Abstract
The purpose of this study was to examine opioid and endocannabinoid mechanisms of exercise-induced hypoalgesia (EIH). Fifty-eight men and women (mean age = 21 yrs) completed three sessions. During the first session, participants were familiarized with the temporal summation of heat pain and pressure pain protocols. In the exercise sessions, following double-blind administration of either an opioid antagonist (50 mg naltrexone) or placebo, participants rated the intensity of heat pulses and indicated their pressure pain thresholds (PPT) and ratings (PPR) before and after 3 minutes of submaximal isometric exercise. Blood was drawn before and after exercise. Results indicated circulating concentrations of two endocannabinoids, N-arachidonylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) as well as related lipids oleoylethanolamide (OEA), palmitoylethanolamide (PEA), N-docsahexaenoylethanolamine (DHEA), and 2-oleoylglycerol (2-OG) increased significantly (p < 0.05) following exercise. PPT increased significantly (p < 0.05) while PPR decreased significantly (p < 0.05) following exercise. Also, temporal summation ratings were significantly lower (p < 0.05) following exercise. These changes in pain responses did not differ between placebo or naltrexone conditions (p > 0.05). A significant association was found between EIH and DHEA. These results suggest involvement of a non-opioid mechanism in EIH following isometric exercise.
Keywords: pain, analgesia, opioids, endocannabinoids
INTRODUCTION
One form of endogenous pain modulation is exercise-induced hypoalgesia (EIH). A number of investigators have reported an attenuation of pain following single episodes of exercise in healthy young adults. Various modes of exercise have been studied including aerobic exercise (e.g., running, cycling), resistance exercise (e.g., lifting weights), and isometric exercise (e.g., static muscle contractions), and several review articles have been published summarizing this research.5,42,43,53 EIH has been characterized by elevations in pain thresholds and tolerances, as well as reductions in pain intensity ratings during and following exercise. Currently, the mechanisms responsible for EIH are poorly understood. Results from the animal research indicate there are multiple analgesia mechanisms including opioid and non-opioid systems that contribute to changes in pain sensitivity resulting from exercise.19,31,44,45,49,69
The most commonly tested hypothesis for EIH is that exercise induces a release of endogenous opioids at either peripheral, spinal, and/or central sites: all of which contribute to pain modulation.29,73 Muscle contractions activate Group III (A-delta) and IV (C) primary afferents in skeletal muscle, and stimulation of these fibers can activate the endogenous opioid system.73 Elevations in peripheral blood beta-endorphin concentrations have been reported in men following exercise,35,73 and it has been suggested that the stimulation of peripheral afferent neurons modulate pain by activating spinal or supraspinal inhibitory mechanisms.63 A number of studies have been conducted in which an opioid antagonist (naltrexone or naloxone) was administered before exercise in both humans and animals. In the human studies, results are equivocal while in the animal studies, opioid antagonists have been found to attenuate the hypoalgesic response following mild exercise (e.g., swimming in warm water) but have not had a consistent effect on hypoalgesia following more severe exercise (e.g., swimming in cold water).5,42 These results indicate that EIH is mediated, in part, by the endogenous opioid system. However, hypoalgesia that is insensitive to opioid antagonists can also occur, which provides evidence for naloxone-insensitive, thus, non-opioid hypoalgesia.
Endocannabinoid-mediated mechanisms have been suggested as an alternative mechanism for EIH.8 The presence of cannabinoid (CB) receptors in the pain processing areas of the brain and spinal cord 23,74 suggests that endocannabinoids, N-arachidonylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), may contribute to the control of pain transmission within the central nervous system through activation of CB1 cannabinoid receptors.31,78 Indeed, considerable data in preclinical and human studies support the hypothesis that exogenous activation of CB receptors produces antinociception.55 In further support of this hypothesis, several investigators have found significant elevations in circulating concentrations of AEA following exercise.11,24,56,57,64 Thus, like the endogenous opioid peptides, endocannabinoids are increased in the circulation during exercise, and CB1 receptor activation produces analgesia. The purpose of this study was to examine opioid and endocannabinoid mechanisms of EIH in men and women.
MATERIALS AND METHODS
Participants
All procedures were approved by the University of Wisconsin Health Sciences Institutional Review Board and informed consent was obtained from each participant prior to data collection. A power analysis was performed to estimate an optimal sample size for detecting a potential difference between men and women in the effects of naltrexone on EIH using a repeated measures design, with an alpha of 0.05, a power of 0.80, and a medium effect of 0.50.39,76 Results from the analysis indicated that 44 participants (22 women and 22 men) would be needed for the study; however, sample size was increased in anticipation of potential subject attrition. Sixty healthy adults (30 women, 30 men) between the ages of 18–40 yrs without a history of major medical problems or routine use of medications were recruited for this study. Participants were required to be free from clinical pain and the use of pain medications. Participants were asked to refrain from caffeine, nicotine, alcohol, and vigorous exercise for at least four hours before arrival to the laboratory and also be free from analgesic medication use for 24 hours prior to testing. Upon completion of the study, subjects were paid $100 for their participation in this research.
Methods and Procedures
A double-blind, placebo-controlled crossover design employing an opioid blockade methodology was utilized. The order of drug administration and placebo was randomized and counterbalanced. The sessions were conducted on separate days in the morning to minimize potential circadian changes in experimental variables. The familiarization session and the two experimental sessions were conducted in the Clinical Research Unit (CRU) located in the University of Wisconsin hospital. To control for potential menstrual cycle effects, the two experimental testing sessions were conducted during the follicular phase of each woman’s menstrual cycle (i.e., days 4–10 after the onset of menses). Carefully scripted instructional sets were used to minimize potential behavioral artifacts (e.g., demand characteristics).
Pain Testing
The testing consisted of inducing pain using pressure and thermal stimuli. Different peripheral and central nociceptive pathways can be evoked by varying noxious stimulation parameters, and it has been suggested that different mechanisms underlie analgesic responses for various types of noxious stimulation.12,18 Pain thresholds were assessed during pressure stimulation by asking participants to indicate when the stimulus was first perceived to be painful by pressing a button attached to a timer out of view of the participant. Pain ratings for pressure and thermal stimuli were obtained using a standard numerical scale.66,77 The scale ranges from 0–100 in increments of 5 with the following verbal descriptors: 20 = “barely painful,” 30 = “very weak pain,” 40 = “weak pain,” 50 = “moderate pain,” 60 = “slightly strong pain,” 70 = “strong pain,” 80 = “very strong pain,” 90 = “nearly intolerable pain,” and 100 = “intolerable pain.”
Pressure stimulus
A pressure stimulus (the force of a 3000 gram mass) was applied to the forefinger of the dominant hand for a maximum of two minutes using a Forgione-Barber Pressure Stimulator.13 Previous research has indicated that this procedure produces a painful sensation but does not cause tissue damage or injury.51 In addition, findings from previous controlled experiments with the pressure device showed no significant changes in pain perception across multiple trials during control conditions but moderate to large changes across trials following exercise.37,38
Thermal stimulus
A thermal stimulus was applied to the thenar eminence of the dominant hand using a computer controlled Medoc sensory analyzer (CHEPS model). The protocol involved administration of brief, repetitive suprathreshold heat pulses to evoke Type IV (C-fiber) pain summation (i.e., temporal summation). Temporal summation of second pain can be evoked by repetitive heat pulses to the skin at frequencies of 0.33 Hz or more. This phenomenon is dependent on activation of peripheral Type IV nociceptors resulting in wind-up of Type IV fiber evoked discharges of dorsal horn neurons.67 A thermode was programmed to deliver pulses that rapidly rose from an adapting temperature to a peak temperature of 51°C at a rate of 30°C/sec, remained at this level for 0.5 sec, and then returned to baseline at a rate of 30°C/sec. Ten heat pulses were delivered to the thenar eminence of the hand. Briefly, the temperature of the thermode increased during the first four stimuli from 35°C (baseline) to 45°C (peak), 36°C to 47°C, 37°C to 48°C, 38°C to 49°C, while the baseline and peak temperatures of the 6 remaining pulses were 38°C and 51°C. All participants were trained to rate the late pain sensations evoked by repetitive thermal stimuli applied to the surface of the hand during a familiarization session described below. Before application of repetitive heat pulses, participants were told that they may or may not feel sensations of pain during the heat pulses and to attend to a possible late sensation of pain beginning 1–2 seconds after each pulse. They were instructed to provide numerical ratings (0–100 scale) of the magnitude of these late sensations of pain which could increase or decrease with stimulus repetitions. Pilot testing using this protocol revealed that the first pulse was rated as non-painful (mean rating = 10) while the last pulse in a train of 10 pulses was rated as painful (mean rating = 40) and was significantly more painful than the fifth pulse indicating that this was a sufficient protocol to evoke temporal summation of heat pain. Also, previous pilot research employing a control condition in which participants sat quietly before a second exposure to the temporal summation protocol indicated that temporal summation did not change significantly between the first and second exposure.39
Procedures
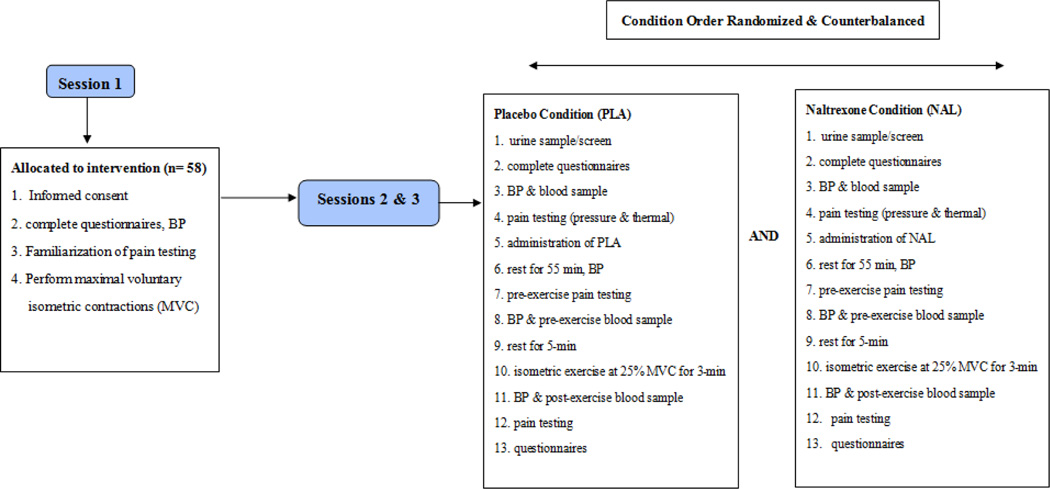
Each participant completed three sessions including a familiarization session and two randomly assigned experimental sessions, and the procedures are summarized in Figure 1.
Figure 1.
Schematic of the Experimental Procedures, BP = blood pressure; min = minutes
Familiarization Session
During the familiarization session, participants completed the informed consent document and six questionnaires consisting of: 1) a Demographic and Pain History Questionnaire, 2) the Pain Catastrophizing Scale (PCS),71 3) the Fear of Pain-III Scale,47 4) the Profile of Mood States Questionnaire,46 5) the Gender Role Expectation of Pain Questionnaire,60 and 6) the Family Environment Scale.50 The Demographic and Pain History Questionnaire was used to collect health, pain, and demographic information. The standard PCS was completed at the beginning of the familiarization session to assess individuals’ tendency to engage in pain-related catastrophizing, while actual pain catastrophizing was assessed following the two experimental sessions. Blood pressure and heart rate were assessed, then participants were exposed to the thermal and pressure stimuli to familiarize them with the pain testing to be completed in subsequent sessions. Next, participants completed isometric exercise testing by performing two maximal voluntary contractions (MVCs). Participants squeezed a hand dynamometer with their dominant hand at maximum strength for 5-seconds, rested for 3-minutes, and then performed another maximal contraction for 5-seconds. The average of the two MVC’s was used to calculate the intensity of exercise to be used in subsequent testing sessions. Isometric exercise was employed because muscle contractions activate Group III (A-delta) and Group IV (C) primary afferents,35,48 and stimulation of afferent fibers in skeletal muscle can result in activation of endogenous pain inhibitory systems.35,73 Prior research in our lab indicated that EIH occurs following isometric exercise, and that isometric handgrip exercise produces ipsilateral and contralateral analgesic responses indicative of a generalized and centrally-mediated response.41
Experimental Sessions
A toxicology screen was performed at the beginning of the two experimental sessions. Participants reported to the CRU upon which time they provided a urine sample in order to screen for recent opioid and cannabinoid use, and for pregnancy in the women. Participants completed a 24-Hour History Questionnaire, the POMS questionnaire, rested for 10-min, and then blood pressure and heart rate were assessed. A research nurse inserted a catheter into the non-dominant arm and blood was drawn through antecubital venipuncture in plasma separating tubes to examine baseline levels of endocannabinoids (AEA and 2-AG) as well as four structural lipid analogs (i.e., palmitoylethanolamide (PEA), oleoylethanolamide (OEA), N-docosahexaenoylethanolamine (DHEA) and 2-oleoylglycerol (2-OG)). Next, participants completed baseline experimental pain testing using the same procedures as were used in the familiarization session. Participants then received an oral dose of either the opioid antagonist (50 mg naltrexone capsule) or a placebo (lactose capsule). Naltrexone (NAL) is an opioid receptor antagonist with particular affinity for µ-Opioid receptors79 which are found both in the central and peripheral nervous system14 The endogenous ligand with the highest affinity to the µ-opioid receptor is β-endorphin.58 The order of placebo and NAL capsules were randomly assigned and administered in a double-blind fashion. The dose of NAL was chosen based on previous studies.1 Both placebo and NAL-containing capsules were administered identically with 8-ounces of water and under observation of the research nurse. Participants sat and rested quietly for 55-minutes to allow for peak plasma concentrations of NAL to occur (side effects, blood pressure and heart rate were monitored every 15-min). Experimental pain testing was completed, blood was drawn immediately, and then a recovery period of 3-minutes followed so as to allow any residual effects of prior stimulation of nociceptors to dissipate (based on our previous research). Participants then performed isometric exercise at 25% MVC for 3-minutes. Muscle pain and ratings of perceived exertion (RPE) were assessed every 30 seconds during exercise using a muscle pain intensity scale designed and validated to specifically measure muscle pain during exercise6, and Borg’s well-validated 6–20 perceived exertion scale.3 Blood pressure and heart rate were assessed and then a blood draw and experimental pain testing was completed immediately post-exercise. Participants completed an in vivo pain catastrophizing scale,10 and then were asked to indicate whether they thought they had received NAL or a placebo during the session.
Plasma endocannabinoids extraction and measurement
All extractions were performed using Bond Elut C18 solid-phase extraction columns (1 mL; Varian Inc, Lake Forest, CA). Plasma samples (0.5 mL each) were thawed and made up to 15% ethanol, to which the internal standards [2H8]-AEA (16.9pmol) and [2H8]-2-AG (46.5 pmol) (Cayman Chemicals, Ann Arbor, MI) were added. Samples were then vortexed and centrifuged at 1000g for 4 min. The supernatant was loaded on C18 columns, which had been conditioned with 1 mL redistilled ethanol and 3 mL of double distilled water (ddH2O). The remaining pellet was washed with 100 µL of 15% ethanol and centrifuged again for 3 min. The resulting supernatant was loaded onto the C18 column. Columns were washed with 5 mL ddH2O and eluted with 1 mL of ethyl acetate. The ethyl acetate layer in the resulting elute was removed and dried under N2. Lipids in the residual ddH2O were extracted by mixing with an additional 1 mL of ethyl acetate, which was added to the original ethyl acetate solution. Once dried, the samples were resuspended in 20 µL of methanol and stored at −80°C. AEA , 2-AG, 2-OG, OEA, PEA, and DHEA were quantified using isotope-dilution, electrospray ionization liquid chromatography/mass spectrometry (LC-ESI-MS) as described previously.54 All of these procedures were performed at the Medical College of Wisconsin under the supervision of Dr. Hillard.
Statistical Analyses
All statistical analyses were conducted using IBM SPSS Statistical Version 20.0, and significance was set at p < 0.05. Descriptive statistics were computed for the variables for the men and women in the placebo and NAL conditions. Data not meeting the normality assumption (i.e., Shapiro-Wilke test) were log transformed. For muscle pain and perceived exertion (RPE) during exercise, the data were analyzed with 2 (sex) × 2 (drug treatment: placebo & NAL) × 6 (time: 30, 60, 90, 120, 150, 180 sec) ANOVAs with repeated measures on the last two factors. Temporal summation of heat pain ratings were assessed at baseline, pre-exercise, and post-exercise, and the data were analyzed with 2 (sex) × 2 (drug treatment) × 3 (trials) × 3 (pulses: 1, 5 & 10) ANOVAs with repeated measures on the last three factors. Pressure pain ratings were analyzed with 2 (sex) × 2 (drug treatment) × 3 (trials) and 4 (time: 30, 60, 90, 120 sec) ANOVAs with repeated measures on the last three factors while pressure pain thresholds were analyzed with a 2 (sex) × 2 (drug treatment) by 3 (trials) ANOVAs with repeated measures on the last two factors. As for the endocannabinoids, the data were analyzed with 2 (sex) × 3 (trials) repeated measures ANOVAs in the placebo condition only (to avoid possible contamination by NAL) to assess whether men and women differed in endocannabinoid responses to exercise. Spearman’s Rho correlational analyses were performed to examine the relationship between endocannabinoids and EIH. In addition, effect sizes were calculated using Cohen’s d.4
RESULTS
A total of 30 men and 30 women with a mean age of 21 yrs (sd=3) participated in this study. Two participants did not complete testing: one woman experienced blood draw complications and one man had trouble swallowing the capsule. Thus, the final sample consisted of 29 men and 29 women. The racial and ethnic make-up of the sample included 33 (57%) Caucasian participants (16 women, 17 men), 10 (17%) African American participants (6 women, 4 men), 8 (14%) Asian American participants (2 women, 6 men), 6 (10%) Latino participants (4 women, 2 men), and 1 (2%) American Indian participant (1 woman). The average maximum grip strength for the women was 22 kg (sd = 7) and the average maximum grip strength for the men was 38 kg (sd = 10).
Ratings of Perceived Exertion and Muscle Pain During Exercise
The results indicated there was a significant time effect (p < 0.05) for ratings of perceived exertion (RPE) with RPE increasing during exercise. The main effects for sex and drug treatment (placebo, NAL) on RPE were not found to be significant (p > 0.05). Similarly, there was a significant time effect (p < 0.05) for muscle pain while the main effects for sex and drug treatment were not significant (p > 0.05). Muscle pain was found to increase significantly during exercise. The results for RPE and muscle pain during exercise are summarized in Table 1.
Table 1.
Means and standard deviations for muscle pain and ratings of perceived exertion during exercise in the placebo and naltrexone conditions; RPE: Ratings of Perceived Exertion; *indicates a significant time effect in both placebo and naltrexone conditions
| Time (s) | 30 | 60 | 90 | 120 | 150 | 180 |
|---|---|---|---|---|---|---|
| RPE* | ||||||
| Placebo | 10 (2.3) | 11.7(2.1) | 13.4 (2.3) | 14.6 (2.5) | 15.8(2.5) | 16.6 (2.5) |
| Naltrexone | 9.9 (2.6) | 11.8 (2.7) | 13.3 (2.8) | 14.9 (2.6) | 16.1 (2.7) | 17.1 (2.6) |
| Muscle Pain* | ||||||
| Placebo | 0.8 (1.0) | 1.4 (1.4) | 2.4 (1.9) | 3.2 (2.6) | 3.9 (3.2) | 4.5 (3.2) |
| Naltrexone | 0.7 (1.3) | 1.4 (1.7) | 2.4 (3.0) | 3.7 (6.6) | 4.3 (5.4) | 5.0 (5.5) |
Experimental Pain
Pressure Pain Thresholds
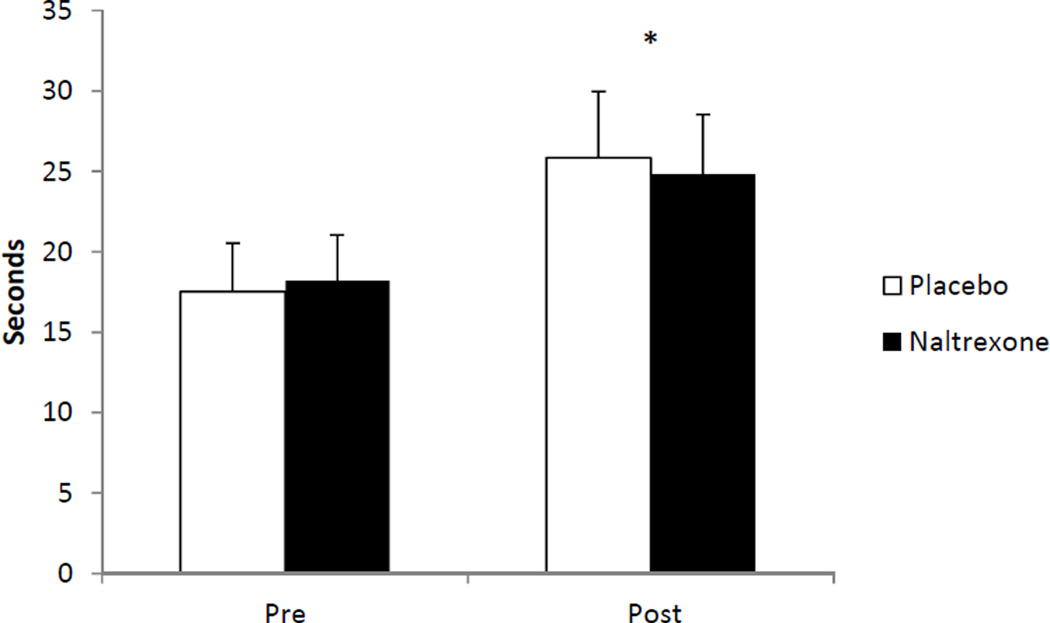
There was a significant trials effect (p < 0.05) for pressure pain thresholds which were found to be higher following exercise in comparison to baseline (i.e., took longer for the stimulus to become painful) in the placebo (d = 0.42) and NAL (d = 0.58) conditions. The main effects for sex and drug treatment, as well as the interactions, were not significant (p > 0.05). The results for pain thresholds are summarized in Figure 2.
Figure 2.
Means and standard errors for pressure pain thresholds pre and post exercise in the placebo and naltrexone conditions; *indicates a significant increase (p < 0.05) post exercise in both conditions. Note: assessments were taken twice before exercise, i.e., baseline and pre-exercise but have been combined in the figure because they were not significantly different.
Pressure Pain Ratings
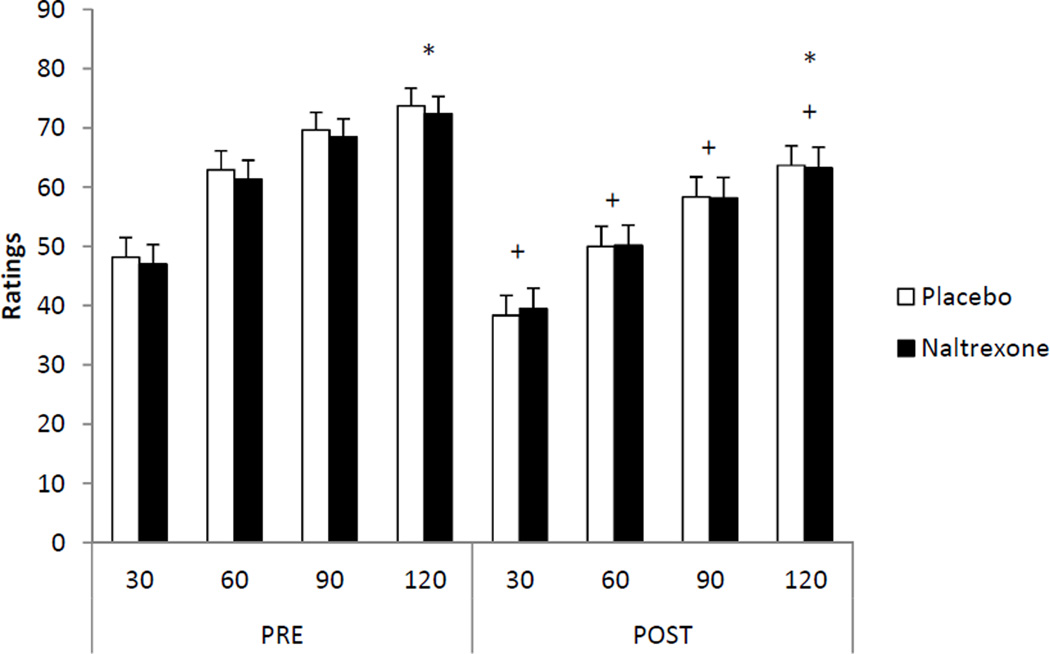
There was a significant time effect (p < 0.05) and a significant trials effect (p < 0.05) for pressure pain ratings. In addition, there was a significant trials × time interaction (p < 0.05) and a significant condition × trials × time interaction (p < 0.05). However, there were no significant main effects for sex or drug treatment (p > 0.05). Pain ratings increased significantly across time during the two minute exposure to the pressure stimulator. Pain ratings were found to be significantly lower following exercise in the placebo (d = 0.64) and NAL (d = 0.50) conditions. The results for pressure pain ratings are illustrated in Figure 3.
Figure 3.
Means and standard errors for pressure pain ratings pre and post exercise in the placebo and naltrexone conditions; *indicates a significant increase in pain ratings (p < 0.05) over the two minutes; +indicates a significant decrease (p < 0.05) in pain ratings at that time from pre to post exercise. Note: assessments were taken twice before exercise, i.e., baseline and pre-exercise but have been combined in the figure because they were not significantly different.
Temporal Summation
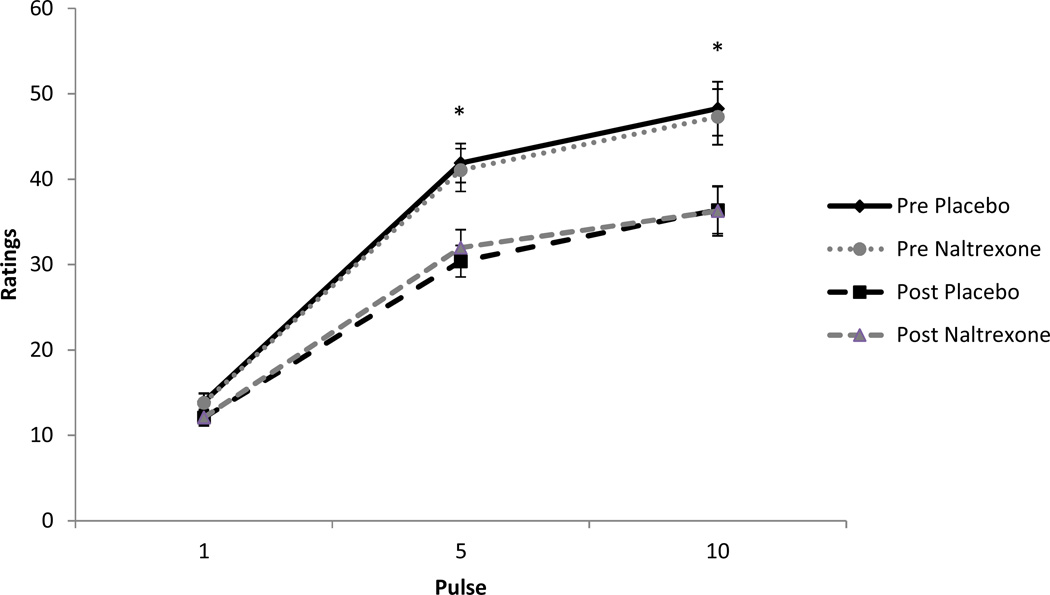
The results for temporal summation ratings of heat pain indicated a significant heat pulse effect (p < 0.05), a significant trials (pre and post-exercise) effect (p < 0.05), and a significant pulse × trials interaction (p < 0.05). However, there were no significant main effects for sex or drug treatment (p > 0.05), and none of the other interaction effects were found to be significant (p > 0.05). Participants rated the first, fifth, and tenth pulses as successively more painful. Men and women experienced a significant decrease in temporal summation ratings of heat pain following exercise in both the placebo (Cohen’s d = 0.50) and NAL (d = 0.48) conditions. The results for temporal summation ratings are illustrated in Figure 4.
Figure 4.
Means and standard errors for temporal summation ratings pre and post exercise in the placebo and naltrexone conditions; *indicates a significant decrease (p < 0.05) in pain ratings at that pulse post exercise. Note: assessments were taken twice before exercise, i.e., baseline and pre-exercise but have been combined in the figure because they were not significantly different.
Lipid Measurements
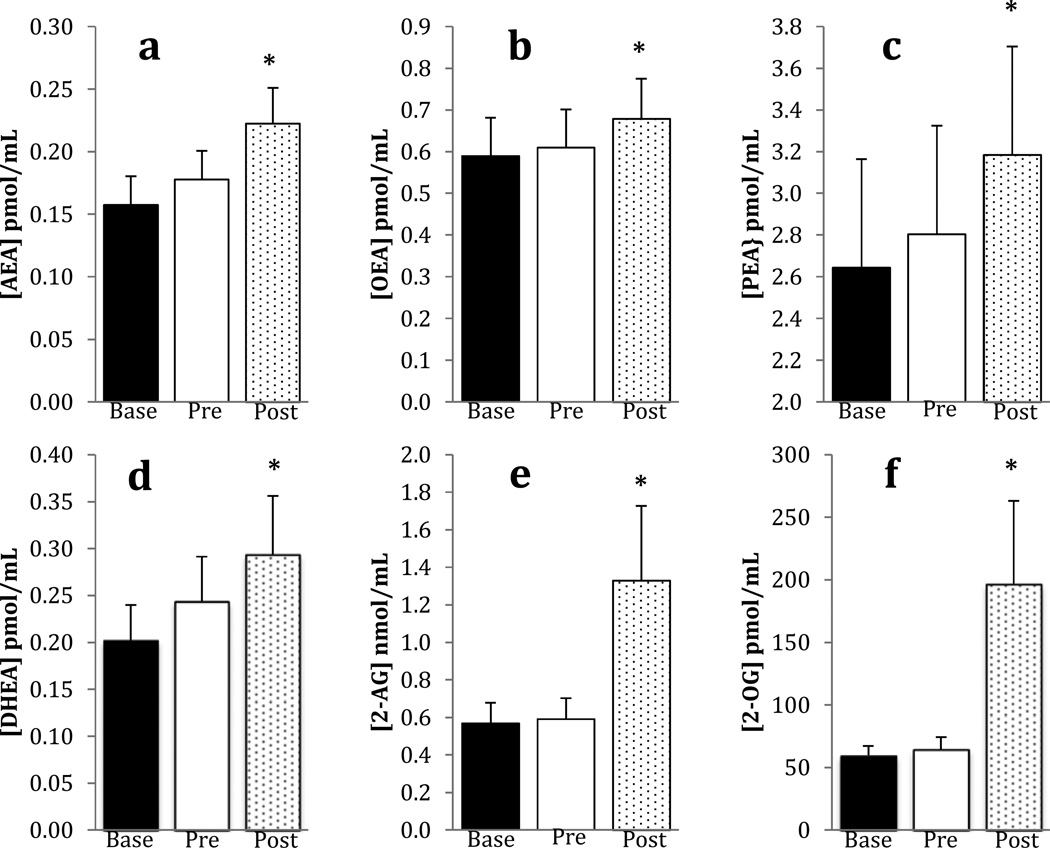
The concentrations of six biogenic lipids were determined in the plasma of participants before and after exercise in the placebo arm of the study. The concentrations of four N-acylethanolamines were measured: the endocannabinoid, AEA, and three structural analogs, DHEA, OEA and PEA. The concentrations of all these lipids were significantly greater following exercise (Figure 5). For each of these lipids, there was a significant trials effect, but the main effect for sex and the sex × trials interaction were not significant (p > 0.05). The concentrations of two acyl-glycerols were also determined: the endocannabinoid 2-AG, and its analog, 2-OG. Both of these lipids were also significantly greater following exercise, but the main effect for sex and the sex × trials interactions were not significantly different (p > 0.05).
Figure 5.
Means and standard errors for biogenic lipids at baseline, pre, and post exercise in the placebo condition only; n = 58; *indicates a significant increase (p < 0.05) in concentration from baseline to post exercise
Relationships Among Biogenic Lipids and EIH
Spearman’s Rho (ρ) correlational analyses were performed to further examine the relationships among the biogenic lipids in the circulation and EIH. A significant correlation was found between DHEA and temporal summation pain ratings. Changes in DHEA concentrations were significantly associated with changes in temporal summation ratings for the men (p = 0.03; ρ = 0.40) and women (p = 0.02; ρ = 0.43).
DISCUSSION
The results from this study indicated that exercise-induced hypoalgesia (EIH) occurred in men and women following isometric exercise. The EIH response was characterized by a reduction in temporal summation of heat pain ratings, lower pressure pain ratings, and higher pressure pain thresholds following exercise. These results indicate that EIH occurred following low intensity (25% MVC) static muscle contractions performed for a short duration (i.e., 3 minutes), and suggest that exercise reduces central sensitivity (i.e., reduced temporal summation) to experimental pain. Currently, the mechanisms responsible for EIH are poorly understood, but the endogenous opioid system has received significant attention. However, results from previous studies in which an opioid antagonist was administered before exercise are equivocal.42 It is unclear why there are conflicting results, but the equivocal findings could be due to methodological differences across studies such as different exercise protocols, different pain induction techniques, differences in the administration of the opioid antagonists, and differences in the characteristics of the samples (e.g., sex, sample size).
Most of the research in this area has involved the testing of men. In several of these studies a mixed sample of men and women were used but the number of women was small, and the data were typically not analyzed for sex differences. In the current study, sample size was estimated prior to the initiation of the study and an equal number of men and women were recruited and tested in both placebo and NAL conditions. The results indicated that EIH occurred following exercise and that administration of an opioid antagonist did not influence EIH responses in men or women. Results were similar for men and women and indicated that pain was attenuated following exercise for all three pain measures (temporal summation of heat pain, pressure pain thresholds, and pressure pain ratings). Very little research has examined whether men and women differ in EIH which is in contrast to a much larger literature in the general pain area indicating sex differences in pain perception. Currently, the results from the limited number of studies which have examined sex differences in EIH are equivocal.28,39,40,68 The results from the present study indicated no significant differences in EIH between men and women, but women were tested during the follicular phase of their menstrual cycle. These results are in agreement with the only other EIH study to control for menstrual cycle phase which also reported no significant sex differences in EIH when women were tested during the follicular phase of their menstrual cycle.39
The results from this study indicated that a comparable EIH response occurred in both the placebo and NAL conditions suggesting that opioids may not be the primary mechanism of EIH in this study. However, we did not measure circulating levels of opioids and cannot be certain that these levels increased as a result of exercise, especially since we used a short duration isometric exercise protocol. Research indicates that properties of the exercise stressor are important in determining which system is activated during exercise (i.e., opioid or non-opioid). In the animal research, it has been shown that by manipulating the parameters of the exercise stressor (e.g., duration of exercise, intermittent vs continuous exercise), it is possible to elicit either opioid or non-opioid hypoalgesia.42
Circulating endocannabinoid concentrations were found to increase significantly following exercise suggesting involvement of a non-opioid mechanism in EIH following isometric exercise. Very little is known about non-opioid hypoalgesia but an endocannabinoid hypothesis has been proposed.8 The endocannabinoid system is a neuromodulatory system composed of cannabinoid receptors (e.g., CB1, CB2), their endogenous ligands (the endocannabinoids AEA and 2-AG), and proteins responsible for their metabolism. CB receptors have been identified throughout the body (e.g., brain, spinal cord, sensory neurons, muscles, endothelial cells, lungs). In particular, the presence of CB receptors in the pain processing areas of the brain and spinal cord.23,74 suggests that endogenous cannabinoids may contribute to the control of pain transmission in the central nervous system.31,78 The two most studied endocannabinoids are the arachidonic acid derivatives, AEA and 2-AG. Non-arachidonate analogs of both AEA and 2-AG are also present in the circulation, usually at far higher concentrations than the endocannabinoids. PEA, OEA, and 2-OG have very low affinity for the CB receptors, particularly compared to the affinities of AEA and 2-AG.26,70 Putative targets of OEA and PEA include the nuclear transcription factor PPARα21 and the transient receptor potential vanilloid channel-1 (TRPV1) channel.2,65 2-OG is a putative agonist for an orphan G protein coupled receptor, GPR119.22 Importantly, the N-acylethanolamines have overlapping mechanisms of synthesis and degradation such that their concentrations often increase and decrease together.26 The same is true for 2-AG and 2-OG.27 The present study confirms these relationships.
The only lipid for which a significant correlation between exercise-induced change and pain ratings occurred was DHEA. DHEA is the ethanolamide of docsoahexenoic acid, which is an omega 3 fatty acid with 22 carbons and 6 double bonds. DHEA binds to CB1 receptors with about 10-fold lower affinity than AEA62, which brings up the possibility that DHEA is a weak endocannabinoid. The only other target for DHEA that has been identified thus far is the transcription regulator, PPARY.61 It is unlikely that PPARY mediates the association of DHEA with pain ratings due to the short time frame of the effects.
Multiple lines of evidence indicate that endocannabinoids play a role in pain modulation. For example, neurophysiological studies have demonstrated that cannabinoids suppress nociceptive processing.20 Also, research indicates hypersensitivity to pain (i.e., hyperalgesia) following pharmacological blockade of CB1 receptors.20 Further, studies employing stimulation-produced analgesia and stress-induced analgesia provide support for the hypothesis that endogenous AEA and 2-AG suppress pain through CB1-dependent mechanisms.20 Thus, it has been hypothesized that the endocannabinoid system may be involved in EIH.8 Only a limited number of studies, however, have been conducted examining endocannabinoid responses to exercise. Sparling et al (2003) provided the first evidence in men that the endocannabinoid system was activated by exercise as evidenced by significant elevations in circulating AEA levels following cycling or running.64 Heyman et al (2011) examined endocannabinoid responses to exercise in trained male cyclists, and found significant elevations in AEA levels, as well as elevations in PEA and OEA levels following exercise.24 Raichlen et al (2013; 2012) conducted two studies examining endocannabinoid responses to exercise. Results from one of the studies indicated significant elevations in AEA levels following running but not walking on a treadmill.56 In another study, Raichlen et al (2013) examined endocannabinoid responses to different intensities of exercise, and found significant elevations in AEA levels following moderate intensity treadmill exercise but not at lower or higher intensities of exercise.57 In addition, Feuerecker et al (2012) examined changes in endocannabinoid levels following hiking at different altitudes in comparison to a passive ascent (i.e., by helicopter), and found significant elevations in AEA levels following hiking at a lower altitude with further increases in AEA levels following hiking at a higher altitude.11 Elevated brain AEA levels have also been found following exercise in an animal study by Hill et al (2010). Eight days of free access to running wheels were found to increase tissue content of AEA in the hippocampus.25 All of these previous studies indicated significant elevations in AEA levels following aerobic exercise. The results of the current study are in general agreement with these earlier studies in that exercise produced significant increases in AEA, as well as OEA, and PEA, but extends the findings to isometric exercise (static muscle contractions). Further, elevations in DHEA and the second endocannabinoid, 2-AG along with 2-OG, occurred following exercise. This is the first study to demonstrate significant elevations in endocannabinoid related lipid concentrations following short duration isometric exercise. CB receptors are densely expressed on peripheral nerve terminals such as A-delta and C primary afferents.30 It appears that static muscle contractions which activate A-delta and C primary afferents produce alterations in circulating concentrations of endocannaboids.
The results from the current study extend the findings from the previous research by including assessments of pain before and following exercise. The results indicated EIH occurred following isometric exercise, and this was in conjunction with significant elevations in circulating concentrations of endocannabinoids. Only a limited number of studies have been conducted to date in which changes in pain and endocannabinoid levels have been examined during or following exercise. Ghafouri et al (2013) examined concentrations of PEA and stearoylethanolamide (SEA) in three groups of women including women with chronic neck/shoulder pain (CNSP), women with chronic widespread pain (CWP), and healthy controls before and following exercise consisting of 20 minutes of repetitive arm movement. Pain was found to increase during arm movement for the women with CNSP and CWP. The increase in pain intensity during arm movement was found to be associated with low levels of PEA and SEA in the women with chronic pain, consistent with the hypothesis that PEA and SEA levels are associated with pain responses.17 Galdino et al (2013) examined the role of the endocannabinoid system in exercise-induced antinociception in rats. Male Wistar rats ran on a rodent treadmill while pressure and thermal nociceptive thresholds were assessed before and following exercise in different conditions (e.g., administration of CB1 and CB2 receptor antagonists, administration of endocannabinoid metabolizing enzyme inhibitors). Aerobic exercise produced a significant increase in pressure and thermal nociceptive thresholds as well as significant increases in AEA, 2-AG, PEA, and OEA, similar to the results of the present study. Further, systemic and central pretreatment with CB1 and CB2 receptor antagonists blocked the antinociceptive response following exercise.16 In addition, systemic and central pretreatment with endocannabinoid metabolizing enzyme inhibitors prolonged the antinociceptive response following exercise. Notably, there were significant increases in CB1 receptors expression in the brains of rats who had exercised. Specifically, CB1 receptors increased in neurons within the dorsolateral and ventrolateral PAG regions in the exercised rats. Similar eCB results were recently reported in another study conducted by Galdino et al. (2014) employing resistance exercise instead of aerobic exercise.15 Thus, the results from these studies provide support for the endocannabinoid hypothesis of EIH.
An interesting question that arises from these studies is the source of the circulating lipids. Recent studies have demonstrated that skeletal muscle expresses mRNA for N-acylethanolamine phosphatidylethanolamine (NAPE)-specific phospholipase D,32 an enzyme known to synthesize the N-acylethanolamines in a calcium-dependent manner.75 Transcript for diacylglycerol lipase (DAGL), the rate limiting step in the synthesis of 2-AG and other 2-acylglycerols,59 is also found in skeletal muscle.7,32 Thus, it is possible that the source of the endocannabinoids and other lipids is the exercising muscle and further research is needed to examine this possibility.
In addition to a possible role in regulation of pain, it is possible that the lipids serve as a link between an acute change in muscle activity and the requirement for additional fuel through diverse metabolic effects. For example, a recent study has demonstrated that DHEA increases glucose uptake by C2C12 myoblasts in culture.33 It is well established that activation of CB1 receptors by AEA and 2-AG increases food consumption,34 however, there is also evidence that CB1 receptor over-activity contributes to insulin resistance in skeletal muscle.9 Interestingly, however, OEA has anorexic properties.72
Strengths of the present study include a double-blind, placebo-controlled crossover design, an optimal sample size of men and women, and control of menstrual cycle phase in the women. A limitation of this study was the lack of a control condition in which participants were exposed to the pain stimuli before and following a non-exercise control condition. However, in our previous research we did not find changes in pain following control conditions consisting of sitting quietly for the same duration as the exercise condition.38,39 Also, isometric exercise was the only exercise stimulus used in this study, thus, the results cannot be generalized to other modes of exercise. Additional research is required examining mechanisms of EIH for aerobic and resistance exercise. Further, the results from the current study can only be generalized to healthy young men and women. It is unclear whether individuals with chronic pain would experience similar results as our healthy young adults. Results from studies examining EIH in individuals with chronic pain are currently equivocal with EIH occurring in some chronic pain conditions (e.g., osteoarthritis, rheumatoid arthritis) but not consistently in others (e.g., fibromyalgia, temporomandibular disorders, painful diabetic neuropathy).36,52 In conclusion, EIH was found to occur following short duration isometric exercise. Administration of an opioid antagonist did not alter this response, but endocannabinoid levels were found to be elevated following exercise. Thus, EIH following short duration isometric exercise may be mediated by a non-opioid mechanism, possibly by cannabinoid receptor activation.
PERSPECTIVE.
Currently, the mechanisms responsible for exercise-induced hypoalgesia (EIH) are unknown. This study provides support for a potential endocannabinoid mechanism of EIH following isometric exercise.
Acknowledgments
This research was supported by NIH grants R21AR057159 and 1UL1RR025011 and by the Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research was conducted at the University of Wisconsin Hospital and Clinics: Clinical Research Unit
Summary: EIH occurred following short duration isometric exercise and endocannabinoid levels were found to be elevated following exercise.
Disclosures: There are no conflicts of interest with this study, none of the authors have anything to disclose. All authors participated in the conduct of this study.
REFERENCES
- 1.al’Absi M. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosino P, Soldovieri MV, Russo C, Taglialatela M. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARα agonist palmitoylethanolamide. Br J Pharmacol. 2013;168:1430–1444. doi: 10.1111/bph.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, Illinois: Human Kinetics. 1998 [Google Scholar]
- 4.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 5.Cook DB, Koltyn KF. Pain and exercise. Int J Sport Psychol. 2000;31:256–277. [Google Scholar]
- 6.Cook DB, O’Connor PJ, Eubanks SA, Smith JC, Lee M. Naturally occurring muscle pain during exercise: assessment and experimental evidence. Med Sci Sports Exerc. 1997;29:999–1012. doi: 10.1097/00005768-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Crespillo A, Suárez J, Bermúdez-Silva FJ, Rivera P, Vida M, Alonso M, Palomino A, Lucena MA, Serrano A, Pérez-Martín M, Macias M, Fernández-Llébrez P, Rodríguez de Fonseca F. Expression of the cannabinoid system in muscle: effects of a high-fat diet and CB1 receptor blockade. Biochem J. 2011;433:175–185. doi: 10.1042/BJ20100751. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sport Med. 2004;38:536–541. doi: 10.1136/bjsm.2004.011718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckardt K, Sell H, Taube A, Koenen M, Platzbecker B, Cramer A, Horrighs A, Lehtonen M, Tennagels N, Eckel J. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia. 2009;52:664–674. doi: 10.1007/s00125-008-1240-4. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 11.Feuerecker M, Hauer D, Toth R, Demetz F, Hölzl J, Thiel M, Kaufmann I, Schelling G, Choukèr A. Effects of exercise stress on the endocannabinoid system in humans under field conditions. Eur J Appl Physiol. 2012;112:2777–2781. doi: 10.1007/s00421-011-2237-0. [DOI] [PubMed] [Google Scholar]
- 12.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Forgione AG, Barber TX. A strain gauge pain stimulator. Psychophysiology. 1971;8:102–106. doi: 10.1111/j.1469-8986.1971.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 14.Galdino G, Romero T, Andrade I, Duarte I, Perez A. Opioid receptors are not involved in the increase of the nociceptive threshold induced by aerobic exercise. Neurosciences. 2014;19:33–37. [PubMed] [Google Scholar]
- 15.Galdino G, Romero T, da Silva JFP, Aguiar D, de Paula AM, Cruz J, Parrella C, Piscitelli F, Duarte I, Di Marzo V, Perez A. Acute resistance exercise induces antinociception by activation of the endocannabinoid system in rats. Anesth Analg. 2014 Jun;:26. doi: 10.1213/ANE.0000000000000340. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galdino G, Romero TRL, Silva JFP, Aguiar DC, de Paula AM, Cruz JS, Parrella C, Piscitelli F, Duarte ID, Di Marzo V, Perez AC. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology. 2013;77C:313–324. doi: 10.1016/j.neuropharm.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Ghafouri N, Ghafouri B, Larsson B, Stensson N, Fowler CJ, Gerdle B. Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain. 2013;154:1649–1658. doi: 10.1016/j.pain.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Consensus Working Group of the Sex and Pain SIG of the IASP G: Studying sex and gender differences in pain and analgesia: a consensus report. 2007;(132 Suppl):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grisel J, Fleshner M, Watkins L, Maier S. Opioid and nonopioid interactions in two forms of stress-induced analgesia. Pharmacol Biochem Behav. 1993;45:161–172. doi: 10.1016/0091-3057(93)90100-8. [DOI] [PubMed] [Google Scholar]
- 20.Guindon J, Hohmann AG. The endocannabinoid system and pain. CNS Neurol Disord Drug Targets. 2009;8:403–421. doi: 10.2174/187152709789824660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen HS. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp Neurol. 2010;224:48–55. doi: 10.1016/j.expneurol.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–E1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- 23.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyman E, Gamelin F-X, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology. 2012;37:844–851. doi: 10.1016/j.psyneuen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, Gorzalka BB, Hillard CJ, Christie BR. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus. 2010;20:513–523. doi: 10.1002/hipo.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillard CJ, Campbell WB. Biochemistry and pharmacology of arachidonylethanolamide, a putative endogenous cannabinoid. J Lipid Res. 1997;38:2383–2398. [PubMed] [Google Scholar]
- 27.Hillard CJ. Endocannabinoids and vascular function. J Pharmacol Exp Ther. 2000;294:27–32. [PubMed] [Google Scholar]
- 28.Hoeger Bement MK, Dicapo J, Rasiarmos R, Hunter SK. Dose response of isometric contractions on pain perception in healthy adults. Med Sci Sports Exerc. 2008;40:1880–1889. doi: 10.1249/MSS.0b013e31817eeecc. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann P, Terenius L, Thorén P. Cerebrospinal fluid immunoreactive beta-endorphin concentration is increased by voluntary exercise in the spontaneously hypertensive rat. Regul Pept. 1990;28:233–239. doi: 10.1016/0167-0115(90)90021-n. [DOI] [PubMed] [Google Scholar]
- 30.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 31.Hohmann AG, Suplita RL. Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8:E693–E708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchins-Wiese HL, Li Y, Hannon K, Watkins BA. Hind limb suspension and long-chain omega-3 PUFA increase mRNA endocannabinoid system levels in skeletal muscle. J Nutr Biochem. 2012;23:986–993. doi: 10.1016/j.jnutbio.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Carlson ME, Watkins BA. Docosahexaenoyl ethanolamide improves glucose uptake and alters endocannabinoid system gene expression in proliferating and differentiating C2C12 myoblasts. Front Physiol. 2014;5:100. doi: 10.3389/fphys.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkham TC. Endocannabinoids and the non-homeostatic control of appetite. Curr Top Behav Neurosci. 2009;1:231–253. doi: 10.1007/978-3-540-88955-7_9. [DOI] [PubMed] [Google Scholar]
- 35.Kjaer M, Secher NH, Bach FW, Galbo H, Reeves DR, Jr, Mitchell JH. Hormonal, metabolic, and cardiovascular responses to static exercise in humans: influence of epidural anesthesia. Am J Physiol Department of Anesthesia, Rigshospitalet, Denmark. 1991;261:E214–E220. doi: 10.1152/ajpendo.1991.261.2.E214. [DOI] [PubMed] [Google Scholar]
- 36.Knauf MT, Koltyn KF. Exercise-induced modulation of pain in adults with and without painful diabetic neuropathy. J Pain. 2014;15:656–663. doi: 10.1016/j.jpain.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koltyn KF, Arbogast RW. Perception of pain after resistance exercise. Br J Sport Med. 1998;32:20–24. doi: 10.1136/bjsm.32.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koltyn KF, Garvin AW, Gardiner RL, Nelson TF. Perception of pain following aerobic exercise. Med Sci Sports Exerc. 1996;28:1418–1421. doi: 10.1097/00005768-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Koltyn KF, Knauf MT, Brellenthin AG. Temporal summation of heat pain modulated by isometric exercise. Eur J Pain. 2013;17:1005–1011. doi: 10.1002/j.1532-2149.2012.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koltyn KF, Trine MR, Stegner AJ, Tobar DA. Effect of isometric exercise on pain perception and blood pressure in men and women. Med Sci Sport Exerc. 2001;33:282–290. doi: 10.1097/00005768-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Koltyn KF, Umeda M. Contralateral attenuation of pain after short-duration submaximal isometric exercise. J Pain. 2007;8:887–892. doi: 10.1016/j.jpain.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Koltyn KF. Analgesia following exercise: a review. Sport Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 43.Koltyn KF. Exercise-induced hypoalgesia and intensity of exercise. Sport Med. 2002;32:477–487. doi: 10.2165/00007256-200232080-00001. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–625. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- 45.Marek P, Mogil JS, Sternberg WF, Panocka I, Liebeskind JC. N-methyl-D-aspartic acid (NMDA) receptor antagonist MK-801 blocks non-opioid stress-induced analgesia. II. Comparison across three swim-stress paradigms in selectively bred mice. Brain Res. 1992;578:197–203. doi: 10.1016/0006-8993(92)90248-8. [DOI] [PubMed] [Google Scholar]
- 46.McNair D, Lorr M, Droppleman L. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 47.McNeil DW, Rainwater AJ., 3rd Development of the fear of pain questionnaire--III. J Behav Med. 1998;21:389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 48.Mense S, Simons D. Muscle pain: understanding its nature, diagnosis, and treatment. Philadelphia, PA: Lippincott Williams & Wilkins; pp. 1–402001. [Google Scholar]
- 49.Mogil JS, Sternberg WF, Balian H, Liebeskind JC, Sadowski B. Opioid and nonopioid swim stress-induced analgesia: a parametric analysis in mice. Physiol Behav. 1996;59:123–132. doi: 10.1016/0031-9384(95)02073-x. [DOI] [PubMed] [Google Scholar]
- 50.Moos R. Family Environment Scale. Menlo Park, CA: Mind Garden, Inc; 1974. [Google Scholar]
- 51.Morgan WP, Horstman DH. Psychometric correlates of pain perception. Percept Mot Skills. 1978;47:27–39. doi: 10.2466/pms.1978.47.1.27. [DOI] [PubMed] [Google Scholar]
- 52.Nijs J, Kosek E, Van Oosterwijck J, Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain to exercise or not to exercise? Pain Physician. 2012;15:ES205–ES213. [PubMed] [Google Scholar]
- 53.O’Connor PJ, Cook DB. Exercise and pain: the neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exerc Sport Sci Rev. 1999;27:119–166. [PubMed] [Google Scholar]
- 54.Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- 55.Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 56.Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the “runner”s high’. J Exp Biol. 2012;215:1331–1336. doi: 10.1242/jeb.063677. [DOI] [PubMed] [Google Scholar]
- 57.Raichlen DA, Foster AD, Seillier A, Giuffrida A, Gerdeman GL. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur J Appl Physiol. 2013;13:869–875. doi: 10.1007/s00421-012-2495-5. [DOI] [PubMed] [Google Scholar]
- 58.Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- 59.Reisenberg M, Singh PK, Williams G, Doherty P. The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2012;367:3264–3275. doi: 10.1098/rstb.2011.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson ME, Riley I, II JL, Myers CD, Papas RK, Wise EA, Waxenberg LB, Fillingim RB. Gender role expectations of pain: Relationship to sex differences in pain. J Pain. 2001;2:251–257. doi: 10.1054/jpai.2001.24551. [DOI] [PubMed] [Google Scholar]
- 61.Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I, Lanzino M, De Amicis F, Sisci D, Mauro L, Aquila S, Catalano S, Bonofiglio D, Andò S. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARγ activation in MCF-7 breast cancer cells. J Cell Physiol. 2013;228:1314–1322. doi: 10.1002/jcp.24288. [DOI] [PubMed] [Google Scholar]
- 62.Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J Med Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- 63.Solomon S. A review of mechanisms of response to pain therapy: why voodoo works. Headache. 2002;42:656–662. doi: 10.1046/j.1526-4610.2002.02155.x. [DOI] [PubMed] [Google Scholar]
- 64.Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- 65.Starowicz K, Makuch W, Korostynski M, Malek N, Slezak M, Zychowska M, Petrosino S, De Petrocellis L, Cristino L, Przewlocka B, Di Marzo V. Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS One. 2013;8:e60040. doi: 10.1371/journal.pone.0060040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staud R, Robinson ME, Vierck CJ, Jr, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. doi: 10.1016/s0304-3959(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 67.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 68.Sternberg WF, Bokat C, Kass L, Alboyadjian A, Gracely RH. Sex-dependent components of the analgesia produced by athletic competition. J Pain. 2001;2:65–74. doi: 10.1054/jpai.2001.18236. [DOI] [PubMed] [Google Scholar]
- 69.Sternberg WF, Liebeskind JC. The analgesic response to stress: genetic and gender considerations. Eur J Anaesthesiol. 1995;10(Suppl):14–17. [PubMed] [Google Scholar]
- 70.Sugiura T, Kodaka T, Kondo S, Tonegawa T, Nakane S, Kishimoto S, Yamashita A, Waku K. 2-Arachidonoylglycerol, a putative endogenous cannabinoid receptor ligand, induces rapid, transient elevation of intracellular free Ca2+ in neuroblastoma × glioma hybrid NG108-15 cells. Biochem Biophys Res Commun. 1996;229:58–64. doi: 10.1006/bbrc.1996.1757. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychol Assess American Psychological Association. 1995;7:524–532. [Google Scholar]
- 72.Thabuis C, Tissot-Favre D, Bezelgues J-B, Martin J-C, Cruz-Hernandez C, Dionisi F, Destaillats F. Biological functions and metabolism of oleoylethanolamide. Lipids. 2008;43:887–894. doi: 10.1007/s11745-008-3217-y. [DOI] [PubMed] [Google Scholar]
- 73.Thoren P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc. 1990;22:417–428. [PubMed] [Google Scholar]
- 74.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 75.Ueda K, Kimura-Sakiyama C, Aihara T, Miki M, Arata T. Calcium-dependent interaction sites of tropomyosin on reconstituted muscle thin filaments with bound Myosin heads as studied by site-directed spin-labeling. Biophys J. 2013;105:2366–2373. doi: 10.1016/j.bpj.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Umeda M, Newcomb LW, Ellingson LD, Koltyn KF. Examination of the dose-response relationship between pain perception and blood pressure elevations induced by isometric exercise in men and women. Biol Psychol. 2010;85:90–96. doi: 10.1016/j.biopsycho.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Vierck CJ, Jr, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 78.Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb Exp Pharmacol. 2005:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- 79.Younger JW, Zautra AJ, Cummins ET. Effects of naltrexone on pain sensitivity and mood in fibromyalgia: no evidence for endogenous opioid pathophysiology. PLoS One. 2009;4:e5180. doi: 10.1371/journal.pone.0005180. [DOI] [PMC free article] [PubMed] [Google Scholar]