawashima M, Faraco J, et al. Common variants in P2RY11 are associated with narcolepsy. Nat Genet. 2011;43:66–71. doi: 10.1038/ng.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilguvar K, Yasuno K, Niemela M, et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet. 2008;40:1472–1477. doi: 10.1038/ng.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Willer CJ, Ding J, et al. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Willer C, Sanna S, et al. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange AW, Keiser AR, Wells JM, et al. Sox17 promotes cell cycle progression and inhibits TGF-beta/Smad3 signaling to initiate progenitor cell behavior in the respiratory epithelium. PLoS One. 2009;4:5711. doi: 10.1371/journal.pone.0005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye YW, Wu JH, Wang CM, et al. Sox17 regulates proliferation and cell cycle during gastric cancer progression. Cancer Lett. 2011;307:124–31. doi: 10.1016/j.canlet.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Lee S, Lee S, et al. Sox17 promotes tumor angiogenesis and destabilizes tumor vessels in mice. J Clin Invest. 2013;123:418–31. doi: 10.1172/JCI64547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qvist P, Huertas P, Nimeno S, et al. CtIP mutations cause Seckel and Jawad syndromes. PLoS Genet. 2011;7(10):e1002310. doi: 10.1371/journal.pgen.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashimoto T, Mesa-Tejada R, Quick CM, et al. Evidence of increased endothelial cell turnover in brain arteriovenous malformations. Neurosurgery. 2001;49:124–31. doi: 10.1097/00006123-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Weinsheimer S, Xu H, Achrol AS, et al. Gene expression profiling of blood in brain arteriovenous malformation patients. Transl Stroke Res. 2011;2:575–587. doi: 10.1007/s12975-011-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto T, Lawton MT, Wen G, et al. Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery. 2004;54:410–423. doi: 10.1227/01.neu.0000103421.35266.71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


