Abstract
Acetylation of histone and non-histone proteins by histone acetyltransferases plays a pivotal role in the expression of pro-inflammatory genes. Given the importance of dietary selenium in mitigating inflammation, we hypothesized that selenium supplementation may regulate inflammatory gene expression at the epigenetic level. The effect of selenium towards histone acetylation was examined in both in vitro and in vivo models of inflammation by chromatin immunoprecipitation (ChIP) assays and immunoblotting. Our results indicated that selenium supplementation, as selenite, decreased acetylation of histone H4 at K12 and K16 in COX-2 and TNF promoters, and of the p65 subunit of the redox sensitive transcription factor NFκB in primary and immortalized macrophages. On the other hand, selenomethionine had a much weaker effect. Selenite treatment of HIV-1 infected human monocytes also significantly decreased the acetylation of H4 at K12 and K16 on the HIV-1 promoter, supporting the downregulation of proviral expression by selenium. A similar decrease in histone acetylation was also seen in the colonic extracts of mice treated with dextran sodium sulfate that correlated well with the levels of selenium in the diet. Bone marrow-derived macrophages from Trspfl/flCreLysM mice that lack expression of selenoproteins in macrophages confirmed the important role of selenoproteins in the inhibition of histone H4 acetylation. Our studies suggest that the ability of selenoproteins to skew the metabolism of arachidonic acid to contribute, in part, to their ability to inhibit histone acetylation. In summary, our studies suggest a new role for selenoproteins in the epigenetic modulation of pro-inflammatory genes.
Keywords: Selenium, p300, epigenetic regulation, inflammatory gene expression, cyclopentenone prostaglandins, selenoproteins
Introduction
Transcription, DNA repair, and replication are associated with changes in chromatin [1, 2]. The nucleosome, a basic unit of chromatin, is composed of dimers of histones H2A, H2B, H3, and H4 and 147 base pairs of DNA are wrapped around the histone core. The highly basic N-terminal tails of the histones are exposed on the surface of the nucleosome, and serve as the main sites for post-translational modifications. Among these, reversible acetylation of histones at lysine residues play a role in controlling the structure of the chromatin [3, 4], where highly acetylated histones make the chromatin more accessible to host factors, signifying active transcription. Histone acetyltransferases (HATs) are the class of enzymes that catalyze the acetylation of histones (and certain non-histone proteins), wherein they transfer the acetyl group from acetyl-CoA to the lysine-amino tails of histones and other proteins at specific lysine residues. Thus, HATs are also referred to as lysine acetyltransferases (KATs). Acetylation of histone H4 at K5, K8, K12 and K16 by HATs such as p300/CBP, Gcn5, PCAF, ATF2, Esa1, Tip60 is known to be involved in transcriptional activation [5–9].
Dysfunction of HATs and the associated acetylation events have been implicated in a wide variety of diseases like neurodegeneration, cancer, HIV-AIDS, and inflammation [10–13]. The association of HAT activity with initiation and progression of cancer has made p300, Gcn5, and PCAF, as attractive targets or for anticancer and anti-inflammatory therapies. Many of the inhibitors of these enzymes are peptide conjugates of CoA or natural products and their derivatives (reviewed in [14]). HAT activity is also essential for the initiation and elongation events during HIV transcription. Acetylation of HIV Tat (transactivator of transcription) by p300 is critical for the elongation of HIV transcripts [15–17]. Inhibition of p300 has been shown in literature to inhibit HIV replication [18].
Dietary selenium modulates epigenetic events like DNA methylation by inhibiting DNA methyltransferases (DNMTs) in a prostate cancer cell line [19]. It has also been suggested that alpha keto-derivatives of organic selenocompounds inhibit histone deacetylase (HDAC) activity in prostate and colon cancer cell lines [20, 21]. Together, HATs and HDACs maintain the balance between gene expression and repression in cells; with acetylation of histones leading to relaxation of the chromatin and active transcription, while deacetylation leads to gene repression and reestablishment of chromatin structure. Little is known about the effects of selenium on HAT activity in immune (non-transformed) cells.
Recently, Gandhi et al have reported a positive correlation between selenium (in the form of selenite) supplementation, and the expression of a critical enzyme in the prostaglandin (PG) biosynthesis pathway, hematopoietic prostaglandin D synthase (H-PGDS), in vitro and ex vivo in murine macrophages, culminating in an increased production of cyclopentenone PGs (CyPGs) [22]. This results in a shift in cyclooxygenase (COX)-mediated prostaglandin production from pro-inflammatory PGE2 to anti-inflammatory CyPGs, Δ12-PGJ2 and 15d-PGJ2 [22]. As a consequence of such a shunting of eicosanoids, supplementation with selenium polarizes macrophages towards alternatively activated (anti-inflammatory) phenotypes [23]. Previous studies from our laboratory have also shown that Cys1438 in the critical substrate-binding site of p300 HAT domain is a target for covalent modification by cyclopentenone prostaglandins (CyPGs), which results in the inhibition of the enzymatic activity of p300 [24]. Our laboratory has also shown that selenoprotein biosynthesis via the cotranslational insertion of Sec (from tRNA[Ser]Sec; Trsp), is essential for the anti-inflammatory effects of selenium, including CyPG production [22]. In the current study, we describe the ability of selenium, via the production of CyPGs and selenoproteins, to inhibit histone acetylation and regulate inflammatory gene expression in vitro in inflamed macrophages and a model of HIV infection, and in vivo in a murine model of dextran sulfate sodium (DSS)-induced inflammatory bowel disease.
Materials and Methods
Analysis of histone acetylation in macrophages
Murine macrophage-like RAW264.7 cells [cultured in DMEM (Invitrogen) containing 5 % FBS (ATCC, 7 nM selenium), 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin] were treated with 100 ng/ml LPS for 2 h, followed by incubation with increasing doses of selenium in the form of sodium selenite, selenomethionine (SeMet; Sigma-Aldrich) or 1, 4-phenylenebis(methylene)selenocyanate (p-XSC; provided by Dr. Shantu Amin, Penn State College of Medicine, Hershey, PA) for 72 h (as indicated), with or without indomethacin (indo; 10 μM, COX inhibitor; Cayman Chemicals) or HQL-79 (25 μM, H-PGDS inhibitor; Cayman Chemicals). Histones were isolated from these cells [24] and analyzed for their acetylation status using anti-H4 acetyl (K5/K8/K12/K16) antibodies (Active Motif). Histone H3 (anti-H3 C-terminal, Active Motif) was used as a control to normalize loading. Murine primary macrophages (bone marrow derived macrophages; BMDMs) isolated from mice (Trspfl/flCreLysM or wild-type litter-mates) maintained on selenium-deficient diets [22] were cultured in DMEM (Invitrogen) in above mentioned media with 10 % (v/v) L929 fibroblasts conditioned medium. Following treatment with the inhibitors (or vehicle as control) for 12 h, the BMDMs were stimulated with 10 ng/ml LPS for 2 h, after which they were cultured with sodium selenite at different concentrations for 72 h, with or without inhibitors. BMDMs were then treated with 100 ng/ml LPS for 12 h and harvested. Histones were isolated and analyzed as described above.
Analysis of histone acetylation in the colon of a DSS-induced murine colitis model
Selenium-deficient (<1 ppb selenium; Def), selenium-adequate (80 ppb as sodium selenite in diet; Ade) and selenium-supplemented (400 ppb; Sup) mice were treated with water containing 4 % (w/v) DSS for 5 days ad libitum. The mice were given regular water for the next 5 days. On the 10th day, the mice were sacrificed and their colons were isolated. Histones were isolated from these colons and analyzed for acetylation of H4K12 by immunoblotting.
Analysis of p65 acetylation
RAW264.7 macrophages were treated with 100 ng/ml LPS for 2 h, followed by incubation with different concentrations of selenium. These cells were harvested and the nuclear and cytoplasmic fractions were separated. The nuclear fractions were analyzed by immunoblotting for the acetylation status of p65 at K310. The blot was also probed for total p65 as control.
Chromatin immunoprecipitation (ChIP) assay
BMDMs and RAW264.7 cells were cultured with or without sodium selenite at indicated concentrations for 72 h, followed by stimulation with 100 ng/ml LPS for 2 h. These cells were harvested post-stimulation and used for crosslinking ChIP (X-ChIP). Briefly, the cells were treated with 1% formaldehyde for 10 m, followed by 125 mM glycine to terminate the crosslinking reaction. The cells were washed with ice-cold phosphate buffered saline (PBS), and harvested. Approximately 10 million cells were lysed with ChIP lysis buffer (50 mM Tris, pH 8.0, 10 mM EDTA, 1% SDS), and subjected to sonication (Diagenode Bioruptor; 30 s ON, 60 s OFF for 20 cycles). 5 μg of the sonicated chromatin, diluted to 1 ml with the ChIP dilution buffer (0.5% triton-X 100, 2 mM EDTA, 20 mM Tris, pH 8.0, 150 mM NaCl) was pre-treated with 5 μl of protein A/G agarose (Origene) for 30 m. The agarose beads were spun down by centrifugation at 1500g for 5 m at 4 °C. The chromatin was now incubated with antibodies to H4 acetyl (ac)-K12 or H4 ac-K16 (2 μl) and 5 μl of protein A/G agarose overnight at 4 °C. 5 μg of chromatin from the samples were also subjected to IP with an unrelated rabbit polyclonal antibody (IgG) as control. The beads were then washed with 300 μl of ChIP wash buffer (0.1% SDS, 1% triton-X 100, 2 mM EDTA, 20 mM Tris, pH 8.0, 150 mM NaCl) thrice, followed by 500 μl of Tris-EDTA buffer (1 mM Tris, pH 8.0, 0.1 mM EDTA) twice. The immunoprecipitated chromatin was eluted off the beads using the ChIP elution buffer (1% SDS, 0.1 M NaHCO3). The samples were subjected to reverse-crosslinking by incubating them with 300 mM NaCl, 0.5 μg of RNase A (Sigma), and 3 units of Proteinase K (New England Biosciences) per ChIP reaction, at 65 °C in a water-bath overnight. DNA was isolated and concentrated using the ChIP DNA clean and concentrator kit (Zymo Research) according to the manufacturer’s instructions. DNA was eluted in 50 μl of elution buffer. The immunoprecipitated DNA was then analyzed by qPCR using primers detecting the promoter regions of COX-2 (forward GAGCAGCGAGCACGTCA, reverse TCCAGTGGGGGCCTAAA) and TNFα (forward CACACACACCCTCCTGATTG, reverse TCGGTTTCTTCTCCATCGC) that encompass a proximal NF-κB response element. U1/HIV-1 cells were incubated without or with selenium (500 nM) for 3 days. These cells were stimulated with 20 ng/ml PMA for 6 h, harvested, and processed for ChIP assay as described above. The immunoprecipitated DNA was analyzed by qPCR with primers targeting the HIV LTR (forward CGAGAGCTGCATCCGGAGTA, reverse GAGGCTTAAGCAGTGGGTTCC). All ChIP data have been presented as fold change in the signal-to-input ratio, compared to control.
HDAC Assay
RAW264.7 macrophages were cultured in different concentrations of selenium (as sodium selenite) for 72 h. These cells were then stimulated with 100 ng/ml LPS for 2 h. The cells were then washed with complete medium thrice, and incubated with or without selenium for an additional 6 h. The cells were harvested and nuclear extracts were prepared. 20 μg of nuclear protein was used in the HDAC assay (performed as per manufacturer’s instructions, Millipore; catalog # 17-320). Briefly, biotin-conjugated histone H4 peptide was labeled with [3H]acetyl CoA using PCAF enzyme (supplied with kit). The radiolabeled peptide was immobilized on streptavidin-agarose beads. Ten thousand counts per minute (CPM) of immobilized peptide was incubated with the nuclear extract in HDAC assay buffer for 24 h at room temperature on an orbital shaker. The beads were pelleted and the supernatant was subjected to scintillation counting to assay the released [3H]acetate.
Statistics
All data have been represented as mean ± SEM of at least three independent experiments. Data have been analyzed by unpaired t test, one-way ANOVA or two-way ANOVA where appropriate, using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, California, USA. *, @, #, $ represent p < 0.05, p < 0.005, p < 0.0005, p < 0.0001, respectively.
Results
Selenium supplementation inhibits histone acetylation in macrophages in vitro
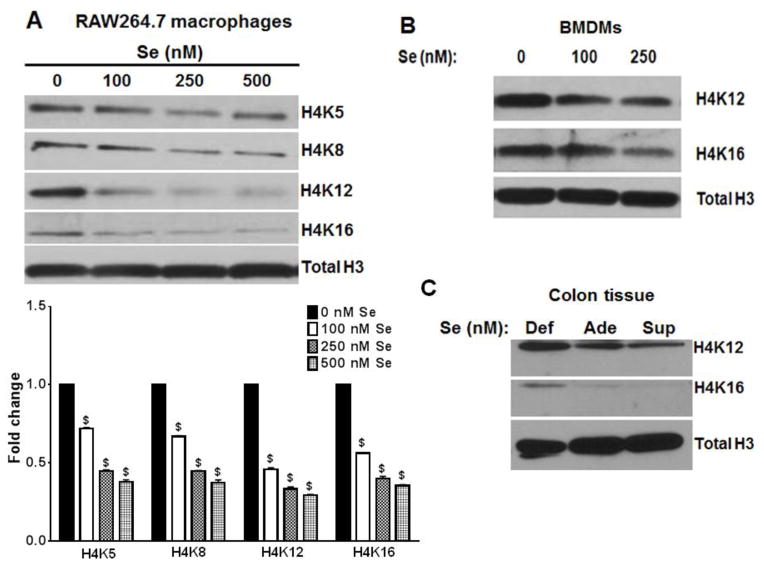
We tested the inhibition of HAT activity upon selenium supplementation in a murine macrophage model of LPS-induced inflammation. RAW264.7 macrophages were treated with LPS, followed by incubation with selenium for 72 h at the indicated concentrations. Histones were isolated from these cells and the acetylation status of histone H4 at positions K5, K8, K12 and K16 were analyzed by immunoblotting (Fig. 1A). Changes in histone H4 K12 acetylation in intact RAW264.7 macrophages treated with LPS was also examined by immunofluorescence (Fig. S1). As shown in Fig. 1A, acetylation of histone H4 in inflamed macrophages was reduced by ~70 % upon selenium supplementation (at 250 nM; as selenite). Primary murine BMDMs isolated from selenium-deficient mice were treated with 10 ng/ml LPS for 2 h and subsequently incubated with various concentrations of selenium for 72 h. The cells were treated with 100 ng/ml LPS for 12 h, and histones were isolated and analyzed for acetylation of histone H4 at positions K12 and K16 as described earlier. Western blot analysis of histones indicated a similar decrease in the acetylation of histone H4 at positions K12 and K16 upon supplementation with selenium, as in RAW264.7 macrophages (Fig. 1B). Immunofluorescence staining of LPS-treated RAW264.7 cultured with 250 nM selenium (as selenite) also showed a ~ 45 % decrease in acetylation of H4K12 compared to those cells cultured without any added selenium (Fig. S1).
FIGURE 1. Selenium supplementation of LPS-stimulated RAW264.7 cells inhibits histone acetylation.
A) (upper panel) RAW264.7 macrophages were treated with 100 ng/ml LPS for 2 h, followed by incubation with selenium (as sodium selenite) for 72 h. Histones were isolated from these cells, and analyzed by immunoblotting. Representative of n = 3 shown. (lower panel) The fold changes in relative (to loading control) densitometric values (compared to 0 nM Se as control for each target) have been plotted as mean ± SEM of three independent experiments. Data was analyzed by two-way ANOVA followed by Dunnett’s multiple comparisons test. $: p<0.0001 compared to the untreated group. B) Primary murine bone marrow-derived macrophages (BMDMs) were treated with 10 ng/ml LPS for 2 h, followed by incubation with selenium for 72 h. This was followed by treatment with 100 ng/ml LPs for 12 h. Histones were isolated and analyzed. Representative of n = 3 shown. C) Histones were isolated from the colonic samples of DSS-treated mice maintained on different selenium diets on day 10 post treatment. Representative of n = 3 shown.
DSS-treatment of selenium supplemented mice shows decreased histone acetylation in the colonic extracts
We further examined the effect of selenium supplementation on the acetylation status of H4K12 in inflamed murine colon tissue following oral DSS treatment as described [25]. It has been reported earlier that colitis induced by DSS treatment results in an increase in the acetylation of histone H4 in mice and rats [26]. Analysis of H4K12 acetylation in the colon tissue from these mice revealed that selenium supplementation decreased the acetylation status of histone H4 at position K12 when compared to selenium-deficient colons (Fig. 1C). This could contribute to a decrease in DSS-induced inflammation due to selenium supplementation (data not shown). Recent studies from our laboratory have indicated that alternatively activated macrophages (M2) play an important role in protecting the colon from DSS-induced inflammation, under selenium supplemented conditions [27].
Selenium supplementation affects histone acetylation at promoters of pro-inflammatory genes and HIV-1 provirus
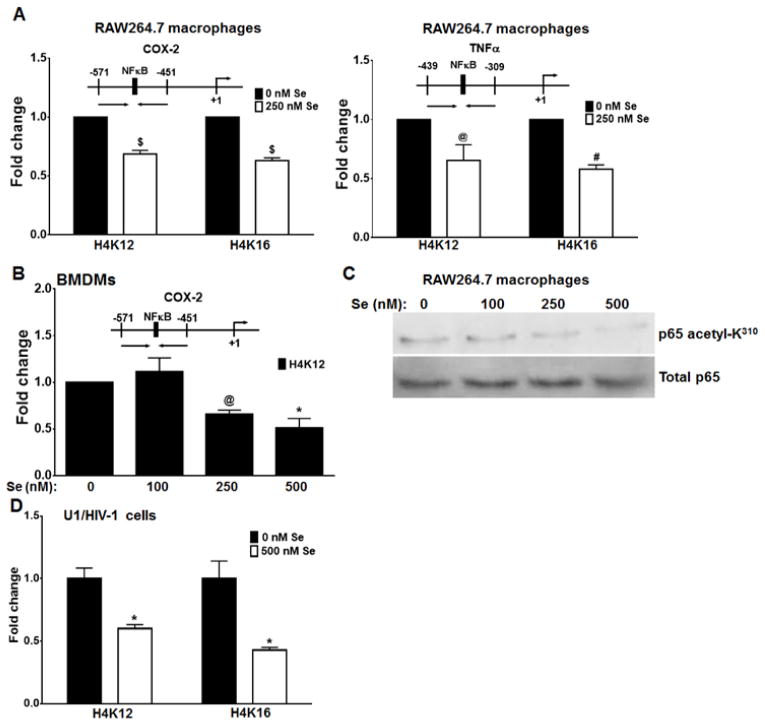
To support our long standing observations on the anti-inflammatory role of selenium [22, 23, 28], we examined the epigenetic regulation of pro-inflammatory gene expression in inflamed macrophages. The promoter regions of two prototypical pro-inflammatory genes, COX-2 and TNFα, were analyzed by ChIP for changes in histone acetylation status. To capture these gene-specific early acetylation events, RAW264.7 macrophages were incubated with, or without 250 nM of sodium selenite for 72 h, following which they were treated with 100 ng/ml LPS for 2 h. The cells were fixed and were subjected to a ChIP assay with anti-K12 acetyl H4 and anti-K16 acetyl H4 antibodies. As shown in Fig. 2A, compared to the selenium-deficient macrophages, LPS-stimulation of selenium-supplemented macrophages exhibited a statistically significant (30–40 %) decrease in the acetylation of H4K12 and H4K16 at the promoters of COX-2 and TNFα. Similarly, we also analyzed inflamed primary murine BMDMs (treated as described earlier) supplemented with different concentrations of selenium, for changes in the acetylation status of H4K12 at the promoter of COX-2. As represented in Fig. 2B, selenium supplementation of inflamed primary macrophages decreased H4K12 acetylation by ~50 % when compared to selenium-deficient macrophages and the decrease was dose-dependent. This data supports previous data from our lab showing a decrease in proinflammatory gene expression in selenium supplemented macrophages stimulated with LPS [29]. Furthermore, to explain the possible mechanism of selenium-dependent modulation of inflammation, we tested the acetylation of the NFκB subunit p65 (RelA) at position K310. Previous reports have shown that p65 is acetylated at K310 by p300/CBP, and that this modification is required for the full transcriptional activity of NFκB [30]. Acetylation of K310 impairs the methylation of residues K314 and K315, which are required for ubiquitination and degradation of p65 [31]. Nuclear extracts from LPS-treated RAW264.7 macrophages, cultured with or without selenium, were analyzed by immunoblotting and probed with an antibody to acetylated p65. Selenium supplementation of inflamed RAW cells inhibited the acetylation of p65 at K310 (Fig. 2C).
FIGURE 2. Epigenetic regulation of pro-inflammatory gene expression by selenium.
A) RAW264.7 macrophages were maintained with or without selenium for 3 days. The cells were treated with 100 ng/ml LPS for 2 h. These were fixed with formaldehyde, lysed, and sonicated to fragment the chromatin. The chromatin was then subjected to immunoprecipitation with antibodies to acetylated histone H4 at positions K12 and K16. The immunoprecipitated chromatin was then analyzed by qPCR using primers (shown with arrows) for the promoter regions of the Ptgs2 (COX-2; left) and Tnfa (TNFα; right). Data analyzed by two-way ANOVA followed by multiple comparisons test. $ and # represent p<0.0001 and p<0.0005, respectively, compared to the untreated group. B) Primary murine BMDMs (cultured with or without indomethacin) were treated with LPS as mentioned earlier, and incubated with different concentrations of selenium. The cells were harvested for ChIP assay to analyze the acetylation status of H4K12 at the COX-2 gene promoter. Data analyzed by unpaired t test, compared to 0 nM Se. $ and * represent p<0.0001 and p<0.05, respectively, compared to the untreated group. C) RAW264.7 macrophages were treated with 100 ng/ml LPS for 2 h, followed by incubation with selenium for 72 h. Nuclear extracts from these cells were analyzed for the acetylation of p65 K310. Total p65 expression was used as control. D) U1/HIV-1 cells, cultured with or without 500 nM selenium, were stimulated with 20 ng/ml PMA for 6 h. Post-stimulation, the cells were harvested for ChIP assay to examine the acetylation status of the HIV-LTR at 4K12 and H4K16. Data (mean ± SEM) was analyzed by t test. *: p<0.05 compared to the untreated group.
We also tested our hypothesis in a model of HIV infection in human macrophages that has been previously reported by our laboratory to be responsive to cellular selenium status [32]. Human U1/HIV-1 cells were incubated with 500 nM selenium (as selenite) for 72 h. The cells were stimulated with PMA for 6 h to initiate transcription and production of HIV virus. Cells were harvested post PMA treatment and processed for ChIP assay as described earlier. The immunoprecipitated DNA was examined by qPCR for acetylation of histone H4 at the HIV-LTR. Selenium supplementation significantly inhibited acetylation of H4K12 and K16 at the HIV promoter by ~50 % (Fig. 2D), suggesting the selenium-dependent epigenetic regulation may be a key factor in impairing proviral expression.
Selenium supplementation does not affect HDAC activity
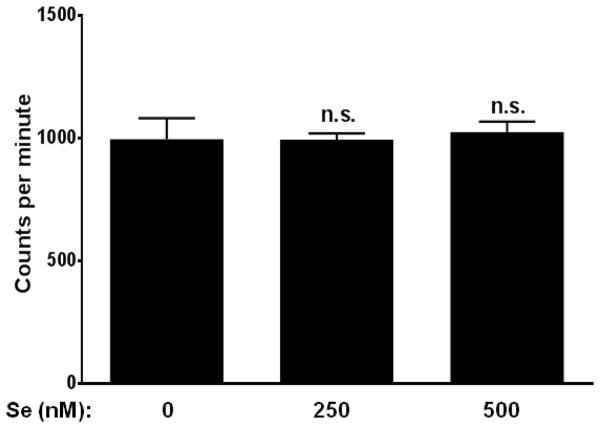
To determine if the reduced acetylation of histones was due to an inhibition in HAT activity or an upregulation in HDAC activity, we tested whether selenium supplementation affects HDAC activity in our model. RAW264.7 macrophages were cultured in different concentrations of selenium and treated with LPS for 2 h. The LPS was removed by changing the culture medium, and the cells were incubated in fresh medium for 6 h. The cells were harvested and the nuclear extract from these cells were used to perform the HDAC assay. Selenium supplementation at 250 nM and 500 nM (as inorganic sodium selenite) had no effect on the HDAC activity in LPS-treated macrophages (Fig. 3).
FIGURE 3. Selenium supplementation does not affect HDAC activity of LPS-stimulated RAW264.7 macrophages.
RAW264.7 macrophages were cultured in different concentrations of selenium for 72 h, and then treated with 100 ng/ml LPS for 2 h. LPS was removed and the cells were incubated for h. Nuclear extracts from these cells were analyzed for HDAC activity. Data analyzed by ordinary one-way ANOVA followed by Dunnett’s multiple comparisons test. n.s., No significant changes were observed.
Selenium supplementation may inhibit the activity of p300 HAT via production of CyPGs
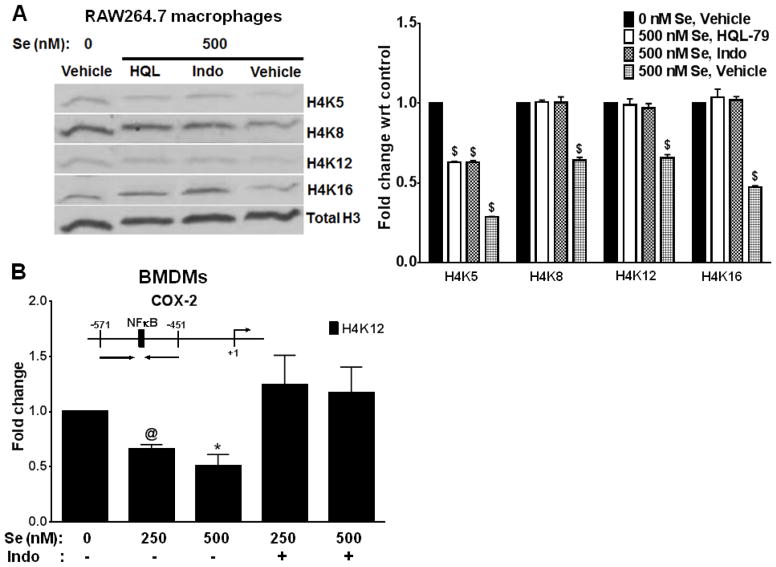
Previous studies from our laboratory have shown that selenium supplementation leads to the preferential upregulation of anti-inflammatory CyPGs in inflamed macrophages [22]. To examine if selenium-dependent shunting of the AA-COX pathway towards production of CyPGs of the J2 series had any effect on the HAT activity, LPS-treated RAW264.7 cells, and primary murine BMDMs, were cultured with selenium (as selenite) in the presence of indomethacin (COX inhibitor) or HQL-79 (H-PGDS inhibitor). Histones were analyzed by immunoblotting to examine histone acetylation (Fig. 4A). BMDMs were also subjected to ChIP assay to examine the acetylation status of H4K12 on the COX-2 promoter (Fig. 4B). The data suggest that inhibition of the AA – COX – H-PGDS pathway of arachidonic acid metabolism in macrophages reverses the inhibition of HAT activity afforded by selenium in these cells, indicating that the inhibitory effect may be dependent on the enhanced production of endogenous CyPGs.
FIGURE 4. Selenium supplementation may inhibit the activity of p300 HAT via production of CyPGs.
A) RAW264.7 macrophages were incubated with inhibitors prior to treatment with 100 ng/ml LPS for 2 h. The cells were washed to remove LPS and then incubated with selenium in the presence of the inhibitors for 72 h. Histones were isolated from these cells, and analyzed by immunoblotting. Representative of n = 3 shown. Densitometry data (right) is shown as mean ± SEM of three independent experiments. Data analyzed by two-way ANOVA followed by Dunnett’s multiple comparisons test. $: p<0.0001 compared to the untreated group. B) Primary murine BMDMs (cultured with or without indomethacin) were treated with LPS as mentioned earlier, and incubated with different concentrations of selenium. The cells were harvested for ChIP assay to analyze the acetylation status of H4K12 at the COX-2 promoter. Data (mean ± SEM) was analyzed by unpaired t test. @ and * represent p<0.0005 and p<0.05, respectively, compared to the untreated group.
Selenoproteins are essential for the inhibitory activity of selenium
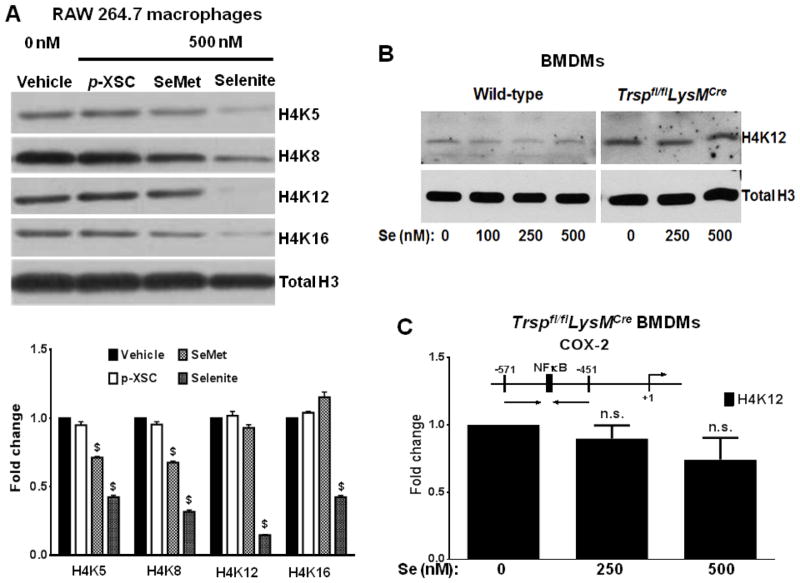
To test the role of selenoproteins in the selenium-dependent inhibition of histone acetylation, selenium-deficient RAW264.7 macrophages were cultured in the presence of 500 nM of sodium selenite, SeMet, or p-XSC for 72 h. p-XSC is an organic, non-bioavailable source of selenium in in-vitro cultures. Upon analysis of histone acetylation by western blotting, we observed a marked difference in the inhibition of histone acetylation in inflamed cells treated with the non-bioavailable form of selenium, when compared to that from cells treated with selenite or SeMet (Fig. 5A). Compared to selenite, SeMet had a much weaker effect. This suggested that the incorporation of selenium into selenoproteins may be essential for the inhibitory effect of selenium on histone acetylation. To further support this paradigm, we used BMDMs from Trspfl/flCreLysM mice that show a complete lack of selenoprotein expression when cultured ex vivo with selenium. Treatment of Trsp knockout BMDMs with LPS followed by supplementation with selenium did not lead to modulation of histone acetylation as seen by immunoblotting (Fig 5B), or ChIP assay (Fig. 5C) when compared to BMDMs from wild-type mice. Taken together, our data strongly suggests that selenoprotein expression plays an important role in the selenium-dependent inhibition of histone acetylation.
FIGURE 5. Selenium bioavailability is important for its inhibitory effect on acetylation.
A) RAW cells were treated with 100 ng/ml LPS for 2 h, followed by incubation with different forms of selenium for 72 h. Histones from these cells were analyzed for acetylation by immunoblotting. Representative of n = 3 shown. The densitometric values (lower) have been plotted as mean ± SEM of three independent experiments. Data was analyzed by two-way ANOVA followed by Dunnett’s multiple comparisons test. $: p<0.0001 compared to the untreated group. B) Wild-type and Trspfl/flCreLysM BMDMs were treated as mentioned earlier. Histones were isolated and analyzed for acetylation of H4K12 by immunoblotting. Representative of n = 3 shown. C) Trspfl/flCreLysM BMDMs were treated with LPS and selenium as mentioned. The cells were harvested for ChIP assay to analyze the acetylation status of H4K12 at the COX-2 promoter. Data analyzed by unpaired t test, compared to 0 nM Se. n.s., No significant changes were observed.
Discussion
Dietary selenium is known to regulate epigenetic events by modulating the activity of DNA and histone modifying enzymes. Inorganic selenium inhibits the activities of HDAC and DNMT1 (inhibiting DNA methylation) in a prostate cancer cell line at relatively higher concentrations leading to the reactivation of GSTP1 expression by de-repressing the promoter [19]. Organic forms of selenium like methylselenocysteine and SeMet can be converted to β-methylselenopyruvate and α-keto-γ-methylselenobutyrate, respectively, to inhibit HDAC activity in human colon and prostate cancer cell lines [20, 21]. To our knowledge, there is no report on the epigenetic regulation of inflammation in immune cells, such as macrophages, by selenium. Our studies, for the first time, show that selenium supplementation inhibits HAT activity in chronically HIV-infected human monocytes as well as inflamed murine macrophages stimulated with bacterial endotoxin LPS.
Our data demonstrating the ability of selenium supplementation to decrease acetylation of p65 (RelA) at K310 further suggests that the inhibition of histone acetylation may be, in part, due to the inhibition of p300 HAT activity. It should be noted that p65 at K310 is a target for acetylation by p300/CBP and such a post-translational modification (PTM) is essential for increasing the transcriptional activity of p65 [30]. Acetylation of p65 at K310 also results in the inhibition of methylation at residues K314 and K315, which are important in the degradation of p65 [31]. Thus, selenium-dependent inhibition of acetylation of p65 (K310) very likely contributes to the inhibition of its transcriptional activity, shown in earlier reports from our laboratory [28, 33]. In addition, HAT activity of p300 is also known to be essential for HIV replication, where it acetylates the Lys50 in the transactivating protein Tat. Such a post-translational modification allows Tat to be released from the transactivation complex to be recycled into the “active” pool of Tat in infected cells [15–17]. p300 also acetylates histones in the nucleosome at the nuc-0 and nuc-1 position on the HIV promoter, thus de-repressing the promoter and allowing access to other transcription factors [34]. Thus, selenium-dependent inhibition of acetylation of the HIV-1 promoter coupled with decreased functionally active NFκB (p65) could contribute to the selenium-dependent inhibition of proviral transcription and replication, as reported earlier from our laboratory [32].
While selenite can be reduced to hydrogen selenide to be used in the biosynthesis of cellular selenoproteins, p-XSC cannot be readily processed by cells in culture, particularly macrophages, which do not express methioninase-γ-lyase. Given that cells incubated with p-XSC do not show a significant increase in selenoprotein biosynthesis [22] when compared to more bioavailable forms, we hypothesize that selenoprotein activity is necessary for the inhibitory effect of selenium supplementation on histone acetylation. It is not surprising that these results corroborate with the histone acetylation pattern seen in LPS-treated BMDMs from Trspfl/flCreLysM mice. Selenoprotein biosynthesis in Trspfl/flCreLysM mice is abrogated due to the downregulation in expression of tRNA[Ser]Sec gene (Trsp), due to which selenocysteine can no longer be incorporated into the selenoproteins. Taken together, these studies strongly suggest that the beneficial effects of selenium are mediated through its incorporation into selenoproteins. It remains to be seen if the activity is restricted to a few selected selenoproteins or the selenoproteome in general.
Our current studies suggest that the inhibitory effect of selenium on HAT activity may, in part, be dependent on the production of Δ12-PGJ2. Inhibition of either COX-2 or H-PGDS activity in macrophages led to a reversal in the acetylation status in selenium-supplemented macrophages. We have previously established that Δ12-PGJ2 inhibits p300 HAT activity by covalently modifying a key Cys residue, Cys1438, in the active site of the enzyme [24]. Earlier studies from our lab have suggested that selenoprotein expression is essential for the production of CyPGs [22]. Taken together, our data suggest that the observed inhibition of HAT activity in these cells may be due to the selenium-dependent upregulation in production of CyPGs. In the current studies we were unsuccessful in demonstrating a covalent Michael adduct of p300 with endogenously produced Δ12-PGJ2 due to technical limitations. However, based on our studies with indomethacin and HQL-79 treatment of macrophages that inhibit the selenium-dependent endogenous production of Δ12-PGJ2, it is likely that sufficient localized concentrations of Δ12-PGJ2 may be generated in sufficient amounts to effectively inhibit p300 activity. Alternatively, it is possible that Δ12-PGJ2 may target upstream signaling pathways, including activation of PPARγ, to negatively modulate the activity of p300 through other PTMs. An additional mechanism of activation of p300 is its phosphorylation at residue Ser1834, which is important for its HAT and transcriptional activity, by the PI3K/Akt pathway [35], which in turn is inhibited by selenium supplementation in prostate cancer cells [36]. It remains to be seen if selenium supplementation can inhibit p300 activity, and possibly other HATs, and regulate inflammatory gene expression through the above pathways in immune and other non-cancerous cells.
In the in vivo studies reported here, we have used sodium selenite as the primary source of selenium. Our in vitro studies with macrophages supplemented with SeMet have only shown modest decreases in acetylation of histone H4 at position K5 and K8, but not at positions K12 and K16, at the concentrations tested. Moreover given the controversy with SeMet usage, as in the Selenium and Vitamin E Cancer Trial (SELECT), where participants were supplemented with 200 μg of selenium (in the form of SeMet) per day [37], we have restricted ourselves to inorganic sodium selenite as a highly bioavailable form of selenium.
In conclusion, we propose that the micronutrient selenium inhibits the activity of p300 HAT in macrophages by a unique mechanism requiring the expression of selenoproteins, where the AA-COX pathway plays a central role. We believe that these studies open a new area in the field of selenoprotein regulation of gene expression, particularly in inflammation and HIV biology, where fatty acid metabolites may serve as key mediators.
Supplementary Material
Highlights.
Selenium supplementation of macrophages decreased acetylation of histone H4
Histone acetylation of many pro-inflammatory gene promoters decreased by selenite
Selenoproteins in macrophages are key for the inhibition of histone H4 acetylation
Eicosanoid metabolism by selenoproteins drive inhibition of histone acetylation
Acknowledgments
Funding Sources: National Institutes of Health PHS grants DK 077152 and CA 162665
We thank Dr. Dolph Hatfield (National Cancer Institute, NIH) for the generous donation of Trspfl/flCreLysM mice and their corresponding wild-type littermates, Dr. Shantu Amin (Penn State College of Medicine) for providing pXSC, and Dr. Ramesh Ramachandran (Penn State University) for help with fluorescence microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Attikum H, Gasser SM. The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol. 2005;6:757–65. doi: 10.1038/nrm1737. [DOI] [PubMed] [Google Scholar]
- 2.Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- 3.Grant PA, Berger SL. Histone acetyltransferase complexes. Semin Cell Dev Biol. 1999;10:169–77. doi: 10.1006/scdb.1999.0298. [DOI] [PubMed] [Google Scholar]
- 4.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Clarke AS, Lowell JE, Jacobson SJ, Pillus L. Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol Cell Biol. 1999;19:2515–26. doi: 10.1128/mcb.19.4.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawasaki H, Schiltz L, Chiu R, Itakura K, Taira K, Nakatani Y, et al. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature. 2000;405:195–200. doi: 10.1038/35012097. [DOI] [PubMed] [Google Scholar]
- 7.Kimura A, Horikoshi M. Tip60 acetylates six lysines of a specific class in core histones in vitro. Genes Cells. 1998;3:789–800. doi: 10.1046/j.1365-2443.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 8.Kuo MH, Brownell JE, Sobel RE, Ranalli TA, Cook RG, Edmondson DG, et al. Transcription-linked acetylation by Gcn5p of histones H3 and H4 at specific lysines. Nature. 1996;383:269–72. doi: 10.1038/383269a0. [DOI] [PubMed] [Google Scholar]
- 9.Schiltz RL, Mizzen CA, Vassilev A, Cook RG, Allis CD, Nakatani Y. Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. J Biol Chem. 1999;274:1189–92. doi: 10.1074/jbc.274.3.1189. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25:552–63. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- 11.Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225–31. doi: 10.1038/sj.onc.1207118. [DOI] [PubMed] [Google Scholar]
- 12.McKinsey TA, Olson EN. Cardiac histone acetylation--therapeutic opportunities abound. Trends Genet. 2004;20:206–13. doi: 10.1016/j.tig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Pumfery A, Deng L, Maddukuri A, de la Fuente C, Li H, Wade JD, et al. Chromatin remodeling and modification during HIV-1 Tat-activated transcription. Curr HIV Res. 2003;1:343–62. doi: 10.2174/1570162033485186. [DOI] [PubMed] [Google Scholar]
- 14.Furdas SD, Kannan S, Sippl W, Jung M. Small molecule inhibitors of histone acetyltransferases as epigenetic tools and drug candidates. Arch Pharm (Weinheim) 2012;345:7–21. doi: 10.1002/ardp.201100209. [DOI] [PubMed] [Google Scholar]
- 15.Kiernan RE, Vanhulle C, Schiltz L, Adam E, Xiao H, Maudoux F, et al. HIV-1 tat transcriptional activity is regulated by acetylation. EMBO J. 1999;18:6106–18. doi: 10.1093/emboj/18.21.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott M, Geyer M, Zhou Q. The control of HIV transcription: keeping RNA polymerase II on track. Cell host & microbe. 2011;10:426–35. doi: 10.1016/j.chom.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott M, Schnolzer M, Garnica J, Fischle W, Emiliani S, Rackwitz HR, et al. Acetylation of the HIV-1 Tat protein by p300 is important for its transcriptional activity. Curr Biol. 1999;9:1489–92. doi: 10.1016/s0960-9822(00)80120-7. [DOI] [PubMed] [Google Scholar]
- 18.Mantelingu K, Reddy BA, Swaminathan V, Kishore AH, Siddappa NB, Kumar GV, et al. Specific inhibition of p300-HAT alters global gene expression and represses HIV replication. Chem Biol. 2007;14:645–57. doi: 10.1016/j.chembiol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Xiang N, Zhao R, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29:2175–81. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JI, Nian H, Cooper AJ, Sinha R, Dai J, Bisson WH, et al. Alpha-keto acid metabolites of naturally occurring organoselenium compounds as inhibitors of histone deacetylase in human prostate cancer cells. Cancer prevention research. 2009;2:683–93. doi: 10.1158/1940-6207.CAPR-09-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nian H, Bisson WH, Dashwood WM, Pinto JT, Dashwood RH. Alpha-keto acid metabolites of organoselenium compounds inhibit histone deacetylase activity in human colon cancer cells. Carcinogenesis. 2009;30:1416–23. doi: 10.1093/carcin/bgp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi UH, Kaushal N, Ravindra KC, Hegde S, Nelson SM, Narayan V, et al. Selenoprotein-dependent Up-regulation of Hematopoietic Prostaglandin D2 Synthase in Macrophages Is Mediated through the Activation of Peroxisome Proliferator-activated Receptor (PPAR) {gamma} J Biol Chem. 2011;286:27471–82. doi: 10.1074/jbc.M111.260547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson SM, Lei X, Prabhu KS. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J Nutr. 2011;141:1754–61. doi: 10.3945/jn.111.141176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravindra KC, Narayan V, Lushington GH, Peterson BR, Prabhu KS. Targeting of histone acetyltransferase p300 by cyclopentenone prostaglandin Delta(12)-PGJ(2) through covalent binding to Cys(1438) Chem Res Toxicol. 2012;25:337–47. doi: 10.1021/tx200383c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679–86. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsaprouni LG, Ito K, Powell JJ, Adcock IM, Punchard N. Differential patterns of histone acetylation in inflammatory bowel diseases. J Inflamm (Lond) 2011;8:1. doi: 10.1186/1476-9255-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaushal N, Kudva AK, Patterson AD, Chiaro C, Kennet MJ, Desai D, et al. Crucial role of macrophage selenoproteins in experimental colitis. J Immunol. 2014;193 doi: 10.4049/jimmunol.1400347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vunta H, Davis F, Palempalli UD, Bhat D, Arner RJ, Thompson JT, et al. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J Biol Chem. 2007;282:17964–73. doi: 10.1074/jbc.M703075200. [DOI] [PubMed] [Google Scholar]
- 29.Zamamiri-Davis F, Lu Y, Thompson JT, Prabhu KS, Reddy PV, Sordillo LM, et al. Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radic Biol Med. 2002;32:890–7. doi: 10.1016/s0891-5849(02)00775-x. [DOI] [PubMed] [Google Scholar]
- 30.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–48. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang XD, Tajkhorshid E, Chen LF. Functional interplay between acetylation and methylation of the RelA subunit of NF-kappaB. Mol Cell Biol. 2010;30:2170–80. doi: 10.1128/MCB.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalantari P, Narayan V, Natarajan SK, Muralidhar K, Gandhi UH, Vunta H, et al. Thioredoxin reductase-1 negatively regulates HIV-1 transactivating protein Tat-dependent transcription in human macrophages. J Biol Chem. 2008;283:33183–90. doi: 10.1074/jbc.M807403200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhu KS, Zamamiri-Davis F, Stewart JB, Thompson JT, Sordillo LM, Reddy CC. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: role of nuclear factor-kappaB in up-regulation. Biochem J. 2002;366:203–9. doi: 10.1042/BJ20020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–20. [PMC free article] [PubMed] [Google Scholar]
- 35.Huang WC, Chen CC. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell Biol. 2005;25:6592–602. doi: 10.1128/MCB.25.15.6592-6602.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Zu K, Warren MA, Wallace PK, Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–52. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- 37.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.