Abstract
Invasive fungal disease constitutes a growing health burden and development of novel antifungal drugs with high potency and selectivity against new fungal molecular targets are urgently needed. Previously, an aminothiazole derivative, designated as 41F5, was identified in our laboratories as highly active against Histoplasma yeast (MIC50 0.4-0.8 µM) through phenotypic high-throughput screening of a commercial library of 3600 purine mimicking compounds (Edwards, JA et al. Antimicrob. Agents Chemother. 2013, 57:4349-5359). Consequently, 68 analogues of 41F5 were designed and synthesized or obtained from commercial sources and their MIC50s of growth inhibition were evaluated in Histoplasma capsulatum to establish a basic Structure-Activity-Relationship (SAR) for this potentially new class of antifungals. The growth inhibiting potentials of smaller subsets of this library were also evaluated in Cryptococcus neoformans and human hepatocyte HepG2 cells, the latter to obtain Selectivity Indices (SIs). The results indicate that a thiazole core structure with a naphth-1-ylmethyl group at the 5-position and cyclohexylamide-, cyclohexylmethylamide-, or cyclohexylethylamide substituents at the 2-position caused the highest growth inhibition of Histoplasma yeast with MIC50s of 0.4 µM. For these analogues, SIs of 92 - >100 indicated generally low host toxicity. Substitution at the 3- and 4-position decreased antifungal activity. Similarities and differences were observed between Histoplasma and Cryptococcus SARs. For Cryptococcus, the naphth-1-ylmethyl substituent at the 5-position and smaller cyclopentylamide or cyclohexylamide groups at the 2-position were important for activity. In contrast, slightly larger cyclohexylmethyl- and cyclohexylethyl substituents markedly decreased activity.
Keywords: Aminothiazoles, Antifungal activity, Structure-Activity-Relationship, Histoplasma capsulatum, Cryptococcus neoformans
1. Introduction
Over the past few decades, systemic and invasive fungal infections have emerged as a significant threat to public health. Invasive fungal infections cause more human deaths than tuberculosis, although the latter has gained more notoriety in the public eye.1, 2 Cryptococcus infections have been estimated to cause over 500,000 deaths annually among immunocompromised individuals.2 Invasive fungal infections are not limited to individuals with compromised immune functions. For example, in the United States, infections with Cryptococcus gattii and Histoplasma capsulatum occur in immunocompetent as well as immunocompromised hosts, classifying these as primary, not just opportunistic, fungal pathogens.3
The shared eukaryotic nature of both the host and pathogen significantly complicates treatment options for fungal disease. Existing antifungals for systemic mycoses target either the fungal membrane sterol ergosterol or cell wall β-glucan.4 Amphotericin B targets sterols directly and triazole-class antifungals impair sterol synthesis. However, both antifungal classes have significant host toxicity, which prohibits general prophylactic use of these antifungals.5 The echinocandins are a third class of fungistatic antifungals recently developed, which target the synthesis of the essential fungal cell wall polysaccharide β-glucan. While better tolerated than amphotericin and the triazoles, the echinocandins lack efficacy against the more virulent fungal pathogens Cryptococcus and Histoplasma.6 Further complicating antifungal treatment is the fact that Cryptococcus and Histoplasma yeasts invade immune cells (e.g., macrophages), and this intracellular location presents additional barriers to drug accessibility and efficacy. Thus, development of antifungal drugs with high potency and selectivity against new cellular targets are urgently needed to combat the growing health burden of invasive fungal disease.
Recently, our group performed a phenotypic high-throughput screen of a purinome-focused library of 3600 compounds with structural similarity to purines or any known purine analogue scaffold.7 Inhibition of Histoplasma yeast growth was used as the screening phenotype. Concurrently, we measured mammalian cytotoxicity using a P388D1 macrophage cell line8 since macrophages are the primary host cell for Histoplasma yeast. Among the 10 hits with the highest selectivity indices (SIs), a subgroup of three structurally related thiazole/thiophene derivatives (41F5, 2F8, 4H2, Figure 1) were identified. The most active compound of this group was the aminothiazole 41F5, which had the lowest MIC50 (0.4-0.8 µM) and the highest SI (63-135) of all tested compounds relative to P388D1 macrophages. Preliminary studies also indicated selective toxicity of 41F5 against Cryptococcus neoformans.7 Thus, the aminothiazole 41F5 has efficacy against Histoplasma capsulatum and Cryptococcus neoformans, two fungal pathogens that have natural resistance against the echinocandin class of antifungals.
Figure 1.
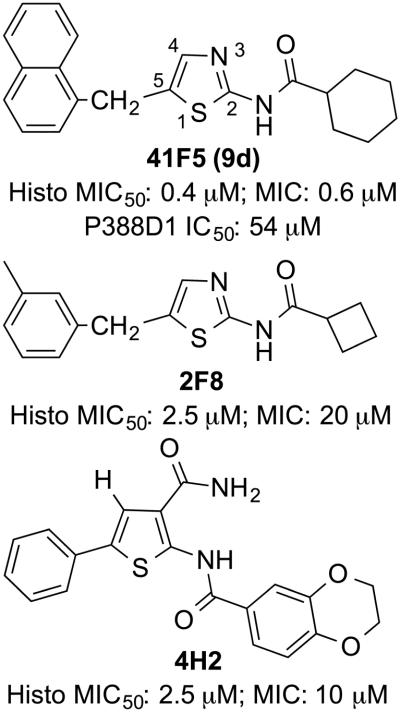
Structurally related thiazole/thiophene hit compounds 41F5, 2F8, and 4H2 identified in a phenotypic high-throughput screen of a purinome-focused library.7
Compounds with aminothiazole scaffold display a wide range of biological activities,9 including antiparasitic-,10 antifungal-,11 antibacterial-,12 antitubucular-,13 antiviral-,14 anticancer-,15, 16 and antiprion17 action. The study described here was carried out to establish the basic anti-Histoplasma and anti-Cryptococcus specific aminothiazole Structure-Activity Relationships (SARs).
2. Results
2.1. Chemistry
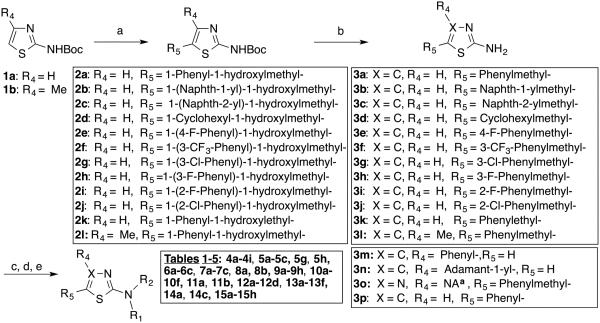
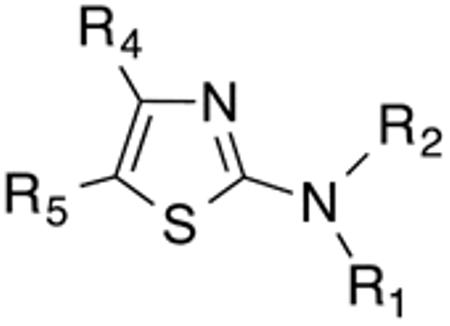
The primary objective of our studies was to establish a Histoplasma SAR for aminothiazoles based on the 41F5 structure (Figure 1). Other objectives were the development of a very basic Cryptococcus SAR for comparison and the evaluation of toxicity of promising novel compounds to hepatocyte (HepG2) cells. For this purpose we synthesized or purchased 68 compounds that are structurally related to 41F5. The thiazole core structure is easily amenable to modification. Due to its abundant use in drug design, numerous synthetic approaches have been developed and many synthetic precursor molecules for thiazole synthesis are commercially available or easily prepared.15, 16, 18, 19 Indeed, the design of this initial library was based to a significant extent on the synthetic feasibility and/or commercial availability of starting materials. Established synthetic procedures are shown in Scheme 1.
Scheme 1.
Reagents and conditions: (a) R5CHO, n-BuLi, THF, −78 °C, 2h; (b) Et3SiH, TFA, DCM, overnight, rt; (c) R2COCl, Et3N, THF, 15 min, rt or (d) R2COOH, EDAC, DMAP, Et3N, DMF/DCM (3/1, v/v), 2h, rt or (e) TFAA, DCM, 30 min, rt. aNot applicable.
The reaction of compounds 1a and 1b with various aldehydes in presence of n-Buli at −78 C afforded compounds 2a-2l in yields ranging from 22% to 59% (Scheme 1). The secondary alcohol functions of compounds 2a-2l were reduced with triethylsilane (SiEt3H) followed by in situ deprotection of the Boc group with trifluoroacetic acid (TFA) in DCM to give compounds 3a-3l in yields ranging from 53% to 91%. It seems that this synthetic method has not been described previously for compounds 3a-3l. Acylation of compounds 3a-3p with various alkyl-, alicyclic-, or aryl acyl chlorides in the presence of triethylamine (TEA) gave products 4b-4f, 4h, 4i, 5h, 6a-6c, 7a-7c, 8a, 8b, 9b-9h, 10a-10f, 11a, 11b, 12a-12d, 13a-13f, 14a, 14c, and 15a-15h.
The reaction of 3a or 3b with trifluoroacetic anhydride (TFAA) at 0 °C followed by stirring for 30 minutes at room temperature yielded 4a and 9a in 56% and 93%, respectively. The reactions of compound 3a with various alicyclic carboxylic acid in presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) and dimethylaminopyridine (DMAP) gave compounds 4g, 5a-5c, and 5g in yields ranging from 20 % to 77%. Carboxylic acids instead of alicyclic acyl chlorides were used for the synthesis of 4g, 5a-5c, and 5g because of their commercial availability.
In order to explore the effect of substituents at the N3 position of the thioazole ring on Histoplasma growth inhibition, compound 4f was exposed to methyl iodide in the presence of sodium hydride according to previously described methods (Scheme 2).20, 21 Surprisingly, the reaction produced a mixture of two products, 14b and 16a, in 38% and 32% yield, respectively. Both compounds have similar HR-MS data indicating mono-methylation. However, for compound 16a, a high field shift to 6.53 pm was observed for the proton at the 4-position in the 1H-NMR spectrum, presumably due to lack of aromaticity in the thiazol-2(3H)-ylidene scaffold, compared to ~ 7.2 pm for the same proton in proton 14b.
Scheme 2.
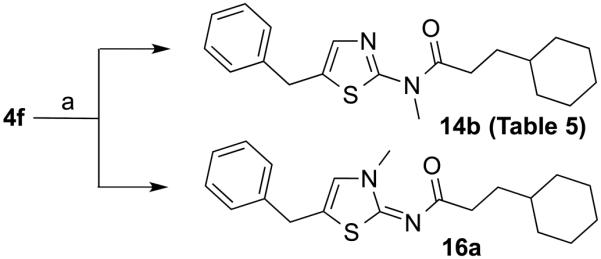
Reagents and conditions: (a) MeI, NaH, THF, 2h, rt.
In order to explore urea-type spacers and also the presence of heteroatoms in the ring system of the 2-position side chain, the reaction of compound 3a with various N-acyl chlorides was carried out in the presence of DMAP (Scheme 3). In contrast to all reactions shown in Scheme 1, this reaction led to compounds that were mono substituted at the amino group in the 2-position (5d-5f) in low yields (5%-8%) and compounds that were disubstituted both at the amino group in the 2-position and at the N3-position of thiazole ring (16b-16d) in yields ranging from 23% to 28%. The HR-MS data of compounds 5d-5f and 16b-16d are in agreement with mono- and disubstitution, respectively. In addition, the 1H-NMR spectra of 16b-16d showed signals for the proton 4-position in the range of 6.5-6.6 pm, which is consistent with the thiazol-2(3H)-ylidene scaffold whereas the signals for the same proton in the 1H-NMR spectra of compounds 5d-5f were at 6.9-7.0 ppm, which indicates a thiazole ring system. Apparently the carbonyl carbon of the N-acyl chloride reagents is sufficiently electrophilic to allow nucleophilic attack both by the nitrogen of the amino group at the 2-position and that at the 3-position of thiazole ring.
Scheme 3.
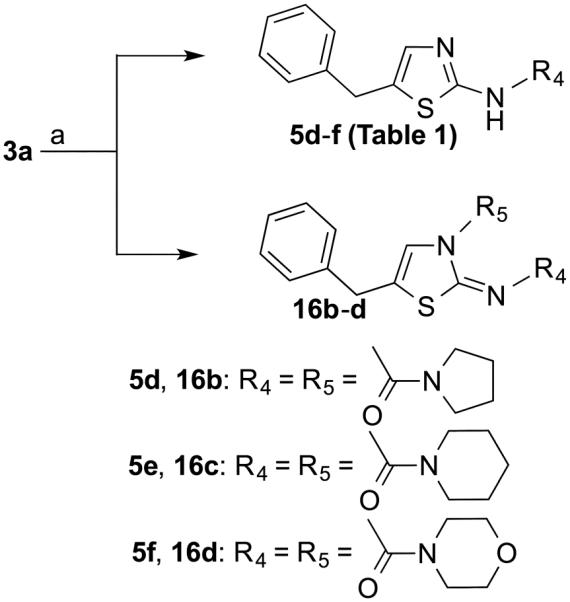
Reagents and conditions: (a) 3-Cyclohexylpropanoyl chloride, TEA, THF, rt.
2.2. Biology
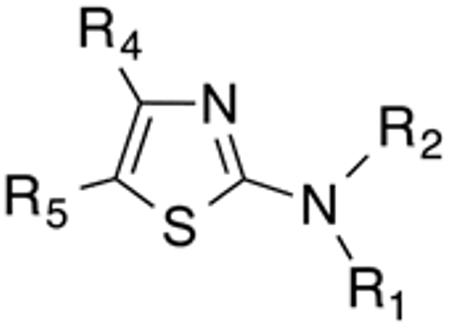
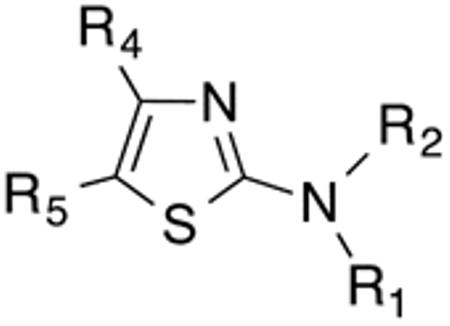
Using 41F5 (Figure 1) as the template, our primary intention was to develop an initial Histoplasma SAR for aminothiazole analogues (Figure 2) by mapping the molecular target's binding pocket through varying dimensions and basic properties of substituents at the 2- and 5-positions of the aminothiazole core. Another objective was to determine the general effect of substitution at the 3- and 4-positions of the ring system on activity. Some other substitution effects were explored as well. For analogues with an Histoplasma MIC50 < 3.5 µM, a very basic Cryptococcus SAR was also developed. In addition, for eleven compounds out of this group, we tested for potential liver toxicity in human hepatocyte HepG2 cells to establish Selectivity Indices (SIs).
Figure 2.
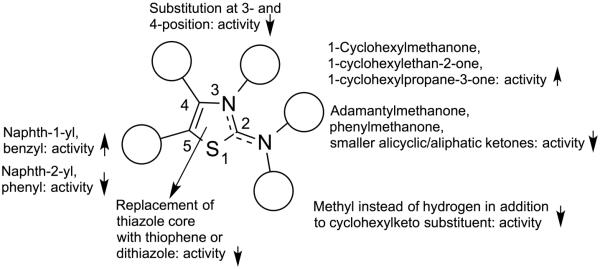
Basic Histoplasma SAR for aminothiazoles.
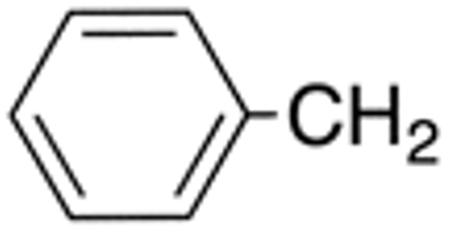
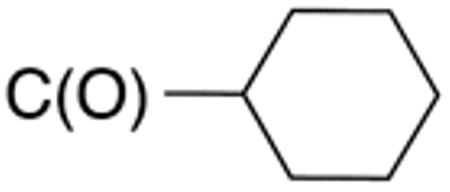
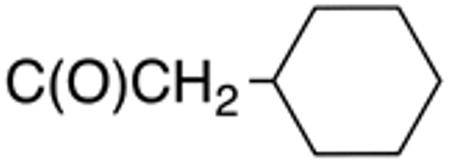
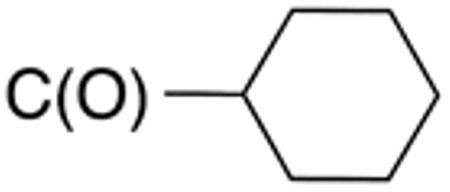
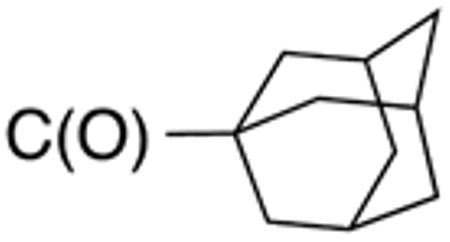
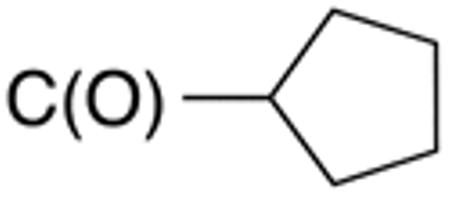
Table 1 and Figure 1 summarize the biological data of aminothiazole derivatives with a benzyl substituent at the 5-position and various types of substituents at the 2-position. Within this group of compounds, those with cyclohexylmethylamide- (4e) and cyclohexylethylamide (4f) substituents at the 2-position had the lowest Histoplasma MIC50- (0.7 µM) and MIC (1.3-2.5 µM) values. Cyclopentylamide- (4c), cyclohexylamide- (4d) and cyclobutylamide (2F8, Figure 1) substitutents caused slightly lower activity (MIC50=1.6-2.5 µM; MIC=5 µM), whereas those with isopropylamide- (4b), tetrahydrothiopyran- (5a), and adamantylamide groups had markedly reduced activity (MIC50=4.8-6.7 µM; MIC=10-20 µM). Interestingly, introduction of an E-ethene function between the amide and the cyclohexyl group (4g) reduced activity significantly compared with a cyclohexylethyl group (4f). Another noticeable finding was that the introduction of O- or N-heteroatoms at various positions of the ring systems at the 2-position reduced activity (5b-5h) even when the ring system was comparable in size to the cyclopentyl or cyclohexyl rings (5b-5f) (MIC50 > 10 µM; MIC >20 µM). Other compounds in Table 1, i.e. 3a (unsubstituted 2-amino group), 4a (2-trifluoromethylamide group) and 4h (5-phenylamide group) had MIC50- and MIC values > 10 µM and >20 µM, respectively.
Table 1.
5-Benzyl-substituted aminothiazole derivatives
|
Histoplasma
capsulatum |
Cryptococcus
neoformans |
HepG 2 cells |
SI (Histo/ HepG2) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Cmpd | R 4 |
R5 | R1 | R2 | MIC50
(μM) |
MIC (μM) |
MIC50
(μM) |
MIC (μM) |
MIC50
(μM) |
|
| 3a | H |
|
H | H | >10 | >20 | nd | nd | nd | nd |
| 4a | H |
|
H | C(O)CF3 | >10 | >20 | nd | nd | nd | nd |
| 4b | H |
|
H | C(O)CH(CH3)2 | 6.7 | 20 | nd | nd | nd | nd |
| 4c | H |
|
H |
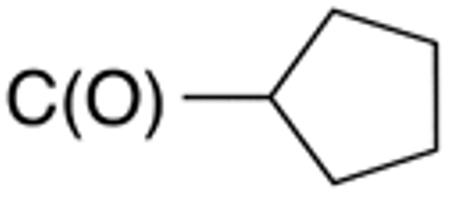
|
1.6±0.1 | 5 | 3.6±0. 9 |
10 | >40 | >24 |
| 4d | H |
|
H |
|
1.6±0.1 | 5 | 1.3±0. 2 |
5 | .>40 | >21 |
| 4e | H |
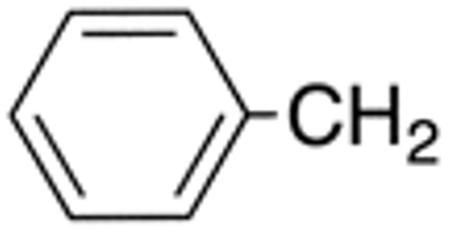
|
H |
|
0.7±0.1 | 2.5 | >10 | >20 | >40 | >44 |
| 4f | H |
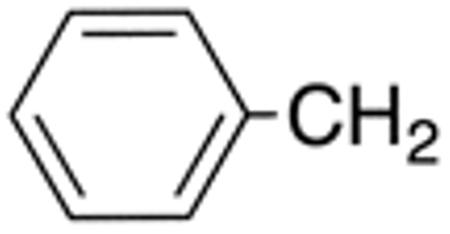
|
H |
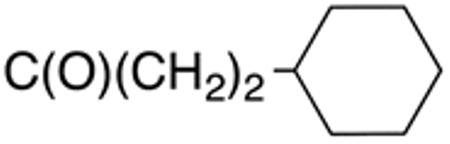
|
0.7±0.1 | 1.3 | >10 | >20 | >40 | >44 |
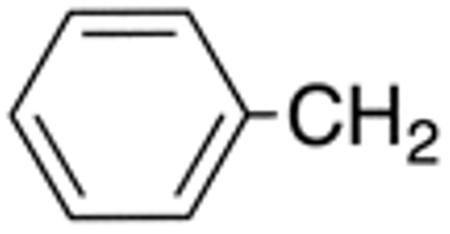
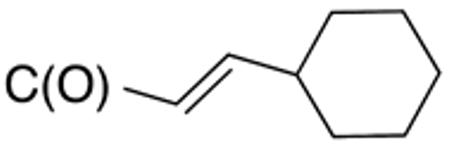
| 4g | H |
|
H |
|
>10 | >20 | nd | nd | nd | nd |
| 4h | H |
|
H |
|
>10 | >20 | nd | nd | nd | nd |
| 4i | H |
|
H |
|
5.0 | 10 | nd | nd | nd | nd |
| 5a | H |
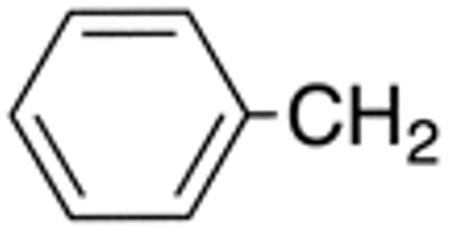
|
H |
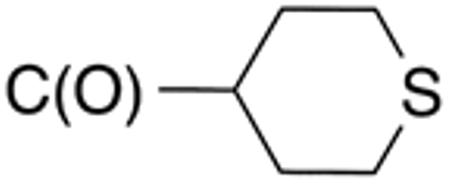
|
4.8 | 10 | nd | nd | nd | nd |
| 5b | H |
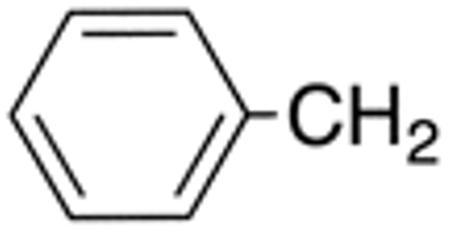
|
H |
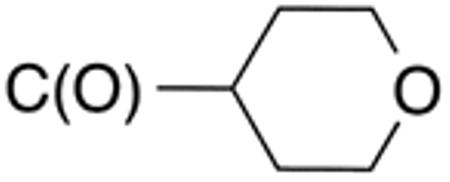
|
>10 | >20 | nd | nd | nd | nd |
| 5c | H |
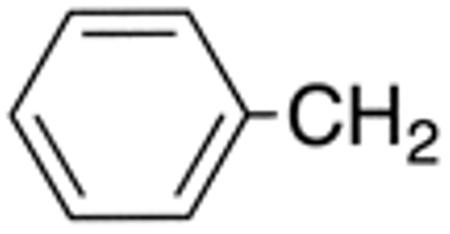
|
H |
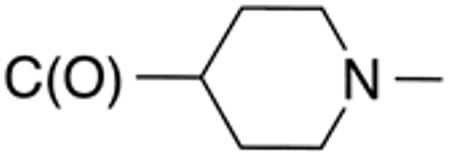
|
>10 | >20 | nd | nd | nd | nd |
| 5d | H |
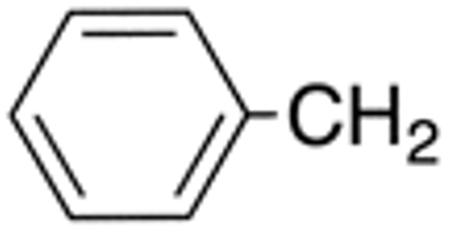
|
H |
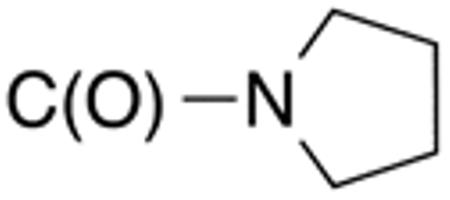
|
>10 | >20 | nd | nd | nd | nd |
| 5e | H |
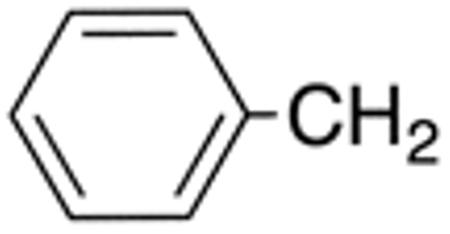
|
H |
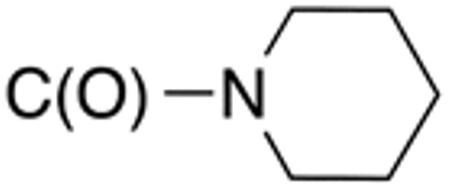
|
>10 | >20 | nd | nd | nd | nd |
| 5f | H |
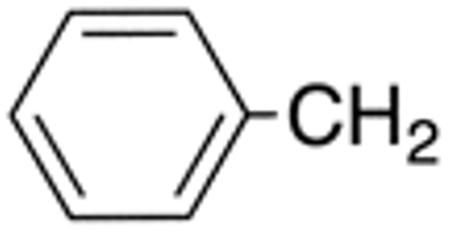
|
H |
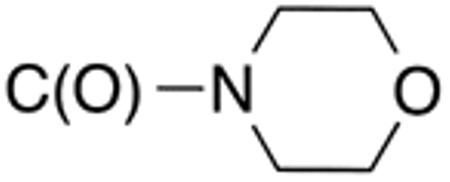
|
>10 | >20 | nd | nd | nd | nd |
| 5g | H |
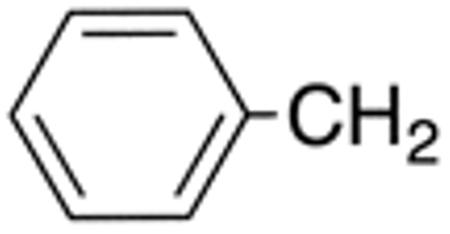
|
H |
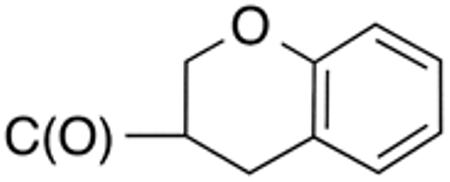
|
>10 | >20 | nd | nd | nd | nd |
| 5h | H |
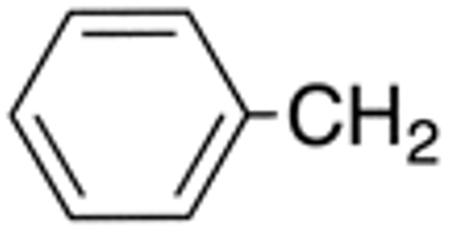
|
H |
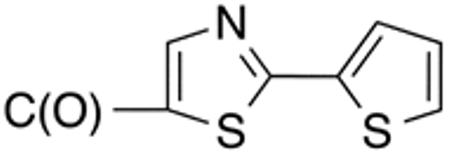
|
>10 | >20 | nd | nd | nd | nd |
1 not determined
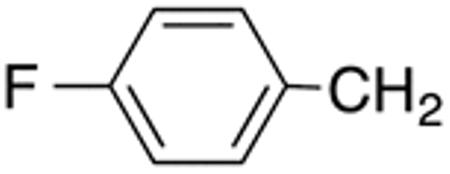
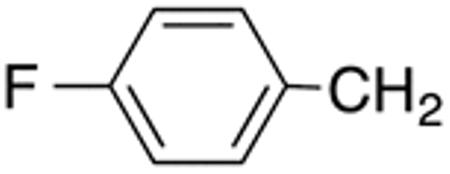
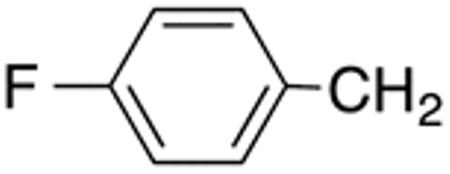
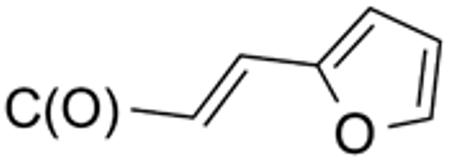
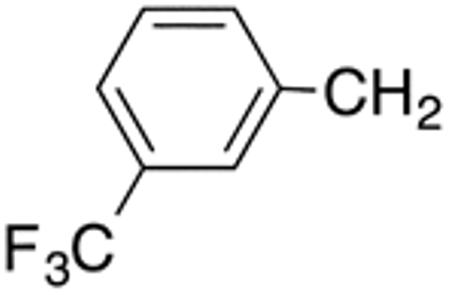
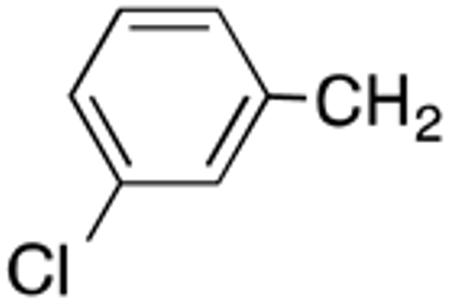
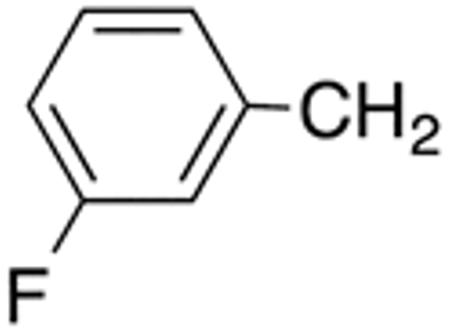
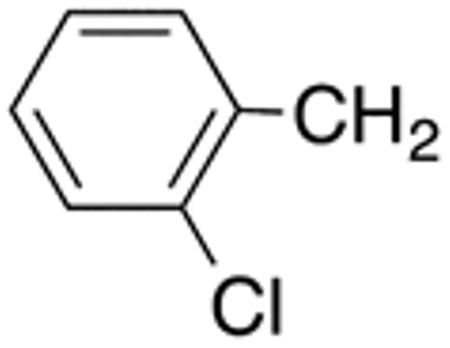
With the compounds shown in Table 2, we explored the effect of substitution at the 5-benzyl group on anti-Histoplasma activity. Aromatic ortho- (8a, 8b) and meta- (7b, 7c) substitution with fluorine and chlorine did not substantially alter activity compared with unsubstituted 4c-4f (Table 1) whereas para-substitution (6a-6d) had a negative impact. Noticeable is the lack of activity observed for 7a with a trifluoromethyl group in meta-position.
Table 2.
5-Benzyl-substituted aminothiazoles with differing substitution pattern at the 5-benzyl group
|
Histoplasma
capsulatum |
Cryptococc
us neoformans |
HepG 2 cells |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Cmp d |
R4 | R5 | R 3 |
R4 | MIC50
(μM) |
MIC (μM) |
MIC5
0 (μM) |
MIC (μM) |
MIC5
0 (μM) |
SI (Histo/ HepG2) |
| 6a | H |
|
H |
|
5.2 | 20 | 1nd | nd | nd | nd |
| 6b | H |
|
H |
|
4.4±1. 0 |
20 | nd | nd | nd | nd |
| 6c | H |
|
H |
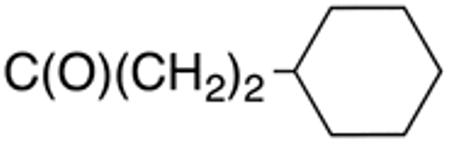
|
>10 | >20 | nd | nd | nd | nd |
| 6d | H |
|
H |
|
>10 | >20 | nd | nd | nd | nd |
| 7a | H |
|
H |
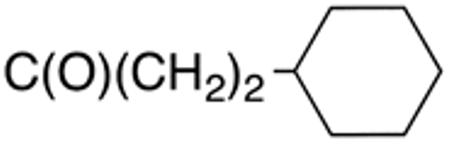
|
>10 | >20 | nd | nd | nd | nd |
| 7b | H |
|
H |
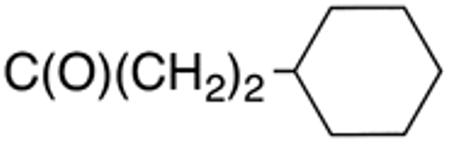
|
1.1±0. 4 |
2.5 | >10 | >20 | 39±2 | 65 |
| 7c | H |
|
H |
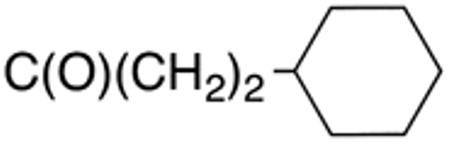
|
0.8±0. 1 |
2.5 | >10 | >20 | 40±2 | 57 |
| 8a | H |
|
H |
|
0.9±0. 3 |
2.5 | >10 | >20 | nd | nd |
| 8b | H |
|
H |
|
1.0±0. 3 |
2.5 | >10 | >20 | nd | nd |
not determined
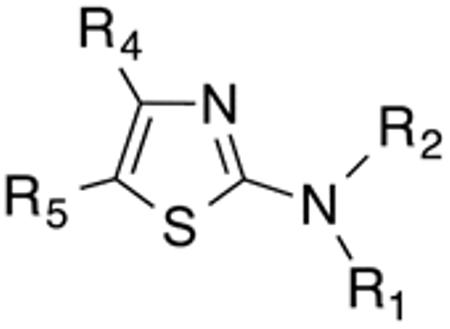
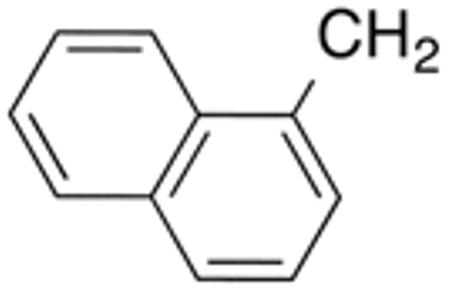
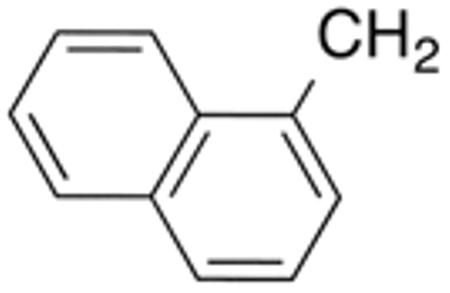
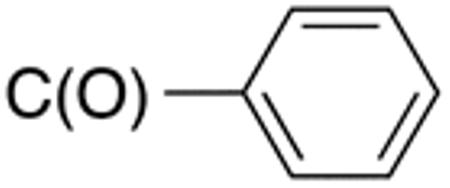
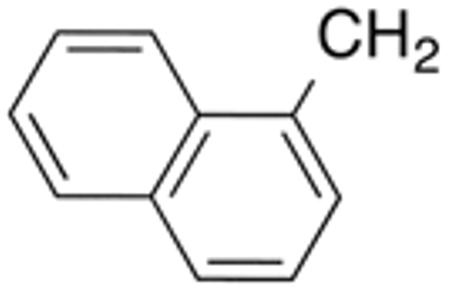
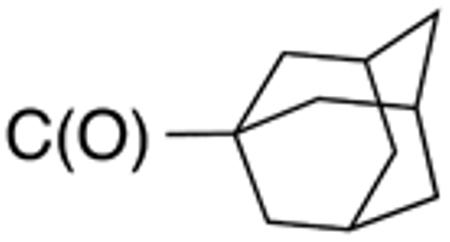
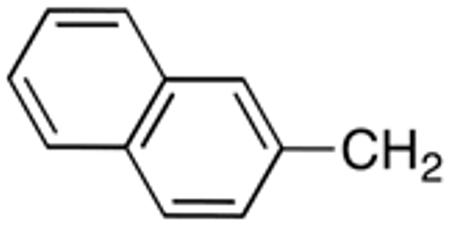
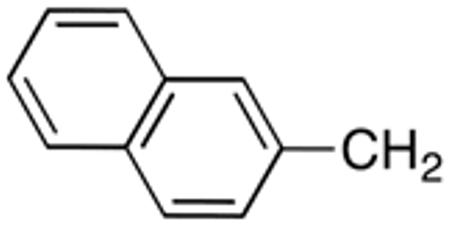
Table 3 displays biological data for compounds with naphth-1-ylmethyl- or naphth-2-ylmethyl group at the 5-position. Within the group of aminothiazole derivatives with a naphth-1-ylmethyl substituent, a similar trend was observed as for compounds with 5-benzyl substituent (Table 1). Compounds with cyclohexylamide- (9d), cyclohexylmethylamide- (9e) and cyclohexylethylamide (9f) groups at the 2-position had the lowest Histoplasma IC50 values (MIC50=0.4 µM; MIC=0.6-1.3 µM) whereas those with smaller isopropylamide- (9b) and cyclopentylamide (9c) substituents had slightly reduced activity. Trifluoromethylamide- (9a), phenylamide- (9g) and adamantylamide (9h) substituents at the 2-position caused markedly reduced activity (MIC50 >10 µM; MIC >20 µM). Except for compounds 10a, with a 2-isopropylamide group (MIC50=4.5 µM; MIC=20 µM), and 10c, with 2-cyclohexylethylamide group (MIC50=5.7 µM; MIC=20 µM), all aminothiazole derivatives with a naphth-2-ylmethyl group at the 5-position had MIC50 values > 10 µM (MIC >20 µM). Comparing naphth-1-ylmethyl substitution at the 5-position with benzyl substitution at the same position, the isopropylamide group at the 2-position had a significant impact on activity, as evidenced by an MIC50 of 1.5 µM (MIC=2.5 µM) for 9b vs. MIC50 of 6.7 µM (MIC=20 µM) for 4b. Overall, compounds 9d-9f, with naphth-1-ylmethyl substituent at the 5-position combined with cyclohexylamide-, cyclohexylmethylamide-, and cyclohexylethylamide substituents, respectively, at the 2-position, had the highest anti-Histoplasma activities (MIC50 = 0.4 µM; MIC =0.6-1.3 µM) of all tested compounds.
Table 3.
5-Napthyl-substituted aminothiazole derivatives
|
Histoplasma
capsulatum |
Cryptococcus
neoformans |
HepG 2 cells |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Cmpd | R 4 |
R5 | R 1 |
R2 | MIC50
(μM) |
MIC (μM) |
MIC50
(μM) |
MIC (μM) |
MIC5
0 (μM) |
SI (Histo/ HepG2) |
| 9a | H |
|
H | C(O)CF3 | >10 | >20 | 1nd | nd | nd | nd |
| 9b | H |
|
H | C(O)CH(CH3)2 | 1.5±0.1 | 2.5 | >10 | >20 | nd | nd |
| 9c | H |
|
H |
|
0.8±0.1 | 1.3 | 0.8±0. 3 |
1.3 | >40 | >57 |
|
9d
(41F5 ) |
H |
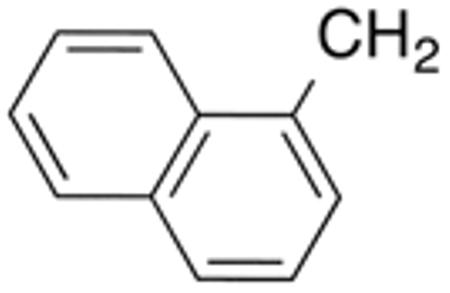
|
H |
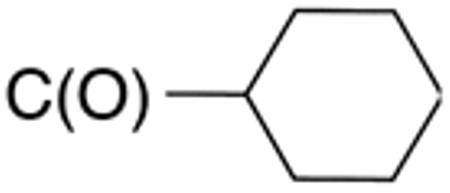
|
0.4±0.0 1 |
0.6 | 0.4±0. 1 |
0.6 | 39±1 | 97 |
| 9e | H |
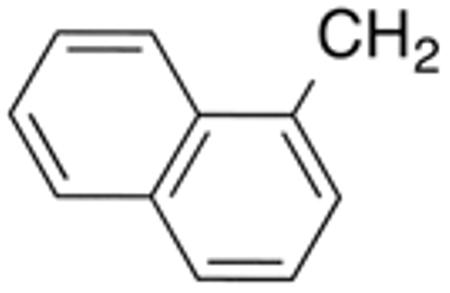
|
H |
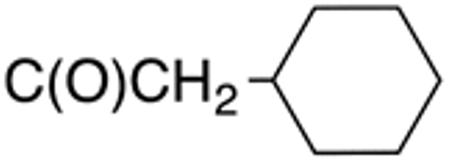
|
0.4±0.0 1 |
0.6 | >10 | >20 | 37±1 | 92 |
| 9f | H |
|
H |
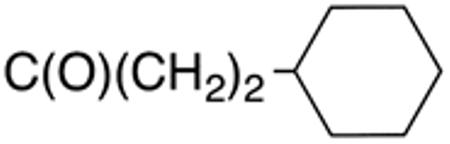
|
0.4±0.0 2 |
1.3 | >10 | >20 | >40 | >100 |
| 9g | H |
|
H |
|
>10 | >20 | nd | nd | nd | nd |
| 9h | H |
|
H |
|
>10 | >20 | nd | nd | nd | nd |
| 10a | H |
|
H | C(O)CH(CH3)2 | 4.5 | 20 | nd | nd | nd | nd |
| 10b | H |
|
H |
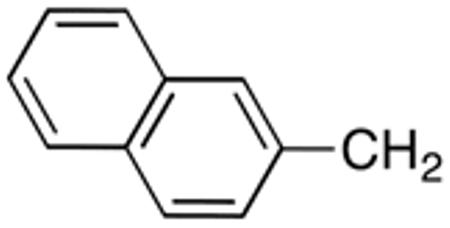
|
>10 | >20 | nd | nd | nd | nd |
| 10c | H |
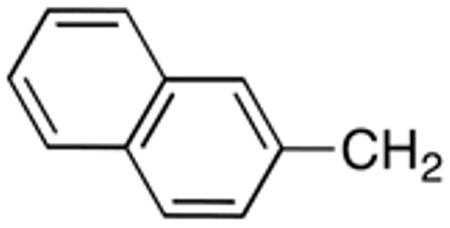
|
H |
|
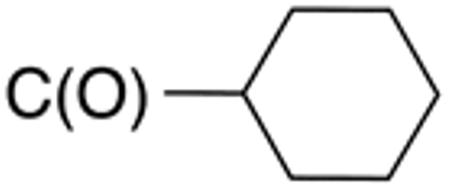
5.7 | 20 | nd | nd | nd | nd |
| 10d | H |
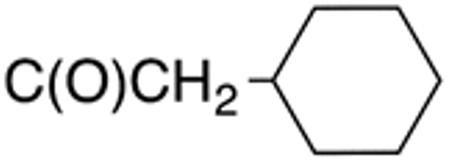
|
H |
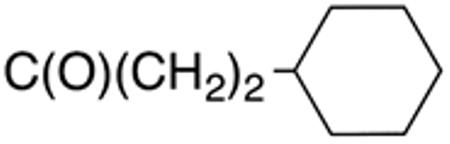
|
>10 | >20 | nd | nd | nd | nd |
| 10e | H |
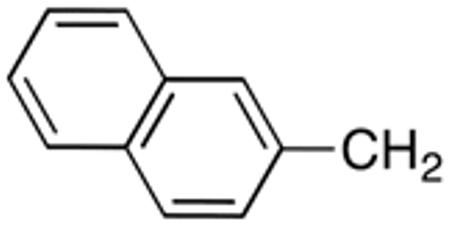
|
H |
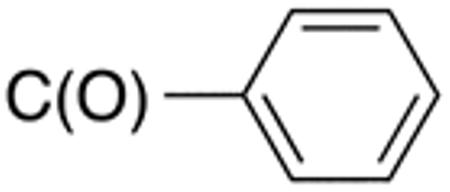
|
>10 | >20 | nd | nd | nd | nd |
| 10f | H |
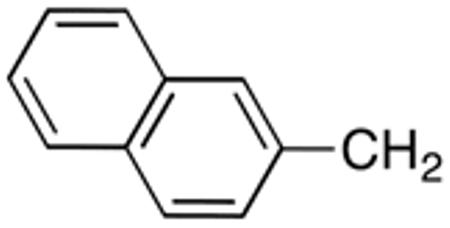
|
H |
|
>10 | >20 | nd | nd | nd | nd |
not determined
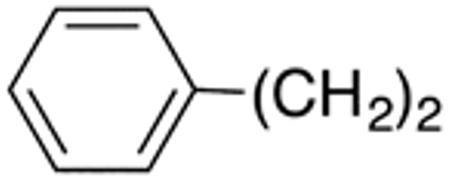
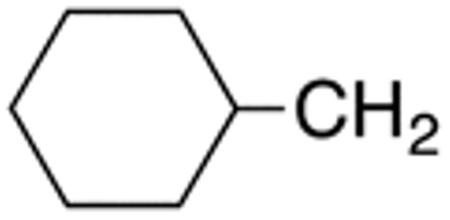
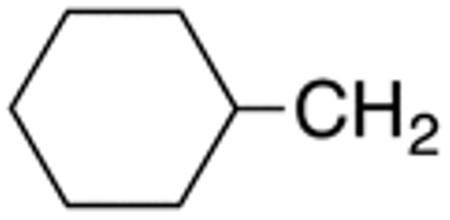
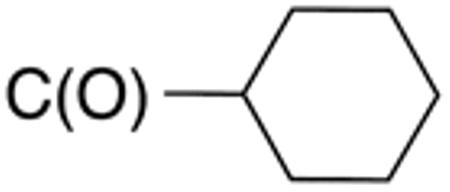
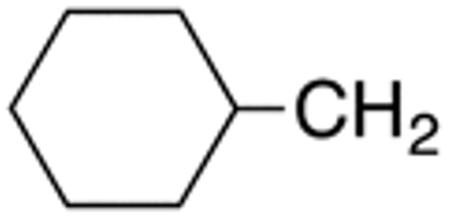
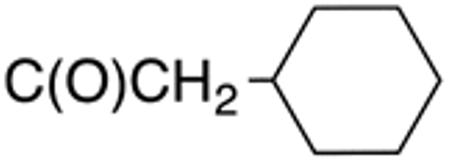
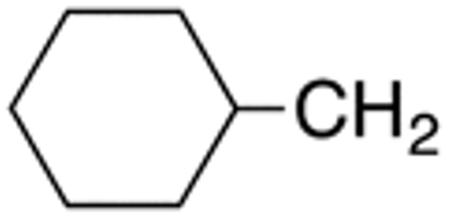
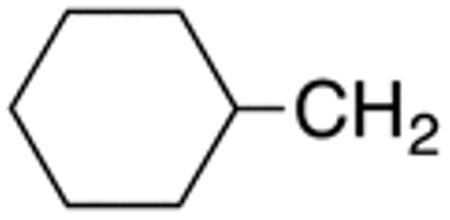
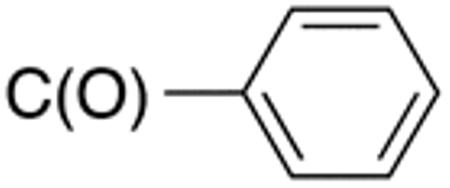
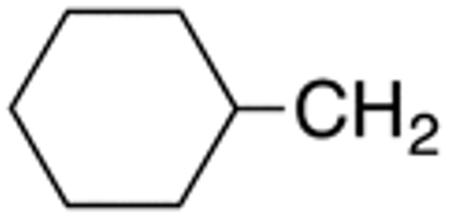
The compounds presented in Table 4 were evaluated to determine the effect of phenylethyl-, phenyl-, and cyclohexylmethyl substituents at the 5-position on Histoplasma growth. Phenylethyl- (11a, 11b) substitution reduced activity only slightly compared to naphth-1-ylmethyl substitution (9d-9f, Table 3) whereas phenyl substitution (12b-12d) had a detrimental impact on activity. An interesting pattern was observed for compounds with cyclohexylmethyl substituent at the 5-position. Those with isopropylamide- (13a), cyclohexylmethylamide- (13c), and adamantylamide (13f) groups at the 2-position had no activity, whereas those with phenylamide (13e) substitutent retained some activity (MIC50=4.6 µM; MIC=10 µM)). In contrast, cyclohexylamide- (13b) and cyclohexylethylamide- (13d) substitution produced high activity (MIC50=0.7-0.9 µM; MIC=2.5-5.0 µM).
Table 4.
Aminothiazole derivatives with phenylethyl,- phenyl-, and cyclohexylmethyl substituents at the 5 position
|
Histoplasma
capsulatum |
Cryptococcus
neoformans |
HepG 2 cells |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Cmp d |
R4 | R5 | R 1 |
R2 | MIC50
(μM) |
MIC (μM) |
MIC50
(μM) |
MIC (μM) |
MIC5
0 (μM) |
SI (Histo/ HepG2) |
| 11a | H |
|
H |
|
0.9±0.1 | 2.5 | 2.7±0. 6 |
5 | 1nd | nd |
| 11b | H |
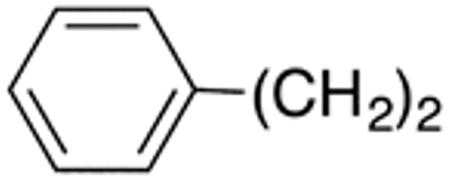
|
H |
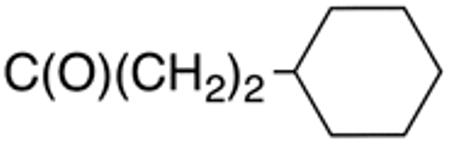
|
0.7±0.1 | 2.5 | >10 | >20 | >40 | >50 |
| 12a | H |
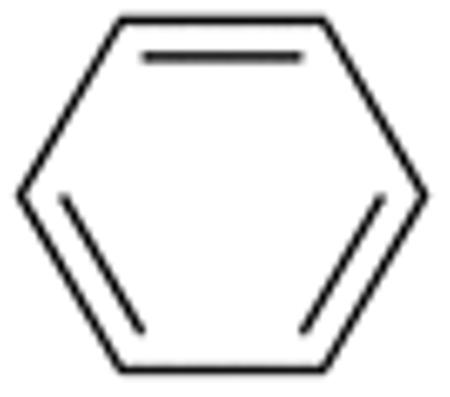
|
H |
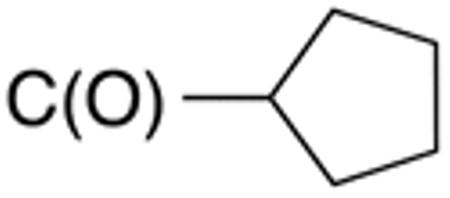
|
>10 | >20 | nd | nd | nd | nd |
| 12b | H |
|
H |
|
>10 | >20 | nd | nd | nd | nd |
| 12c | H |
|
H |
|
>10 | >20 | nd | nd | nd | nd |
| 12d | H |
|
H |
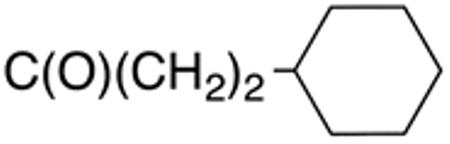
|
>10 | >20 | nd | nd | nd | nd |
| 13a | H |
|
H | C(O)CH(CH3)2 | >10 | >20 | nd | nd | nd | nd |
| 13b | H |
|
H |
|
0.7±0.1 1 |
2.5 | >10 | >20 | nd | nd |
| 13c | H |
|
H |
|
>10 | >20 | nd | nd | nd | nd |
| 13d | H |
|
H |
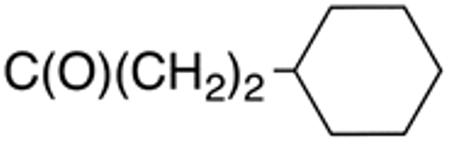
|
0.8±0.1 | 5 | 9.6 | >20 | nd | nd |
| 13e | H |
|
H |
|
4.6 | 10 | nd | nd | nd | nd |
| 13f | H |
|
H |
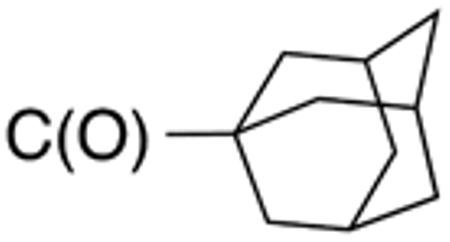
|
>10 | >20 | nd | nd | nd | nd |
not determined
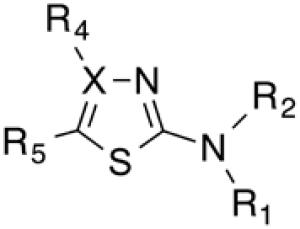
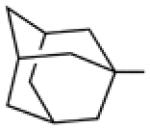
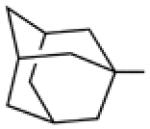
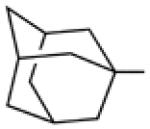
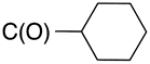
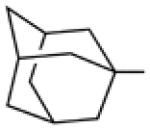
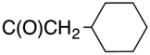
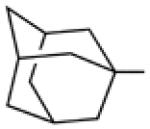
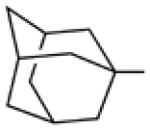
Biological data for compounds with varying substitution patterns, including groups at the 3- or -4 position, are shown in Table 5, Schemes 2 & 3, and Figure 1. Compounds with substituents at the 3- (16a-16d, Schemes 2 and 3 [data not shown]) and 4- (14c, 15a-15h, Table 5) positions had reduced anti-Histoplasma activity or were inactive. Some aminothiazole derivatives with bulky adamantyl substitution at the 4-position in combination with hydrogen at the 5-position retained moderate activity (15d, 15e, 15g, 15h) with MIC50 values ranging from 2.5 µM to 7.4 µM (MIC=5-20 µM). In contrast, compounds with 4-benzyl group and hydrogen at the 5-position (15a, 15b) had no activity. Disubstitution at the amide nitrogen (14b) as well as replacement of thiazole with thiadiazole (14a), and possibly also thiophene (4H2, Figure 1), abolished or reduced activity. In addition, the simultaneous presence of a hydroxymethyl linker at the 5-position and a Boc group at the 2-position (2a, Table 5) resulted in lack of activity. Interestingly, compounds 15e-15h (Table 5) and 13b-13e (Table 4) showed similar activity patterns. In both sub-series, 2-cyclohexylmethylamide substitution (13c, 15f) abolished activity whereas the cyclohexylamide- (13b, 15e), cyclohexylethylamide- (13d, 15g), and phenyl (13e, 15h) homologues retained activity.
Table 5.
Aminothiazole derivatives with varying substitution patterns
|
Histoplasma
capsulatum |
Cryptococcus
neoformans |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Cmp d |
X | R4 | R5 | R1 | R2 | MIC50
(μM) |
MIC (μM) |
MIC5
0 (μM) |
MIC (μM) |
| 2a | C | H |
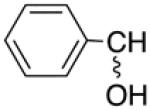
|
H | C(O)OC(CH3)3 | >10 | >20 | 1nd | nd |
| 14a | N | – |
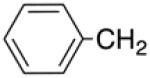
|
H |
|
>10 | >20 | nd | nd |
| 14b | C | H |
|
Me |
|
>10 | >20 | nd | nd |
| 14c | C | Me |
|
H |
|
>10 | >20 | nd | nd |
| 15a | C |
|
H | H |
|
>10 | >20 | nd | nd |
| 15b | C |
|
H | H |
|
>10 | >20 | nd | nd |
| 15c | C |
|
H | H | C(O)CH(CH3)2 | >10 | >20 | nd | nd |
| 15d | C |
|
H | H |
|
7.4 | 20 | nd | nd |
| 15e | C |
|
H | H |
|
2.5±0.1 | 5 | >10 | >20 |
| 15f | C |
|
H | H |
|
>10 | >20 | nd | nd |
| 15g | C |
|
H | H |
|
5.2 | 20 | nd | nd |
| 15h | C |
|
H | H |
|
3.1±0.0 8 |
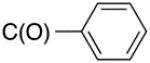
10 | 8.3 | 20 |
not determined
Although only a very basic SAR was established for efficacy against Cryptococcus, we noted some similarities but also differences to the Histoplasma SAR. As in the case of the Histoplasma SAR, compounds with cyclopentaneamide- (4c, 9c) and cyclohexaneamide groups (4d, 9d) at the 2-position had anti-Cryptococcus activity. In contrast to the Histoplasma SAR, however, the compounds with larger cyclohexylmethylamide- (4e, 9e) and cyclohexylethylamide (4f, 9f) substituents had lost activity against Cryptococcus. The same is the case for the smaller isopropyl group (9b). At the 5-postion, the naphth-1-ylmethyl group (9c, 9d) resulted in highest activity where as the benzyl- (4c, 4d) and phenylethyl (11a) groups caused moderate loss of activity.
SIs between >21(4d) and >100 (9f) indicate generally low host toxicity of the aminothiazole antifungals. Interestingly, commercially-available compound 6d (Table 2) did not have any antifungal activity against Histoplasma. However, the same compound caused in vitro proliferation inhibition of DU-145 human prostate cancer cells with an IC50 of 15 nM.15 This compound, with host cell toxicity but no antifungal activity, highlights the good specificity for fungi of the aminothiazole analogues presented here.
Most of the active aminothiazoles are very lipophilic and do not contain functional groups that are ionizable under physiological conditions. For example, 9d has a CLogP of 5.67 (ChemBioDraw 13.0.2.3020). Thus, in the case of some of the compounds that were tested in more detail, slight (4c, 7c, 9d, 9e) to moderate (4e, 7b, 9c) precipitation at high compound concentrations (generally >40 µM) was noticed during the antifungal assays in the test vials even though the media contained 1% DMSO. Solubility could be improved by increasing the DMSO concentrations. However, at concentrations above 1%, DMSO itself inhibited fungal growth.
3. Summary and Conclusions
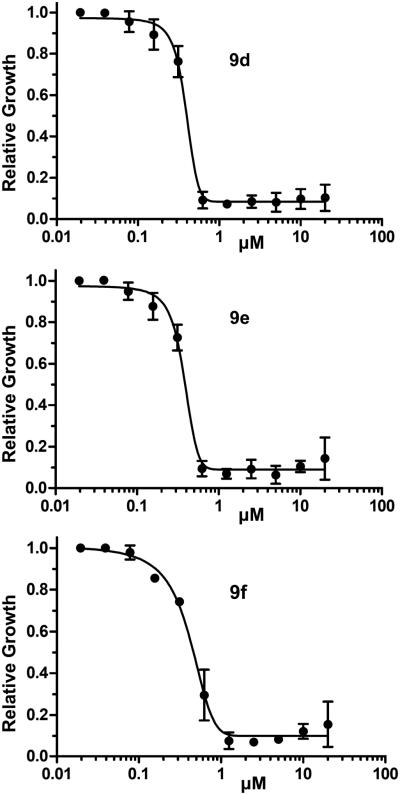
The Histoplasma SAR studies resulted in the lowest MIC50 and MIC values (0.4 µM; MIC=0.6-1.3 µM) for compounds 9d (41F5), 9e, and 9f. These compounds have a naphth-1-ylmethyl group at the 5-position and a cyclohexylamide-, cyclohexylmethylamide-, and cyclohexylethylamide substituent, respectively, at the 2-position. The MIC values of these compounds are approximately 1-2 µM (Figure 3), which is in the range of effective in vitro concentrations of some clinically established antifungal-22 and antiparasitic drugs.23 The highest SI (> 100) was observed for compound 9f. Overall, SIs ranged from >21 to >100, which indicates the possibility of low host toxicity of these novel aminothiazole antifungals and significant differences to, or even absence of, equivalent mammalian targets for the tested aminothiazole antifungals. This hypothesis is also supported by the finding that compound 6d, which is structurally related to 41F5, did not have antifungal activity although it proved to be a strong inhibitor of in vitro proliferation of DU-145 human prostate cancer cells.15 It was previously observed that 9d (41F5) has inhibitory rather than microbicidal action.7 It remains to be determined weather the newly tested compounds have the same activity profile.
Figure 3.
Dose-response curves for compounds 9d-9f.
Previous studies have shown that 9d (41F5) does not inhibit the in vitro growth of Candida albicans, Aspergillus fumigatus, and Blastomyces dermatitidis.7 Strikingly, 9d (41F5), and to a slightly lesser extend 9c, have antifungal activity against two phylogenetically diverged organisms, i.e. the basidiomycete Cryptococcus and the ascomycete Histoplasma, yet there are subtle differences in the SAR for these fungi. We suspect that aminothiazoles target a common molecule in both fungal species, but that the binding site has diverged between members of different phyla within the kingdom Fungi. This suggests the possibility that this class of antigfungals has potential to inhibit the same molecular target also in other fungal pathogens (e.g., Candida, Aspergillus, or Blastomyces) based on appropriate structural modifications. Preliminary studies indicate that there is no synergism beyond additive effects between 9d (41F5) and fluconazole (Fractional Inhibitory Concentration index [FIC] ~ 0.7), nor between 9d (41F5) and caspofungin (FIC ~ 1.1) suggesting that the aminothiazole antifungal target is not involved in ergosterol biosynthesis or cell wall β-glucan synthesis.24 Thus, the possibility of a new molecular target in the area of fungal therapy seems likely, warranting further investigation.
4. Experimental Methods
4. 1. Chemistry
4.1.1. General chemical procedures
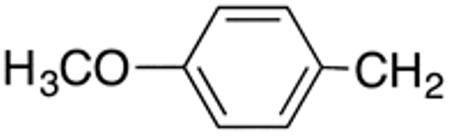
1H- and 13C-NMR spectra were obtained on a Bruker DRX 400 at The Ohio State University College of Pharmacy (400 MHz for 1H and 100 MHz for 13C). Chemical shifts (δ) are reported in ppm from internal deuterated chloroform or methanol. Coupling constants are reported in Hz. 13C-NMR spectra are fully decoupled. High Resolution–Electrospray ionization (HR-ESI) mass spectra were obtained on a Micromass LCT spectrometer at The Ohio State University Campus Chemical Instrumentation Center, Columbus, OH. Silica gel 60 (0.063-0.200 mm), used for gravity column chromatography, and silica gel 60 (0.015-0.049 mm), used for flash column chromatography, were purchased from Dynamic Adsorbents Inc., Norcross, USA). Reagent-grade solvents were used for column chromatography. Pre-coated aluminum-backed TLC plates with silica gel 60 F254 (0.25-mm layer thickness) from Sigma-Aldrich were used for TLC. Compound visualization for TLC was achieved by UV light. Anhydrous solvents, starting materials, including compounds 1a, 1b, 3m, 3n, and 3o, and other chemicals were purchased from standard commercial suppliers. 3-(2-Furyl)-N-[5-(4-methoxybenzyl)-1,3-thiazol-2-yl]acrylamide (6d) was purchased from Chembridge, San Diego, CA, USA. Compounds 3c, 3d, 4b, 4d, 6a, 6b, 9b, 9d, 12a, 12b, 14a, and 15b are commercially available. However, no synthetic procedures and analytical data are published. These compounds were synthesized and analyzed as described in sections 4.1.3. and 4.1.4. Compounds 2f,25 3a,26 3b,27 3e,15 3f,25 3g,28 3h,28 3i,29 3j,29 3k,18 3l,18 4h,30 15a,20 and 15h31 are commercially available and/or have been reported before. These compounds were synthesized and analyzed as described in sections 4.1.2., 4.1.3. and 4.1.4. using partially different methods as described previously. Compound 3p was prepared according to a procedure described by Hardgrave et al.32 (2E)-3-Cyclohexyl-2-propenoic acid, used for the synthesis of 4g, was prepared according procedures previously described.33 All reactions were carried under Argon atmosphere.
4.1.2. General procedure for the synthesis of compounds 2a-2l.
n-BuLi (2.5 M in THF, 1.4 eq.) was added drop wise to a mixture of compound 1a or 1b (1 eq.) and aldhehyde (1.2 eq.) in THF (~ 20 mL) at −78 C for 2h. The reaction was quenched by adding a saturated aqueous solution of NH4Cl, extracted with EtOAc, washed with water and then brine. The organic phase was dried over anhydrous Na2SO4, evaporated, and the residue was purified by silica gel column chromatography.
4.1.2.1. tert-Butyl{5-[hydroxy(phenyl)methyl]thiazol-2-yl}carbamate (2a)
White solid; Rf 0.49 (DCM/MeOH, 19/1); yield 59%. 1H NMR (400 MHz, CDCl3): δ 7.29-7.48 (m, 5H), 7.07 (s, 1H), 6.01 (s, 1H), 2.20 (s, 1H), 1.48 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 162.3, 152.8, 142.3, 135.2, 134.2, 128.8, 128.3, 126.2, 82.3, 70.6, 28.3. MS (HR-ESI) for C15H18N2O3SNa [(M +Na)+], calcd: m/z 329.0936, found: m/z 329.0917.
4.1.2.2. tert-Butyl{5-[hydroxy(naphthalen-1-yl)methyl]thiazol-2-yl}carbamate (2b)
White solid; Rf 0.55 (DCM/MeOH, 19/1); yield 30%. 1H NMR (400 MHz, CDCl3): δ 11.71 (br s, 1H), 7.97 (m, 1H), 7.82 (m, 3H), 7.46 (m, 3H), 6.95 (s, 1H), 6.67 (s, 1H), 2.21 (s, 1H), 1.34 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 162.0, 137.7, 135.0, 134.4, 134.0, 130.3, 129.0, 126.5, 125.9, 125.5, 123.8, 123.6, 82.0, 67.8, 28.2. MS (HR-ESI) for C19H20N2OSNa [(M+Na)+], calcd: m/z 379.1092, found: m/z 379.1089.
4.1.2.3. tert-Butyl-{5-[hydroxy(naphthalen-2-yl)methyl]thiazol-2-yl}carbamate (2c)
White solid; Rf 0.54 (DCM/MeOH, 19/1); yield 30%. NMR (400 MHz, CDCl3): δ 11.83 (s, 1H), 7.93 (s, 1H), 7.84 (m, 3H), 7.49 (m, 3H), 7.06 (s, 1H), 6.14 (s, 1H), 2.17 (s, 1H), 1.39 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 162.4, 139.7, 135.1, 134.6, 133.3, 128.7, 128.3, 127.8, 126.5, 126.4, 124.9, 124.3, 82.2, 70.7, 28.2. MS (HR-ESI) for C19H20N2O3SNa [(M+Na)+], calcd: m/z 379.1092, found: m/z 379.1087.
4.1.2.4. tert-Butyl-{5-[hydroxy(cyclohexyl)methyl]thiazol-2-yl}carbamate (2d)
White solid; Rf 0.35 (DCM/MeOH, 9/1); yield 38%. 1H NMR (400 MHz, CDCl3): δ 7.20 (s, 1H), 4.61 (d, J = 6.83 Hz, 1H), 2.03 (m, 1H), 1.64-1.79 (m, 5H), 1.59 (s, 9H), 0.98-1.29 (m, 5H). 13C NMR (100 MHz, CDCl3): δ 161.8, 152.7, 134.4, 132.3, 82.7, 73.2, 45.2, 29.3, 29.0, 28.4, 26.0. MS (HR-ESI) for C15H24N2O3SNa [(M+Na)+], dalcd: m/z 335.1405, found: m/z 335.1397.
4.1.2.5. tert-Butyl-{5-[hydroxy-(4-fluorophenyl)methyl]thiazol-2-yl}carbamate (2e)
White solid; Rf 0.5 (DCM/MeOH, 19/1); yield 46%. 1H NMR (400 MHz, CDCl3): δ 11.85 (br s, 1H), 7.44 (m, 2H), 7.04-7.09 (m, 2H), 6.0 (s, 1H), 2.19 (s, 1H) 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 168.7, 159.4, 143.5, 140.1, 139.4, 132.9, 119.3, 118.9, 82.7, 69.1, 24.3. MS (HR-ESI) for C18H22N2OSNa [(M+Na)+], calcd: m/z 347.0842, found: m/z 347.0839.
4.1.2.6. tert-Butyl-{5-[hydroxy(3-trifluoromethylphenyl)methyl]thiazol-2-yl}carbamate (2f)
White solid; Rf 0.52 (DCM/MeOH, 19/1); yield 31%. 1H NMR (400 MHz, CDCl3): δ 11.95 (br s, 1H), 7.75 (s, 1H), 7.67 (m, 1H), 7.59 (m, 1H), 7.52 (m, 1H), 7.04 (s, 1H), 6.08 (s, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 163.0, 153.13, 143.6, 134.9, 134.6, 131.6, 131.2, 129.9, 129.5, 125.3, 123.3, 82.6, 70.1, 28.5. MS (HR-ESI) for C16H17F3N2O3S [(M+Na)+], calcd: m/z 397.0812, found: m/z 397.0810.
4.1.2.7. tert-Butyl-{5-[hydroxy(3-chlorophenyl)methyl]thiazol-2-yl)carbamate (2g)
White solid; Rf 0.51 (DCM/MeOH, 19/1); yield 53%.1H NMR (400 MHz, CDCl3): δ 11.91 (s, 1H), 7.39 (s, 1H), 7.33 (m, 3H), 6.01 (s, 1H), 2.19 (s, 1H), 1.49 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 162.6, 152.9, 144.4, 134.7, 134.6, 134.5, 130.0, 128.4, 126.4, 124.4, 82.3, 69.9, 28.3. MS (HR-ESI) for C15H17ClN2O3S [(M+Na)+], calcd: m/z 363.0546, found: m/z 363.0547.
4.1.2.8. tert-Butyl-{5-[hydroxy(3-fluorophenyl)methyl]thiazol-2-yl}carbamate (2h)
White solid; Rf 0.5 (DCM/MeOH, 19/1); yield 58%. 1H NMR (400 MHz, CDCl3): δ 11.68 (br s, 1H), 7.34 (m, 1H), 7.22 (m, 2H), 7.14 (s, 1H), 7.03 (m, 1H), 6.0 (s, 1H), 2.19 (s, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 164.3, 162.5, 161.9, 144.9, 134.6, 130.3, 121.8, 115.3, 115.0, 113.4, 113.2, 82.3, 69.9, 28.3. MS (HR-ESI) for C15H17FN2OS [(M+H)+]. Calcd: m/z 347.0842. Found: m/z 347.0845.
4.1.2.9. tert-Butyl-{5-[hydroxyl(2-fluorophenyl)methyl]thiazol-2-yl}carbamate (2i)
White solid; Rf 0.51 (DCM/MeOH, 19/1); yield 46%. 1H NMR (400 MHz, CDCl3): δ 11.95 (s, 1H), 7.64 (t, J = 7.61 Hz, 1H), 7.29-7.35 (m, 1H), 7.21 (t, J = 7.5 Hz, 1H), 7.03-7.10 (m, 2H), 6.29 (s, 1H), 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 162.3, 161.0, 158.5, 152.9, 134.4, 133.8, 124.7, 124.6, 115.7, 115.5, 82.1, 64.7, 28.3. MS (HR-ESI) for C15H17FN2O3S [(M+H)+], calcd: m/z 325.1022, found: m/z 325.1024.
4.1.2.10. tert-Butyl-{5-[hydroxyl(2-chlorophenyl)methyl]thiazol-2-yl}carbamate (2j)
White solid; Rf 0.49 (DCM/MeOH, 19/1); yield 48%. 1H NMR (400 MHz, CDCl3): δ 11.97 (s, 1H), 7.81 (m, 1H), 7.33-7.44 (m, 2H), 7.21-7.32 (m, 1H), 7.09 (s, 1H), 6.41 (s, 1H), 1.52 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 162.3, 152.9, 139.9, 134.9, 133.3, 132.1, 129.7, 129.3, 127.5, 127.5, 82.2, 67.2, 28.3. MS (HR-ESI) for C15H17ClN2O3S[(M+Na)+], calcd: m/z 363.0546, found: m/z 363.0551.
4.1.2.11. tert-Butyl-{5-[1-hydroxyl(2-phenyl)ethyl]thiazol-2-yl}carbamate (2k)
White solid; Rf 0.47 (DCM/MeOH, 19/1); yield 35%. 1H NMR (400 MHz, CDCl3): δ 7.19-7.30 (m, 5H), 7.07 (s, 1H), 5.11 (t, J = 6.5 Hz, 1H), 3.13 (d, J = 6.8 Hz, 2H), 1.56 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 161.5, 152.9, 137.3, 134.3, 133.5, 129.6, 128.8, 127.1, 82.3, 69.6, 45.7, 28.4. MS (HR-ESI) for C16H20N2O3S[(M+Na)+], calcd: m/z 343.1092, found: m/z 343.1082.
4.1.2.12. tert-Butyl-{5-[hydroxy(phenyl)methyl]-4-methylthiazol-2-yl}carbamate (2l)
White solid; Rf 0.53 (DCM/MeOH, 19/1); yield 22%. 1H NMR (400 MHz, CDCl3): δ 7.31-7.44 (m, 5H), 6.03 (s, 1H), 2.29 (s, 3H), 2.01 (s, 1H), 1.51 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 160.0, 152.6, 143.1, 142.9, 128.7, 128.3, 128.0, 126.0, 82.6, 69.6, 28.3, 15.1. MS (HR-ESI) for C16H20N2O3S [(M+Na)+]. Calcd: m/z 343.1092. Found: m/z 343.1102.
4.1.3. General procedure of the synthesis of compounds 3a-3l.
A mixture of compounds 2a-2l (1 eq.), triethylsilane (8 eq.), and TFA (14 eq.) in DCM (~ 20 mL) was stirred overnight at room temperature. The mixture was evaporated and the residue was treated with a saturated solution of aqueous NaHCO3. The aqueous layer was extracted with DCM, dried over anhydrous Na2SO4, filtered, and evaporated. The residue was purified by silica gel column chromatography.
4.1.3.1. 5-Benzyl-2-thiazolamine (3a)
White solid; Rf 0.38 (DCM/MeOH, 19/1); yield 71%. 1H NMR (400 MHz, CDCl3): δ 7.18-7.34 (m, 5H), 6.78 (s, 1H), 4.92 (br s, 2H), 3.88 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 167.5, 139.9, 135.7, 128.7, 128.5, 128.2, 126.8, 33.5. MS (HR-ESI) for C10H10N2S [(M )+], calcd: m/z 191.0643, found: m/z 191.0618.
4.1.3.2. 5-(1-Naphthalenylmethyl)-2-thiazolamine (3b)
White solid; Rf 0.42 (DCM/MeOH, 19/1); yield 60%. 1H NMR (400 MHz, CDCl3): δ 8.03 (m, 1H), 7.87 (m, 1H), 7.82 (m, 1H), 7.49 (m, 2H), 7.41 (m, 1H), 7.28 (m, 1H), 6.71 (s, 1H), 6.47 (br s, 2H), 4.31 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 168.2, 134.4, 134.1, 131.6, 129.0, 128.2, 126.9, 126.6, 126.1, 125.7, 123.6, 30.8. MS (HR-ESI) for C14H12N2S [(M+H)+], calcd: m/z 241.0799, found: m/z 241.0803.
4.1.3.3. 5-(2-Naphthalenylmethyl)-2-thiazolamine (3c)
White solid; Rf 0.45 (DCM/MeOH, 19/1); yield 53%. 1H NMR (400 MHz, CDCl3): δ 7.79-7.83 (m, 3H), 7.67 (s, 1H), 7.47 (m, 2H), 7.36 (d, J = 7.4 Hz, 1H), 6.87 (s, 1H), 4.82 (br s, 2H), 4.13 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 167.4, 137.4, 136.0, 133.7, 132.5, 128.4, 127.8, 127.0, 126.8, 126.3, 125.8, 33.7. MS (HR-ESI) for C14H12N2S [(M+H)+], calcd: m/z 241.0799, found: m/z 241.0796.
4.1.3.4. 5-(Cyclohexylmethyl)-2-thiazolamine (3d)
White solid; Rf 0.31 (DCM/MeOH, 19/1); yield 91%. 1H NMR (400 MHz, CDCl3): δ 6.69 (s, 1H), 4.92 (br s, 2H), 2.51 (d, J = 7.0 Hz, 2H), 1.67 (m, 5H), 1.43 (m, 1H), 1.09-1.32 (m, 3H), 0.91-1.04 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 166.7, 135.5, 128.0, 39.7, 35.0, 33.0, 26.5, 26.3. MS (HR-ESI) for C10H16N2O [(M+H)+], calcd: m/z 197.1112, found: m/z 197.1104.
4.1.3.5. 5-(4-Fluorobenzyl)-2-thiazolamine (3e)
White solid; Rf 0.39 (DCM/MeOH, 19/1); yield 77%. 1H NMR (400 MHz, CDCl3): δ 7.21 (m, 2H), 7.03 (m, 2H), 6.78 (s, 1H), 4.83 (br s, 2H), 3.88 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 168.9, 164.5, 162.0, 137.1, 131.1, 129.5, 117.0, 34.1 MS (HR-ESI) for C10H9FN2S [(M+H)+], calcd: m/z 209.0549, found: m/z 209.0546.
4.1.3.6. 5-[3-(Trifluoromethyl)benzyl]-2-thiazolamine (3f)
White solid; Rf 0.38 (DCM/MeOH, 19/1); yield 55%. 1H NMR (400 MHz, CDCl3): δ 7.41-7.53 (m, 4H), 6.79 (s, 1H), 5.21 (br s, 2H), 4.03 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 168.0, 140.8, 136.24, 131.9, 131.2, 130.9, 129.2, 126.5, 125.2, 123.7, 33.2. MS (HR-ESI) for C11H9F3N2S [(M+H)+], calcd: m/z 259.0504, found: m/z 259.0517.
4.1.3.7. 5-(3-Chlorobenzyl)-2-thiazolamine (3g)
White solid; Rf 0.37 (DCM/MeOH, 19/1); yield 77%. 1H NMR (400 MHz, CDCl3): δ 7.22 (m, 3H), 7.08 (s, 1H), 5.02 (br s, 2H), 4.01 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 167.7, 141.9, 136.2, 134.5, 129.9, 128.6, 127.0, 126.6, 33.1. MS (HR-ESI) for C10H9ClN2S [(M+H)+], calcd: m/z 225.0253, found: m/z 225.0252.
4.1.3.8. 5-(3-Fluorobenzyl)-2-thiazolamine (3h)
White solid; Rf 0.39 (DCM/MeOH, 19/1); yield 75%. 1H NMR (400 MHz, CDCl3): δ 7.24-7.31 (m, 1H), 7.02 (d, J = 7.8 Hz, 1H), 6.90-6.97 (m, 2H), 6.83 (s, 1H), 4.98 (br s, 2H), 3.97 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 167.9, 164.6, 142.7, 136.5, 130.4, 127.4, 124.3, 115.8, 114.0, 33.4 MS (HR-ESI) for C10H9FN2S [(M+H)+], calcd: m/z 209.0549, found: m/z 209.0547.
4.1.3.9. 5-(2-Fluorobenzyl)-2-thiazolamine (3i)
White solid; Rf 0.38 (DCM/MeOH, 19/1) yield 82%. 1H NMR (400 MHz, CDCl3): δ 7.19 (m, 2H), 7.01-7.09 (m, 2H), 6.78 (s, 1H), 4.92 (br s, 2H), 4.03 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 167.4, 162.0, 159.5, 136.2, 128.6, 126.5, 124.4, 115.6, 115.4, 26.4. MS (HR-ESI) for C10H9FN2S [(M+Na)+], calcd: m/z 231.0368, found: m/z 231.0359.
4.1.3.10. 5-(2-Chlorobenzyl)-2-thiazolamine (3j)
White solid; Rf 0.38 (DCM/MeOH, 19/1); yield 77%. 1H NMR (400 MHz, CDCl3): δ 7.39 (m, 1H), 7.19-7.33 (m, 3H), 6.82 (s, 1H), 4.94 (br s, 2H), 4.11 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 167.5, 137.6, 136.4, 133.8, 130.4, 129.7, 128.3, 127.2, 126.1, 31.0. MS (HR-ESI) for C10H9ClN2S[(M+H)+], calcd: m/z 225.0253, found: m/z 225.0192.
4.1.3.11. 5-Phenethyl-2-thiazolamine (3k)
White solid; Rf 0.38 (DCM/MeOH, 19/1); yield 61%. 1H NMR (400 MHz, CDCl3): δ 7.33 (m, 2H), 7.24 (m, 3H), 6.67 (s,1H), 4.98(br s, 2H), 2.81-2.93 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 166.9, 140.8, 134.7, 128.6, 128.5, 128.2, 126.3, 37.6, 29.1. MS (HR-ESI) for C11H12N2S[(M+H)+], calcd: m/z 205.0799, found: m/z 205.0801.
4.1.3.12. 5-Benzyl-4-methyl-2-thiazolamine (3l)
White solid; Rf 0.4 (DCM/MeOH, 19/1); yield 81%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.28 (m, 5H), 4.83 (br s, 2H), 3.91 (s, 2H), 2.17 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 164.9, 143.6, 140.4, 128.7, 128.2, 126.6, 120.1, 32.3, 14.9 MS (HR-ESI) for C11H12N2S [(M+H)+], calcd: m/z 205.0799, found: m/z 205.0789.
4.1.4. General procedure for the synthesis of compounds 4b-4f, 4h, 4i, 5h, 6a-6c, 7a-7c, 8a, 8b, 9b-9h, 10a-10f, 11a, 11b, 12a-12d, 13a-13f, 14a, 14c, and 15a-15h.
To a mixture of compounds 3a-3p (1 eq.) and acid chloride (1 eq.) in THF (~ 20 mL), triethylamine (3 eq.) was added. The mixture was stirred at room temperature for 15 min followed by filtration. The solvents were evaporated and the residue was purified by column chromatography.
4.1.4.1. N-(5-Benzylthiazol-2-yl)isobutyramide (4b)
White solid; Rf 0.5 (DCM); yield 61%. 1H NMR (400 MHz, CDCl3): δ 11.70 (s, 1H), 7.24-7.33 (m, 5H), 7.11 (s, 1H), 4.11 (s, 2H), 2.69 (m, 1H), 1.28 (s, 3H), 1.26 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 175.1, 159.3, 139.5, 133.3, 132.3, 128.9, 128.6, 126.9, 35.4, 33.2, 19.4. MS (HR-ESI) for C14H16N2OSNa [(M+Na)+], calcd: m/z 283.0881, found: m/z 283.0881.
4.1.4.2. N-(5-Benzylthiazol-2-yl)cyclopentanecarboxamide (4c)
White solid; Rf 0.53 (DCM); yield 60%. 1H NMR (400 MHz, CDCl3): δ 11.82 (s, 1H), 7.25-7.34 (m, 5H), 7.06 (s, 1H,), 4.10 (s, 2H), 2.82 (m, 1H), 1.90-1.95 (m, 4H), 1.78-1.80 (m, 2H), 1.62-1.65 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 174.4, 159.4, 139.4, 133.2, 132.2, 128.9, 128.6, 126.9, 45.5, 33.2, 30.5, 26.1. MS (HR-ESI) for C16H18N2OSNa [(M+Na)+], calcd: m/z 309.1038, found: m/z 309.1037.
4.1.4.3 N-(5-Benzylthiazol-2-yl)cyclohexanecarboxamide (4d)
White solid; Rf 0.52 (DCM); yield 66%. 1H NMR (400 MHz, CDCl3): δ 11.97 (s, 1H), 7.24-7.32 (m, 5H), 7.05 (s, 1H), 4.08 (s, 2H), 2.33 (m, 1H), 1.81-1.93 (m, 4H), 1.71 (m, 1H), 1.52-1.64 (m, 2H), 1.15-1.28 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 174.4, 159.5, 139.2, 133.1, 132.2, 128.9, 128.8, 126.9, 44.9, 33.2, 29.2, 25.7. MS (HR-ESI) for C17H20N2OSNa [(M+Na)+], calcd: m/z 323.1194, found: m/z 323.1115.
4.1.4.4. N-(5-Benzylthiazol-2-yl)-2-cyclohexylacetamide (4e)
White solid; Rf 0.51 (DCM); Yield 71%. 1H NMR (400 MHz, CDCl3): δ 11.69 (s, 1H), 7.18-7.33 (m, 5H), 7.08 (s, 1H), 4.10 (s, 2H), 2.32 (d, 1H, J = 7.13 Hz), 1.73-1.89 (m, 5H,), 0.91-1.34 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 170.6, 159.3, 139.2, 132.6, 132.2, 128.9, 128.7, 127.0, 44.3, 35.3, 33.2, 26.3, 26.2, 26.1 MS (HR-ESI) for C18H22N2OSNa [(M+Na)+], calcd: m/z 337.1350, found: m/z 337.1366.
4.1.4.5. N-(5-Benzylthiazol-2-yl)-3-cyclohexylpropanamide (4f)
White solid; Rf 0.55 (DCM); yield 71%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.29 (m, 5H), 7.07 (s, 1H), 4.13 (s, 2H), 2.51 (t, J = 7.9 Hz, 2H), 1.57-1.73 (m, 7H), 1.16-1.31 (m, 4H), 0.91-1.03 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 179.8, 171.7, 138.9, 132.2, 131.4, 128.9, 128.6, 127.1, 37.4, 37.3, 32.3, 26.7, 26.6, 26.4, 26.3. MS (HR-ESI) for C19H24N2OS [(M+H)+], calcd: m/z 329.1688, found: m/z 329.1686.
4.1.4.6. N-(5-Benzylthiazol-2-yl)benzamide (4h)
White solid; Rf 0.71 (DCM); yield 63%. 1H NMR (400 MHz, CDCl3): δ 7.96 and 7.21-7.64 (m, 10H), 6.78 (s, 1H), 4.06 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 165.6, 159.4, 139.4, 133.9, 132.8, 129.1, 128.9, 128.8, 128.7, 128.6, 128.1, 126.9, 33.1. MS (HR-ESI) for C17H14N2OSNa [(M+Na)+], calcd: m/z 317.0724, found: m/z 317.0740.
4.1.4.7. N-(5-Benzylthiazol-2-yl)adamantane-1-carboxamide (4i)
White solid; Rf 0.49 (DCM); yield 66%. 1H NMR (400 MHz, CDCl3): δ 9.84 (s, 1H), 7.21-7.35 (m, 5H), 4.11 (s, 2H), 2.14 (m, 3H), 1.91 (m, 6H), 1.73 (m, 6H). 13C NMR (100 MHz, CDCl3): 175.8, 158.4, 139.5, 133.8, 132.3, 128.8, 128.6, 126.9, 41.2, 38.9, 36.5, 33.1, 28.1. MS (HR-ESI) for C21H24N2OSNa [(M+Na)+], calcd: m/z 375.1507, found: m/z 375.1515.
4.1.4.8. N-(5-Benzylthiazol-2-yl)-2-(thiophen-2-yl)thiazole-5-carboxamide (5h)
White solid; Rf 0.58 (hexanes/EtOAc, 7/1); yield 65 %.1H NMR (400 MHz, CDCl3): δ 10.43 (br s, 1H), 8.19 (s, 1H), 7.61 (d, J = 3.5 Hz, 1H), 7.47 (d, J = 4.8 Hz, 1H), 7.24-7.41 (m, 6H), 7.12 (d, J = 3.6 Hz, 1H), 4.11 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 157.9, 156.5, 148.3, 139.5, 136.0, 135.2, 133.1, 129.3, 128.9, 128.7, 128.2, 128.1, 126.9, 33.2. MS (HR-ESI) for C18H13N3OS3[(M+Na)+], calcd: m/z 406.0118, found: m/z 406.0156.
4.1.4.9. N-[5-(4-Fluorobenzyl)thiazol-2-yl]cyclopentanecarboxamide (6a)
White solid; Rf 0.47 (DCM); yield 75%. 1H NMR (400 MHz, CDCl3): δ 11.83 (s, 1H), 7.18-7.21 (m, 2H), 6.96-7.03 (m, 3H), 4.03 (s, 2H), 2.8 (m, 1H), 1.92 (m, 4H), 1.77-1.79 (m, 2H), 1.60-1.63 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 174.5, 163.1, 160.7, 136.2, 133.2, 132.1, 130.1, , 115.7 45.5, 32.4, 30.5, 26.1. MS (HR-ESI) for C16H17FN2OS [(M+Na)+], calcd: m/z 327.0943, found: m/z 327.0933.
4.1.4.10. N-[5-(4-Fluorobenzyl)thiazol-2-yl]cyclohexanecarboxamide (6b)
White solid; Rf 0.43 (DCM); yield 78%. 1H NMR (400 MHz, CDCl3): δ 12.02 (s, 1H), 7.2-7.26 (m, 2H), 6.97-7.02 (m, 3H), 4.05 (s, 2H), 2.36 (m, 1H), 1.79-1.89 (m, 4H), 1.67 (m, 1H), 1.49-1.62 (m, 2H), 1.11-1.30 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 174.3, 163.2, 160.7, 135.0, 133.2, 132.1, 130.3, 115.6, 45.0, 32.4, 29.2, 28.3, 25.7. MS (HR-ESI) for C17H19FN2OS [(M+Na)+], calcd: m/z 341.1100, found: m/z 341.1080.
4.1.4.11. N-[5-(4-Fluorobenzyl)thiazol-2-yl]-3-cyclohexanepropanamide (6c)
White solid; Rf 0.44 (DCM); yield 66%.1H NMR (400 MHz, CDCl3): δ 12.11 (br s, 1H), 7.22 (m, 2H), 7.01 (m, 3H), 4.03 (s, 2H), 2.51 (m, 2H), 1.59-1.73 (m, 6H), 1.12-1.31 (m, 5H), 0.84-1.03 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 171.7, 163.1, 160.6, 135.0, 132.9, 131.9, 130.1, 115.7, 37.3, 33.8, 33.1, 32.5, 32.2, 26.6, 26.3. MS (HR-ESI) for C19H23FN2OS [(M+Na)+], calcd: m/z 369.1413, found: m/z 369.1427.
4.1.4.12. N-{5-[3-(Trifluoromethyl)benzyl]thiazol-2-yl}-3-cyclohexanepropanamide (7a)
White solid; Rf 0.49 (DCM); yield 80%. 1H NMR (400 MHz, CDCl3): δ 12.31 (br s, 1H), 7.39-7.52 (m, 4H), 7.11 (s, 1H), 4.08 (s. 2H), 2.51 (t, J = 8.0 Hz, 2H), 1.61-1.73 (m, 7H), 1.11-1.38 (m, 4H), 0.91-1.02 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.7, 159.8, 140.3, 133.5, 132.0, 131.4, 131.1, 130.8, 129.3, 125.3, 123.9, 37.4, 33.8, 33.1, 32.9, 32.5, 26.6, 26.3. MS (HR-ESI) for C20H23F3N2OS [(M+Na)+], calcd: m/z 419.1378, found: m/z 419.1381.
4.1.4.13. N-[5-(3-Chlorobenzyl)thiazol-2-yl]-3-cyclohexanepropanamide (7b)
White solid; Rf 0.55 (DCM); yield 71%. 1H NMR (400 MHz, CDCl3): δ 12.36 (s, 1H), 7.24 (m, 3H), 7.13 (m, 2H), 4.07 (s, 2H), 2.5 (t, J = 7.6 Hz, 2H), 1.62-1.72 (m, 7H), 1.14-1.26 (m, 4H), 0.91-0.96 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.7, 159.8, 134.7, 133.3, 131.0, 130.1, 128.7, 127.2, 126.7, 37.4, 33.8, 33.1, 32.7, 32.5, 26.6, 26.4, 26.3. MS (HR-ESI) for C19H23ClN2OS [(M+H)+], calcd: m/z 363.1298, found: m/z 363.1302.
4.1.4.14. 3-Cyclohexane-N-[5-(3-fluorobenzyl)thiazol-2-yl]-propanamide (7c)
White solid; Rf 0.51 (DCM); yield 64%. 1H NMR (400 MHz, CDCl3): δ 12.37 (s, 1H), 7.32 (m, 1H), 7.13 (s, 1H), 7.01 (m, 1H), 6.92 (m, 2H), 4.06 (s, 2H), 2.5 (t, J = 7.7 Hz, 2H), 1.70 (m, 7H), 1.12-1.19 (m, 4H), 0.91-0.99 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.7, 164.4, 161.9, 159.8, 141.8, 133.3, 131.1, 130.3, 124.2, 115.5, 114.0, 37.4, 33.8, 33.1, 32.8, 32.5, 26.6, 26.3. MS (HR-ESI) for C19H23FN2OS [(M+Na)+], calcd: m/z 369.1413, found: m/z 369.1413.
4.1.4.15. N-[5-(2-Chlorobenzyl)thiazol-2-yl]-3-cyclohexanepropanamide (8a)
White solid; Rf 0.53 (DCM); yield 76%.1H NMR (400 MHz, CDCl3): δ 12.01 (br s, 1H), 7.44 (m, 1H), 7.28 (m, 1H), 7.21 (m, 2H), 7.14 (s, 1H), 4.17 (s, 2H), 2.52 (t, J = 7.6 Hz, 2H), 1.61-1.69 (m, 7H), 1.23-1.31 (m, 4H), 0.91 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.6, 159.2, 156.6, 137.2, 134.0, 133.7, 130.7, 129.9, 128.5, 127.3, 37.4, 33.8, 33.2, 32.5, 30.8, 26.6, 26.3. MS (HR-ESI) for C19H23ClN2OS [(M+H)+], calcd: m/z 363.1298, found: m/z 363.1299.
4.1.4.16. 3-Cyclohexyl-N-[5-(2-fluorobenzyl)thiazol-2-yl]propanamide (8b)
White solid; Rf 0.49 (DCM); yield 67%. 1H NMR (400 MHz, CDCl3): δ 11.91 (s, 1H), 7.34 (m, 2H), 7.01-7.09 (m, 3H), 4.11 (s, 2H), 2.49 (m, 2H), 1.51-1.68 (m, 7H), 1.11-1.23 (m, 4H), 0.92-0.99 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.8, 162.3, 159.5, 133.7, 130.9, 129.1, 126.7, 124.7, 116.0, 115.8, 37.6, 34.1, 33.4, 33.3, 32.8, 26.9, 26.6. MS (HR-ESI) for C19H23FN2OS[(M+Na)+], calcd: m/z 369.1413, found: m/z 369.1404.
4.1.4.17. N-[5-(1-Naphthalenylmethyl)thiazol-2-yl]isobutyramide (9b)
White solid; Rf 0.55 (DCM); yield 74%.1H NMR (400 MHz, CDCl3): δ 12.01 (s, 1H), 8.03 (m, 1H), 7.91 (m, 1H), 7.83 (m, 1H), 7.37-7.49 (m, 4H), 7.01 (s, 1H), 4.52 (s, 2H), 2.61 (m, 1H), 1.17 and 1.19(d s, 6H). 13C NMR (100 MHz, CDCl3): δ 175.2, 159.2, 135.2, 134.1, 133.4, 132.0, 131.7, 129.0, 128.0, 126.9, 126.4, 125.9, 125.7, 123.7, 35.2, 30.6, 19.3. MS (HR-ESI) for C18H18N2OSNa [(M+Na)+], calcd: m/z 333.1038, found: m/z 333.1044.
4.1.4.18. N-[5-(1-Naphthalenylmethyl)thiazol-2-yl]cyclopentanecarboxamide (9c)
White solid; Rf 0.52 (DCM); yield 68%. 1H NMR (400 MHz, CDCl3): δ 11.93 (s, 1H), 8.02 (m, 1H), 7.91 (m, 1H), 7.79 (m, 1H), 7.38-7.51 (m, 4H), 7.03 (s, 1H), 4.54 (s, 2H), 2.71 (m, 1H), 1.69-1.77 (m, 6H), 1.53 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 174.4, 159.1, 135.1, 134.2, 133.4, 131.9, 131.7, 129.0, 128.0, 126.4, 125.9, 125.7, 123.8, 45.4, 30.6, 30.4, 26.0. MS (HR-ESI) for C20H20N2OSNa [(M+Na)+], calcd: m/z 359.1194, found: m/z 359.1200.
4.1.4.19. N-[5-(1-Naphthalenylmethyl)thiazol-2-yl]cyclohexanecarboxamide (9d)
White solid; Rf 0.53 (DCM); yield 63%.1H NMR (400 MHz, CDCl3): δ 12.33 (s, 1H), 8.04 (m, 1H), 7.93 (m, 1H), 7.81 (m, 1H), 7.44-7.48 (m, 4H), 6.81 (s, 1H), 4.53 (s, 2H), 2.21 (m, 1H), 1.69 (m, 2H), 1.41-1.77 (m, 5H), 1.11 (m, 1H), 0.83 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 174.4, 159.3, 134.9, 134.2, 133.3, 131.7, 128.9, 127.2, 126.4, 125.9, 125.7, 123.9, 44.8, 30.5, 29.1, 25.6, 25.5. MS (HR-ESI) for C21H22N2OS [(M+H)+], calcd: m/z 351.1531, found: m/z 351.1539.
4.1.4.20. N-[5-(1-Naphthalenylmethyl)thiazol-2-yl]-2-cyclohexaneacetamide (9e)
White solid; Rf 0.54 (DCM); yield 71%. 1H NMR (400 MHz, CDCl3): δ 12.26 (s, 1H), 8.03 (m, 1H), 7.81-7.89 (m, 1H), 7.82 (m, 1H), 7.51 (m, 2H), 7.44 (m, 2H), 6.91 (s, 1H), 4.53 (s, 2H), 2.21 (d, J= 7.25 Hz, 2H), 1.81 (m, 1H), 1.63 (m, 5H), 1.01-1.23 (m, 3H), 0.84 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 170.6, 159.1, 134.9, 134.2, 133.3, 131.7, 129.0, 128.0, 127.1, 126.4, 126.0, 125.7, 123.8, 44.1, 35.2, 33.1, 30.5, 26.2, 26.0. MS (HR-ESI) for C22H24N2OSNa [(M+Na)+], calcd: m/z 387.1507, found: m/z 387.1499.
4.1.4.21. N-[5-(1-Naphthalenylmethyl)thiazol-2-yl]-3-cyclohexanepropanamide (9f)
White solid; Rf 0.56 (DCM); yield 67%. 1H NMR (400 MHz, CDCl3): δ 8.01 (m, 1H), 7.94 (m, 1H), 7.82 (m, 1H), 7.39-7.49 (m, 4H), 7.03 (s, 1H), 4.54 (s, 2H), 2.42 (t, J = 7.8 Hz, 2H), 1.51-1.73 (m, 7H), 1.21 (m, 4H), 0.94 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.7, 159.4, 134.7, 134.2, 131.9, 131.6, 129.1, 128.2, 127.0, 126.5, 126.0, 125.7, 123.6, 37.2, 33.7, 33.1, 32.3, 30.5, 26.6, 26.2. MS (HR-ESI) for C23H26N2OSNa [(M+Na)+], calcd: m/z 401.1668, found: m/z 401.1675.
4.1.4.22. N-[5-(1-Naphthalenylmethyl)thiazol-2-yl]benzamide (9g)
White solid; Rf 0.65 (DCM); yield 59%. 1H NMR (400 MHz, CDCl3): δ 12.68 (s, 1H), 7.93-87.99 (m, 2H), 7.81 (m, 3H), 7.53 (m, 2H), 7.32-7.44 (m, 3H), 7.12-7.23 (m, 2H), 6.41 (s, 1H), 4.49 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 166.1, 159.3, 135.0, 134.4, 134.2, 133.0, 132.5, 131.7, 129.0, 128.6, 128.1, 128.0, 127.1, 126.4, 125.9, 125.7, 123.9, 30.5. MS (HR-ESI) for C21H16N2OS [(M+H)+], calcd: m/z 345.1062, found: m/z 345.1067.
4.1.4.23. N-[5-(1-Naphthalenylmethyl)thiazol-2-yl]adamantane-1-carboxamide (9h)
White solid; Rf 0.55 (DCM); yield 65%.1H NMR (400 MHz, CDCl3): δ 9.78 (s, 1H), 8.03 (m, 1H), 7.88 (m, 1H), 7.83 (m, 1H), 7.42-7.53 (m, 4H), 7.11 (s, 1H), 4.53 (s, 2H), 2.01 (m, 3H), 1.93 (m, 6H), 1.72 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 175.8, 158.1, 135.2, 134.1, 132.0, 131.7, 128.9, 127.9, 127.0, 126.4, 125.9, 125.7, 123.8, 41.1, 38.8, 36.4, 30.5, 28.0. MS (HR-ESI) for C25H26N2OSNa [(M+Na)+], calcd: m/z 425.1664, found: m/z 425.1667.
4.1.4.24. N-(5-(2-Naphthalenylmethyl)thiazol-2-yl]isobutyramide (10a)
White solid; Rf 0.55 (DCM); yield 64%. 1H NMR (400 MHz, CDCl3): δ 12.03 (br s, 1H), 7.84 (m, 3H), 7.67 (s, 1H), 7.41-7.53 (m, 2H), 7.41 (dd, J = 8.4 Hz, J = 1.7 Hz, 1H), 7.21 (s, 1H), 4.32 (s, 2H), 2.71 (m, 1H), 1.26 (s, 3H), 1.25 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 175.2, 160.0, 137.0, 133.7, 133.2, 132.5, 132.1, 128.6, 127.8, 126.9, 126.4, 125.9, 36.7, 34.7, 20.8. MS (HR-ESI) for C18H18N2OSNa [(M+Na)+], calcd: m/z 333.1038, found: m/z 333.1034.
4.1.4.25. N-[5-(2-Naphthalenylmethyl)thiazol-2-yl]cyclohexanecarboxamide (10b)
White solid; Rf 0.52 (DCM); yield 69%.1H NMR (400 MHz, CDCl3): δ 12.22, (br s, 1H), 7.81 (m, 3H), 7.74 (s, 1H), 7.52 (m, 2H), 7.32 (m, 1H), 7.04 (s, 1H), 4.21 (s, 2H), 2.33 (m, 1H), 1.76 (m, 2H), 1.70 (m, 2H), 1.51 (m, 3H), 1.03-1.21 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 174.4, 159.8, 136.3, 133.7, 132.6, 132.2, 132.0, 128.7, 127.8, 127.7, 127.2, 127.0, 126.4, 125.9, 44.9, 33.3, 29.2, 25.6. MS (HR-ESI) for C21H22N2OSNa [(M+Na)+], calcd: m/z 373.1351, found: m/z 373.1364.
4.1.4.26. N-[5-(2-Naphthalenylmethyl)thiazol-2-yl]-2-cyclohexaneacetamide (10c)
White solid; Rf 0.53 (DCM); yield 78%. 1H NMR (400 MHz, CDCl3): δ 12.12 (s, 1H), 7.81 (m, 3H), 7.68 (s, 1H), 7.51 (m, 2H), 7.35 (d, J = 8.9, 1H), 7.09 (s, 1H), 4.21 (s, 2H), 2.31 (d, J = 7.2 Hz, 2H), 1.83 (m, 1H), 1.81-1.93 (m, 5H), 1.22 (m, 2H), 1.01 (m, 1H), 0.93 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 170.7, 159.6, 136.4, 133.7, 132.6, 132.1, 128.7, 127.8, 127.1, 126.9, 126.4, 125.9, 44.2, 35.2, 33.3, 33.2, 26.2, 26.0. MS (HR-ESI) for C22H24N2OSNa [(M+Na)+], calcd: m/z 387.1507, found: m/z 387.1502.
4.1.4.27. N-[5-(2-Naphthalenylmethyl)thiazol-2-yl)-3-cyclohexanepropanamide (10d)
White solid; Rf 0.54 (DCM); yield 76%.1H NMR (400 MHz, CDCl3): δ 12.17 (s, 1H), 7.82 (m, 3H), 7.72 (s, 1H), 7.49 (m, 2H), 7.41 (d, J = 8.4 Hz, 1H), 7.10 (s, 1H), 4.21 (s, 2H), 2.53 (t, J = 7.6 Hz, 2H) 1.62-1.73 (m, 7H), 1.14-1.21 (m, 4H), 0.91 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.16, 159.5, 136.8, 133.7, 133.1, 132.5, 132.0, 128.6, 127.8, 127.0, 126.9, 126.4, 125.9, 37.3, 33.8, 33.3, 33.1, 32.5, 26.6, 26.2. MS (HR-ESI) for C23H26N2OSNa [(M+Na)+], calcd: m/z 401.1668, found: m/z 401.1658.
4.1.4.28. N-[5-(2-Naphthalenylmethyl)thiazol-2-yl]benzamide (10e)
White solid; Rf 0.56 (DCM); yield 72%.1H NMR (400 MHz, CDCl3): δ 12.53 (br s, 1H), 7.91-8.03 (m, 2H), 7.81 (m, 3H), 7.78 (s, 1H), 7.54 (m, 2H), 7.31 (m, 3H), 6.67 (s, 1H), 4.21 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 167.2, 161.0, 138.2, 135.6, 135.1, 134.7, 134.2, 134.0, 133.9, 133.5, 131.5, 130.0, 129.5, 129.2, 128.5, 127.8, 127.3, 34.6. MS (HR-ESI) for C21H16N2OSNa [(M+Na)+], calcd: m/z 367.0881, found: m/z 367.0898.
4.1.4.29. N-[5-(2-Naphthalenylmethyl)thiazol-2-yl)adamantane-1-carboxamide (10f)
White solid; Rf 0.64 (DCM); yield 77%.1H NMR (400 MHz, CDCl3): δ 9.73 (s, 1H), 7.81 (m, 3H), 7.70 (s, 1H), 7.43-7.51 (m 2H), 7.39 (d, J = 8.6 Hz, 1H), 7.21 (s, 1H), 4.22 (s, 2H), 2.18 (m, 3H), 2.01 (s, 1H), 1.89 (s, 5H), 1.74 (m, 6H). 13C NMR (100 MHz, CDCl3): δ175.7, 158.4, 137.0, 134.1, 133.7, 132.5, 132.2, 128.6, 127.8, 127.0, 126.3, 125.8, 41.1, 38.9, 36.4, 33.3, 28.0. MS (HR-ESI) for C25H26N2OSNa [(M+Na)+], calcd: m/z 425.1664, found: m/z 425.1672.
4.1.4.30. N-(5-Phenethylthiazol-2-yl)cyclopentanecarboxamide (11a)
White solid; Rf 0.45 (DCM); yield 71%. 1H NMR (400 MHz, CDCl3): δ 12.11 (s, 1H), 7.33 (m, 2H), 7.21 (m, 3H), 6.93 (s, 1H), 3.12 (m, 2H), 3.01 (m, 2H), 2.85 (m, 1H), 1.91 (m, 4H), 1.82 (m, 2H), 1.61 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 174.5, 158.8, 140.6, 132.5, 131.9, 128.6, 126.4, 45.4, 37.6, 30.5, 28.7, 26.2. MS (HR-ESI) for C17H20N2OS [(M+Na)+], calcd: m/z 323.1194, found: m/z 323.1148.
4.1.4.31. N-(5-Phenethylthiazol-2-yl)-3-cyclohexanepropanamide (11b)
White solid; Rf 0.47 (DCM); yield 79%. 1H NMR (400 MHz, CDCl3): δ 12.24 (s, 1H), 7.21-7.33 (m, 5H), 7.03 (s, 1H), 3.11 (m, 2H), 3.01 (m, 2H), 2.53 (t, J = 7.6 Hz), 1.59-1.73 (m, 7H), 1.11-1.31 (m, 4H), 0.89-1.03 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.6, 158.7, 140.6, 132.7, 132.0, 128.5, 126.5, 37.6, 37.4, 33.8, 33.2, 32.6, 28.8, 26.6, 26.3. MS (HR-ESI) for C20H26N2OS [(M+H)+], calcd: m/z 365.1664, found: m/z 365.1664.
4.1.4.32. N-(5-Phenylthiazol-2-yl)cyclopentanecarboxamide (12a)
White solid; Rf 0.43 (DCM); yield 75%. 1H NMR (400 MHz, CDCl3): δ 12.52 (s, 1H), 7.62 (m, 3H), 7.43 (t, J = 7.7 Hz, 2H), 7.31 (m, 1H), 3.01 (m, 1H), 2.05 (m, 4H), 1.78 (m, 2H), 1.70 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 174.8, 159.5, 132.9, 131.9, 131.4, 129.3, 128.0, 126.3, 45.5, 30.6, 26.2. MS (HR-ESI) for C15H16N2OS[(M+Na)+], calcd: m/z 295.0881, found: m/z 295.0886.
4.1.4.33. N-(5-Phenylthiazol-2-yl)cyclohexanecarboxamide (12b)
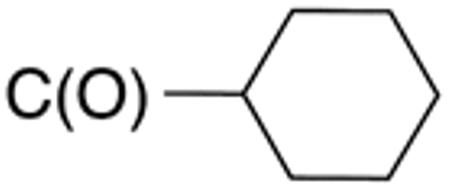
White solid; Rf 0.41 (DCM); yield 69%. 1H NMR (400 MHz, CDCl3): δ 12.33 (s, 1H), 7.51 (m, 2H), 7.43 (m, 2H), 7.30 (m, 1H), 2.53 (m, 1H), 2.01 (m, 2H), 1.89 (m, 2H), 1.61-1.78 (m, 3H), 1.19-1.51 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 174.5, 159.4, 132.9, 131.8, 131.5, 129.3, 128.0, 126.2, 45.0, 29.3, 25.9, 25.7. MS (HR-ESI) for C16H18N2OS[(M+H)+], calcd: m/z 309.1038, found: m/z 309.1037.
4.1.4.34. N-(5-Phenylthiazol-2-yl)-2-cyclohexaneacetamide (12c)
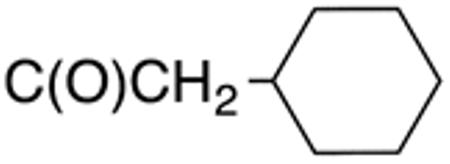
White solid; Rf 0.43 (DCM); yield 78%. 1H NMR (400 MHz, CDCl3): δ 12.39 (s, 1H), 7.61 (s, 1H), 7.58 (d, J = 7.8 Hz, 2H), 7.45 (t, J = 6.8 Hz, 1H), 7.32 (t, J = 7.6 Hz, 1H), 2.50 (d, J = 7.12 Hz, 2H), 2.0 (m, 1H), 1.84 (d, J = 12.27 Hz, 2H), 1.72 (m, 3H), 1.01-1.39 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 170.8, 159.2, 132.9, 131.6, 131.5, 129.3, 128.1, 126.3, 44.3, 36.4, 33.4, 26.2, 26.1. MS (HR-ESI) for C17H20N2OS[(M+Na)+], calcd: m/z 323.1194, found: m/z 323.1182.
4.1.4.35. N-(5-Phenylthiazol-2-yl)-3-cyclohexanepropanamide (12d)
White solid; Rf 0.45 (DCM); yield 66%. 1H NMR (400 MHz, CDCl3): δ 12.61 (s, 1H), 7.59 (s, 1H), 7.51 (d, J = 7.7 Hz, 2H), 7.43 (t, J = 7.8 Hz, 2H), 7.31 (m, 1H), 2.55 (t, J = 7.9 Hz, 2H), 1.61-1.84 (m, 7H), 1.09-1.41 (m, 4H), 0.89-1.02 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.8, 159.5, 132.8, 131.6, 131.4, 129.3, 128.0, 126.2, 37.5, 34.0, 33.2, 32.6, 26.6, 26.3. MS (HR-ESI) for C18H13N2OS[(M+H)+], calcd: m/z 315.1531, found: m/z 315.1517.
4.1.4.36. N-[5-(Cyclohexylmethyl)thiazol-2-yl]isobutyramide (13a)
White solid; Rf 0.33 (DCM); yield 61%.1H NMR (400 MHz, CDCl3): δ 12.08 (br s, 1H), 7.01 (s, 1H), 2.73 (m, 1H), 2.60 (d, J = 7.01 Hz, 2H), 1.68-1.76 (m, 4H), 1.52 (m, 1H), 1.17-1.28 (m, 10H), 0.90-0.99 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 175.2, 158.8, 133.0, 131.7, 39.8, 35.4, 34.6, 33.1, 26.5, 26.3, 19.4. MS (HR-ESI) for C14H22N2OSNa [(M+Na)+], calcd: m/z 289.1351, found: m/z 289.1352.
4.1.4.37. N-[5-(cyclohexylmethyl)thiazol-2-yl]cyclohexanecarboxamide (13b)
White solid; Rf 0.32 (DCM); yield 64%. 1H NMR (400 MHz, CDCl3): δ 12.08 (s, 1H), 7.01 (s, 1H), 2.73 (d, J = 6.9 Hz, 2H), 2.43 (m, 1H), 1.78-2.02 (m, 4H), 1.51-1.79 (m, 8H), 1.11-1.29 (m, 7H), 1.0 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 174.4, 158.8, 133.0, 131.6, 45.0, 39.7, 34.6, 33.1, 29.8, 29.3, 26.5, 26.3, 25.8. MS (HR-ESI) for C17H26N2OSNa [(M+Na)+], calcd: m/z 329.1664, found: m/z 329.1661.
4.1.4.38. N-[5-(Cyclohexylmethyl)thiazol-2-yl]-2-cyclohexaneacetamide (13c)
White solid; Rf 0.35 (DCM); yield 64%. 1H NMR (400 MHz, CDCl3): δ 12.19 (br s, 1H), 7.03 (s, 1H), 2.62 (d, J = 7.2 Hz, 2H), 2.39 (d, J = 6.7 Hz, 2H), 1.89 (m, 1H), 1.61-1.84 (m, 10H), 1.51 (m, 1H), 1.09-1.31 (m, 6H), 0.91-0.99 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 170.7, 158.7, 133.0, 131.6, 44.3, 39.7, 35.4, 34.6, 33.3, 33.2, 33.1, 26.5, 26.3, 26.1. MS (HR-ESI) for C18H28N2OSNa [(M+Na)+], calcd: m/z 343.1820, found: m/z 343.1820.
4.1.4.39. N-[5-(Cyclohexylmethyl)thiazol-2-yl]-3-cyclohexanepropanamide (13d)
White solid; Rf 0.36 (DCM); yield 60%.1H NMR (400 MHz, CDCl3): δ 12.29 (br s, 1H), 7.03 (s, 1H), 2.62 (d, J = 7.0 Hz, 2H), 2.52 (t, J = 7.6 Hz, 2H), 1.57-1.69 (m, 11H), 1.53 (m, 1H), 1.22-1.31 (m, 8H), 0.89-1.0 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 171.6, 158.8, 133.0, 131.6, 39.7, 37.5, 34.6, 33.9, 33.2, 33.1, 32.7, 26.7, 26.5, 26.3, 26.2. MS (HR-ESI) for C19H30N2OSNa [(M+Na)+], calcd: m/z 335.2157, found: m/z 335.2164.
4.1.4.40. N-[5-(Cyclohexylmethyl)thiazol-2-yl]benzamide (13e)
White solid; Rf 0.45 (DCM); yield 65%. 1H NMR (400 MHz, CDCl3): δ 8.11 (d, J = 7.5 Hz, 2H), 7.57 (m, 1H), 7.51 (m, 2H), 2.63 (d, J = 7.1 Hz, 2H), 1.71-1.84 (m, 4H), 1.52 (m, 1H), 1.11-1.29 (m, 4H), 0.92-1.0 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 165.7, 158.9, 133.7, 132.7, 130.1, 128.9, 128.5, 128.2, 39.7, 34.5, 33.1, 26.5, 26.3. MS (HR-ESI) for C17H20N2OSNa [(M+Na)+], calcd: m/z 323.1194, found: m/z 323.1197.
4.1.4.41. N-[5-(Cyclohexylmethyl)thiazol-2-yl]adamantane-1-carboxamide (13f)
White solid; Rf 0.34 (DCM); yield 72%.1H NMR (400 MHz, CDCl3): δ 7.11 (s, 1H), 2.61 (d, J = 6.97 Hz, 2H), 2.11 (m, 3H), 1.93 (m, 6H), 1.62-1.84 (m, 9H), 1.22-1.33 (m, 6H), 0.81-1.0 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 175.9, 158.3, 131.9, 131.3, 41.3, 39.7, 38.9, 36.4, 34.5, 33.0, 28.0, 26.4, 26.2. MS (HR-ESI) for C21H30N2OSNa [(M+Na)+], calcd: m/z 381.1977, found: m/z 381.1970.
4.1.4.42. N-(5-Benzyl-1,3,4-thiadiazol-2-yl)-3-cyclohexylpropanamide (14a)
White solid; Rf 0.56 (DCM); yield 85%. 1H NMR (400 MHz, CDCl3): δ 13.21 (s, 1H), 7.29-7.40 (m, 5H), 4.31 (s, 2H), 2.83 (m, 2H), 1.57-1.80 (m, 7H), 1.11-1.41 (m, 5H), 0.90-1.0 (m, 2H).13C NMR (100 MHz, CDCl3): δ 172.6, 164.1, 161.3, 137.1, 129.1, 128.9, 127.6, 37.4, 36.4, 34.0, 33.1, 32.7, 32.1, 29.8, 26.7, 26.4. MS (HR-ESI) for C18H23N3OS [(M+Na)+], calcd: m/z 352.1455, found: m/z 352.1460.
4.1.4.43. N-(5-Benzyl-4-methylthiazol-2-yl)-3-cyclohexylpropanamide (14c)
White solid; Rf 0.5 (DCM); yield 51%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.33 (m, 5H), 4.0 (s, 2H), 2.41 (t, J = 7.8 Hz, 2H), 2.31 (s, 3H), 1.63-1.71 (m, 6H), 1.22-1.34 (m, 4H), 0.90 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 170.7, 155.3, 141.8, 139.8, 128.8, 128.4, 126.8, 124.8, 37.2, 34.0, 33.1, 32.6 32.2, 29.9, 26.6, 26.3, 14.8. MS (HR-ESI) for C20H26N2OS [(M+Na)+], calcd: m/z 365.1664, found: m/z 365.1665.
4.1.4.44. N-(4-Phenylthiazol-2-yl)cyclopentanecarboxamide (15a)
White solid; Rf 0.44 (DCM); yield 63%. 1H NMR (400 MHz, CDCl3): δ 11.10 (s, 1H), 7.81 (d, J = 7.1, 2H), 7.41 (t, J = 7.5 Hz, 2H), 7.33 (t, J = 7.1, 1H), 7.11 (s, 1H), 2.32 (m, 1H), 1.54 (m, 4H), 1.51 (m, 2H), 1.34 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 175.1, 159.8, 149.9, 134.5, 129.1, 128.4, 126.4, 108.0, 45.3, 30.4, 25.9. MS (HR-ESI) for C18H22N2OSNa [(M+Na)+], calcd: m/z 295.0881, found: m/z 295.0887.
4.1.4.45. N-(4-Phenylthiazol-2-yl)-3-cyclohexanepropanamide (15b)
White solid; Rf 0.46 (DCM); yield 59%. 1H NMR (400 MHz, CDCl3): δ 11.43 (s, 1H), 7.81 (d, J = 6.5 Hz, 2H),7.43 (t, J = 7.5 Hz, 2H), 7.31 (t, J = 6.5 Hz, 1H), 7.19 (s, 1H), 2.01 (t, J = 7.7 Hz, 2H), 1.63 (m, 3H), 1.31-1.40 (m , 4H), 1.11 (m, 3H), 0.83 (m, 1H), 0.69 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 172.1, 159.9, 149.6, 134.4, 129.1, 128.5, 126.3, 107.9, 37.1, 33.5, 32.8, 32.0, 26.5, 26.3. MS (HR-ESI) for C18H22N2OSNa [(M+Na)+], calcd: m/z 337.1351, found: m/z 337.1338.
4.1.4.46. N-[4-(Adamantan-1-yl)thiazol-2-yl]isobutyramide (15c)
White solid; Rf 0.5 (DCM); yield 65%. 1H NMR (400 MHz, CDCl3): δ 8.81 (br s, 1H), 6.53 (s, 1H), 2.59 (m, 1H), 2.09 (m, 3H), 1.92 (m, 6H), 1.74 (m, 6H), 1.31 (d, J = 7.3 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 174.3, 161.3, 157.0, 106.2, 42.2, 36.9, 26.3, 35.8, 29.8, 28.1, 19.3. MS (HR-ESI) for C17H24N2OSNa [(M+Na)+], calcd: m/z 327.1507, found: m/z 327.1505.
4.1.4.47. N-[4-(Adamantan-1-yl)thiazol-2-yl]cyclopentanecarboxamide (15d)
White solid; Rf 0.53 (DCM); yield 79%. 1H NMR (400 MHz, CDCl3): δ 8.91 (br s, 1H), 6.53 (s, 1H), 2.67 (m, 1H), 2.08 (m, 3H), 1.93 (m, 10H), 1.81 (m, 8H), 1.63 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 173.7, 161.2, 157.2, 106.0, 45.8, 42.2, 36.9, 36.3, 30.4, 28.7, 26.1. MS (HR-ESI) for C19H26N2OSNa [(M+Na)+], calcd: m/z 353.1664, found: m/z 353.1675.
4.1.4.48. N-[4-(Adamantan-1-yl)thiazol-2-yl]cyclohexanecarboxamide (15e)
White solid; Rf 0.55 (DCM); yield 61%. 1H NMR (400 MHz, CDCl3): δ 9.33 (br s, 1H), 6.51 (s, 1H), 2.32 (m, 1H), 1.83-2.11 (m, 20 H), 1.65-1.55 (m, 2H), 1.31 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 173.6, 161.1, 157.5, 105.0, 45.3, 42.1, 36.9, 36.3, 29.4, 28.7, 25.7, 25.6. MS (HR-ESI) for C20H28N2OSNa [(M+Na)+], calcd: m/z 367.1820, found: m/z 367.1823.
4.1.4.49. N-[4-(Adamantan-1-yl)thiazol-2-yl]-2-cyclohexylacetamide (15f)
White solid; Rf 0.54 (DCM); yield 68%. 1H NMR (400 MHz, CDCl3): δ 9.01 (s, 1H), 6.53 (s, 1H), 2.28 (s, 2H), 2.11 (s, 3H), 1.87 (s, 6H), 1.76 (m, 14H), 1.31 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 172.7, 164.0, 159.9, 107.8, 47.4, 45.0, 39.7, 39.1, 38.2, 36.0, 31.5, 28.9, 25.6. MS (HR-ESI) for C21H30N2OSNa [(M+Na)+], calcd: m/z 381.1977, found: m/z 381.1989.
4.1.4.50. N-[4-(Adamantan-1-yl)thiazol-2-yl]-3-cyclohexylpropanamide (15g)
White solid; Rf 0.51 (DCM); yield 72%. 1H NMR (400 MHz, CDCl3): δ 9.33 (s, 1H), 6.51 (s, 1H), 2.44 (t, J = 8.1 Hz ,2H), 2.11 (s, 3H), 1.91 (m, 6H), 1.64-1.79 (m, 12 H), 1.21-1.29 (m, 5H), 0.82-0.99 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 171.0, 161.1, 157.5, 105.0, 42.1, 36.9, 36.3, 34.1, 33.1, 32.5, 28.9, 26.5, 26.3. MS (HR-ESI) for C22H32N2OSNa [(M+Na)+], calcd: m/z 395.2133, found: m/z 395.2143.
4.1.4.51. N-[4-(Adamantan-1-yl)thiazol-2-yl]benzamide (15h)
White solid; Rf 0.64 (DCM); yield 65%. 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 7,2 Hz, 2H), 7.41-7.63 (m, 3H), 6.61 (s, 1H), 2.07 (m, 3H), 2.01 (m, 6H), 1.83 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 164.6, 161.4, 157.9, 132.9, 132.4, 129.1, 127.6, 106.5, 42.14, 37.0, 36.4 28.7. MS (HR-ESI) for C20H22N2OSNa [(M+Na)+], calcd: m/z 361.1351, found: m/z 361.1350.
4.1.5. General procedure for the synthesis of compounds 4a and 9a.
TFAA (1.4 eq.) was added drop wise to a solution of compound 3a or 3b (1 eq.) in DCM (3 mL) at 0 °C. The reaction mixture was stirred at room temperature for 30 min. Subsequently, the solvent was evaporated, the residue treated with a saturated aqueous solution of NaHCO3, extracted with EtOAc, and washed with water and then brine. The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by sílica gel column chromatography.
4.1.5.1. N-(5-Benzylthiazol-2-yl)trifluoroacetamide (4a)
White solid; Rf 0.41 (DCM); yield 56%. 1H NMR (400 MHz, CDCl3): δ 13.77 (br s, 1H), 7.18-7.31 (m, 5H), 7.11 (s, 1H), 4.09 (s, 2H). 13C NMR (100 MHz, CDCl3): δ 167.2, 161.2, 137.8, 132.3, 129.2, 128.6, 127.5, 124.6, 115.4, 33.6. MS (HR-ESI) for C12H9F3N2OS [(M+Na)+], calcd: m/z 309.0285, found: m/z 309.0278.
4.1.5.2. N-[5-(Naphthalen-1-ylmethyl)thiazol-2-yl]trifluoroacetamide (9a)
White solid; Rf 0.55 (DCM); yield 93%. 1H NMR (400 MHz, CDCl3): δ 13.86 (br s, 1H), 7.81-8.03 (m, 3H),7.42-7.48 (m, 4H), 7.11 (s, 1H), 4.51, (s, 2H). 13C NMR (100 MHz, CDCl3): δ 166.8, 161.5, 134.2, 133.6, 132.1, 131.4, 129.2, 128.6, 127.2, 126.7, 126.2, 125.7, 124.8, 123.3, 115.4, 30.9. MS (HR-ESI) for C16H11F3N2OS [(M+Na)+], calcd: m/z 359.0442, found: m/z 359.0440.
4.1.6. General procedure for the synthesis of compounds 4g, 5a-5c, and 5g.
To a mixture of compound 3a (1 eq.), alicyclic carboxylic acid (1.3 eq.), DMAP (0.45 eq), triethylamine (1.8 eq.) in DCM/DMF (~ 2 mL 3:1, v:v) was added EDAC (1.8 eq.) at room temperature. The reaction mixture was stirred for 2h, diluted with EtOAc, and washed with brine, aqueous HCl (0.2 N), and aqueous sodium bicarbonate solution. The organic phase was dried over anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography.
4.1.6.1. (E)-N-(5-Benzylthiazol-2-yl)-3-cyclohexylacrylamide (4g)
White solid; Rf 0.69 (DCM/MeOH, 97/3); yield 77%. 1H NMR (400 MHz, CDCl3): δ 11.92 (br s, 1H), 7.22-7.33 (m, 5H), 7.11 (s, 1H), 7.01 (dd, J = 15.5 Hz, J = 6.4 Hz, 1H), 6.03 (d, J = 15.2 Hz, 1H), 4.13 (s, 2H), 2.23 (m, 1H), 1.71-1.94 (m, 5H), 1.01-1.41 (m, 5H). 13C NMR (100 MHz, CDCl3): δ 163.9, 154.0, 139.3, 133.5, 128.9, 128.7, 127.0, 119.5, 40.8, 33.2, 31.8, 26.1, 25.9. MS (HR-ESI) for C19H22N2OS[(M+Na)+], calcd: m/z 349.1351, found: m/z 349.1356.
4.1.6.2. N-(5-Benzylthiazol-2-yl)tetrahydro-2H-thiopyran-4-carboxamide (5a)
White solid; Rf 0.55 (DCM/MeOH, 99/1); yield 39%. 1H NMR (400 MHz, CDCl3): δ 12.31 (s, 1H), 7.32 (m, 2H), 7.21 (m, 3H), 7.03 (s, 1H), 4.09 (s, 1H), 2.71 (m, 2H), 2.53 (m, 2H), 2.38 (m, 2H), 2.19 (m, 1H), 1.91-2.03 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 172.9, 159.5, 139.0, 133.0, 132.9, 129.0, 128.7, 127.1, 44.4, 33.2, 30.0, 27.8. MS (HR-ESI) for C16H18N2OS2[(M+H)+], calcd: m/z 341.0758, found: m/z 341.0745.
4.1.6.3. N-(5-Benzylthiazol-2-yl)tetrahydro-2H-pyran-4-carboxamide (5b)
White solid; Rf 0.49 (DCM/MeOH, 19/1); yield 40%. 1H NMR (400 MHz, CDCl3): δ 12.13 (s, 1H), 7.33-7.41 (m, 2H), 7.22 (m, 3H), 7.02 (s, 1H), 4.11 (s, 2H), 4.04 (d, J = 10.9 Hz, 2H), 3.33 (t, J = 10.9 Hz, 2H), 2.61-2.73 (m, 1H), 1.88-2.02 (m, 2H), 1.70 (d, J = 13.0 Hz, 2H) 13C NMR (100 MHz, CDCl3): δ 172.6, 159.4, 139.1, 132.9, 132.8, 129.0, 128.7, 127.1, 67.1, 41.88, 33.2, 28.8. MS (HR-ESI) for C16H18N2O2S [(M+Na)+], calcd: m/z 325.0987, found: m/z 325.0976.
4.1.6.4. N-(5-Benzylthiazol-2-yl)-1-methylpiperidine-4-carboxamide (5c)
White solid; Rf 0.53 (DCM/MeOH, 4/1); yield 20%. 1H NMR (400 MHz, CD3OD): δ 7.21-7.34 (m, 5H), 7.11 (s, 1H), 4.03 (s, 2H), 2.91 (m, 2H), 2.48 (m, 1H), 2.31 (s, 3H), 2.11-2.21 (m, 2H), 1.78-1.91 (m, 4H). 13C NMR (100 MHz, CD3OD): δ 175.0, 159.3, 141.4, 135.4, 133.9, 129.8, 129.5, 127.8, 55.8, 46.3, 42.6, 33.7, 29.2. MS (HR-ESI) for C17H21N3OS[(M+H)+], calcd: m/z 316.1484, found: m/z 316.1479.
4.1.6.5. N-(5-Benzylthiazol-2-yl)chromane-3-carboxamide (5g)
White solid; Rf 0.73 (DCM/MeOH, 97/3); yield 21%. 1H NMR (400 MHz, CDCl3): δ 7.01-7.31 (m, 8H), 6.93 (m, 2H), 4.49 (m, 1H), 4.21 (t, J = 10.1 Hz, 1H), 4.01 (s, 2H), 3.23-3.31 (m, 1H), 3.11-3.219 (m, 1H), 3.0 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 171.3, 168.8, 159.9, 155.4, 140.7, 134.7, 134.4, 131.3, 129.9, 129.3, 128.4, 122.6, 121.6, 118.4, 68.3, 41.3, 34.5, 29.5. MS (HR-ESI) for C20H18N2O2S[(M+Na)+], calcd: m/z 373.0987, found: m/z 373.0995.
4.1.7. Synthesis of compound 14b and 16a.
To an ice-cooled mixture of NaH (40 mg, 60% in mineral oil, 1.6 mmol) in THF (10 mL) was added drop wise a solution of compound 4f (0.25g, 0.76 mmol) in THF (5 mL). The mixture was stirred at room temperature for 20 min, again cooled to 0 °C, and methyl iodide (120 µL, 1.9 mmol) was added drop wise. The mixture was then stirred at room temperature for 2 h. Water (5 mL) was added to quench the reaction and the mixture was extracted with DCM. The combined organic layers were dried over anhydrous Na2SO4, evaporated, and the residue was separated/purified by silica gel chromatography to afford products 14b and 16a.
4.1.7.1. N-(5-Benzylthiazol-2-yl)-3-cyclohexyl-N-methylpropanamide (14b)
White solid; Rf 0.69 (hexane/EtOAc, 7/3); yield 38%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.34 (m, 6H), 4.11 (s, 2H), 3.72 (s, 3H), 2.63 (m, 2H), 1.56-1.83 (m, 7H), 1.11-1.39 (m, 4H), 0.91-1.04 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 172.6, 159.5, 139.9, 134.0, 133.9, 128.8. 128.6, 126.7, 37.3, 34.5, 33.2, 33.1, 32.3, 32.0, 26.6, 26.3. MS (HR-ESI) for C20H26N2OS[(M+H)+], calcd: m/z 343.1844, found: m/z 343.1830.
4.1.7.2. (E)-N-(5-Benzyl-3-methylthiazol-2(3H)-ylidene)-3-cyclohexylpropanamide (16a)
White solid; Rf 0.49 (hexane/EtOAc, 7/3); yield 32%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.43 (m, 5H), 6.53 (s, 1H), 3.89 (s, 2H), 3.62 (s, 3H), 2.51 (m, 2H), 1.62-1.83 (m, 7H), 1.09-1.34 (m, 4H), 0.81-1.0 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 183.3, 166.9, 138.1, 129.0, 128.8, 127.2, 125.7, 122.6, 37.6, 37.5, 35.4, 33.9, 33.5, 33.4, 26.8, 26.5. MS (HR-ESI) for C20H26N2S [(M+H)+], calcd: m/z 343.1844, found: m/z 343.1827.
4.1.8. General procedure for the synthesis of compounds 5d-5f and 16b-16d.
A mixture of compound 3a (1 eq.), acid chloride (1 eq.), and DMAP (1.2 eq.) in DCM (~2 mL) was stirred for 2h at room temperature. The solvent was evaporated and the residue was purified by silica gel column chromatography.
4.1.8.1. N-(5-Benzylthiazol-2-yl)pyrrolidine-1-carboxamide (5d)
White solid; Rf 0.51 (EtOAc/hexanes, 1/1); yield 5%. 1H NMR (400 MHz, CDCl3): δ 8.03 (br s, 1H), 7.21-7.39 (m, 4H), 7.01 (s, 1H), 4.11 (s, 2H), 3.48 (s, 4H), 2.03 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 159.9, 152.1, 139.8, 133.7, 131.31, 128.8, 128.6, 126.8, 46.0, 33.2, 29.9. MS (HR-ESI) for C15H17N3OS[(M+Na)+], calcd: m/z 310.0990, found: m/z 310.0991.
4.1.8.2. N-(5-Benzylthiazol-2-yl)piperidine-1-carboxamide (5e)
White solid; Rf 0.49 (EtOAc/hexanes, 1/1); yield 8%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.33 (m, 5H), 7.02 (s, 1H), 4.04 (s, 2H), 3.41 (m, 4H), 1.47-1.68 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 161.7, 153.1, 139.7, 133.3, 128.8, 128.6, 126.8, 45.3, 33.2, 29.9, 25.7, 24.4. MS (HR-ESI) for C16H19N3OS[(M+H)+], calcd: m/z 302.1327, found: m/z 302.1326.
4.1.8.3. N-(5-Benzylthiazol-2-yl)morpholine-4-carboxamide (5f)
White solid; Rf 0.54 (DCM/MeOH, 19/1); yield 7%. 1H NMR (400 MHz, CDCl3): δ 9.51 (br s, 1H), 7.32 (m, 5H), 6.93 (s, 1H), 4.03 (s, 2H), 3.69 (m, 4H), 3.54 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 162.8, 155.6, 140.9, 133.7, 132.8, 130.2, 130.0, 128.3, 68.0, 45.69, 34.5, 31.3. MS (HR-ESI) for C15H17N3O2S[(M+H)+], calcd: m/z 304.1120, found: m/z 304.1127.
4.1.8.4. N-[5-Benzyl-3-(pyrrolidine-1-carbonyl)thiazol-2(3H)-ylidene]pyrrolidine-1-carboxamide (16b)
White solid; Rf 0.72 (EtOAc/hexanes, 1/1); yield 25%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.43 (m, 5H), 6.61 (s, 1H), 3.93 (s, 2H), 3.41-3.69 (m, 8H), 1.87-2.01 (m, 8H). 13C NMR (100 MHz, CDCl3): δ 164.0, 160.7, 150.4, 137.7, 129.0, 128.9, 127.1, 124.9, 117.9, 47.7, 46.6, 45.5, 34.2, 25.3, 24.9. MS (HR-ESI) for C20H24N4O2S [(M+Na)+], calcd: m/z 407.1518, found: m/z 407.1587.
4.1.8.5. N-[5-Benzyl-3-(piperidine-1-carbonyl)thiazol-2(3H)-ylidene]piperidine-1-carboxamide (16c)
White solid; Rf 0.68 (EtOAc/hexanes, 1/1); yield 27%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.34 (m, 5H), 6.51 (s, 1H), 3.83 (s, 2H), 3.51-3.73 (m, 6H), 3.18 (m, 2H), 1.41-1.83 (m, 12H). 13C NMR (100 MHz, CDCl3): δ 166.3, 161.0, 150.9, 137.7, 128.9, 128.8, 127.1, 125.1, 118.2, 45.6, 43.8, 34.2, 26.4, 26.0, 24.9, 24.2. MS (HR-ESI) for C22H28N4O2S [(M+Na)+], calcd: m/z 435.1831, found: m/z 435.1821.
4.1.8.6. N-(5-Benzyl-3-(morpholine-4-carbonyl)thiazol-2(3H)-ylidene)morpholine-4-carboxamide (16d)
White solid; Rf 0.64 (DCM/MeOH, 19/1) yield 23%. 1H NMR (400 MHz, CDCl3): δ 7.21-7.34 (m, 5H), 6.56 (s, 1H), 3.81 (s, 2H), 3.49-3.77 (m, 16H). 13C NMR (100 MHz, CDCl3): δ 166.0, 161.1, 150.8, 137.4, 129.0, 128.9, 127.3, 125.8, 118.2, 67.0, 66.5, 45.1, 43.3, 34.2. MS (HR-ESI) for C20H24N4O4S [(M+Na)+], calcd: m/z 439.1416, found: m/z 439.1413.
4.2. Biology
4.2.1. Fungal strains and culture
The Histoplasma capsulatum strain used in this study was the wild-type strain G217B (ATCC 26032). Histoplasma cells were maintained as yeasts by growth in Histoplasma-macrophage media (HMM)34 at 37ºC in 95% air/5% CO2. The Cryptococcus strain used was the wild-type MATα serotype A strain H99 (kindly provided by Tamara Doering). Cryptococcus yeasts were cultured in YPD medium at 37ºC for routine maintenance or in HEPES-buffered RPMI for growth in microtiter plates for dose response measurements. For determination of the concentration of drug that inhibits 50% fungal growth (MIC50), Histoplasma or Cryptococcus yeasts were cultured in 96-well microtiter plates with a two-fold dilution series of each compound from 40 µM to 0.078 µM in 100 µL of their respective growth medium. Wells were inoculated with 2×105 Histoplasma yeasts or 2×102 Cryptococcus yeasts (determined by hemocytometer counts) and plates were agitated twice daily for improved aeration. Yeast growth was monitored by absorbance at 595 nm over 5 days (Histoplasma) or 4 days (Cryptococcus) using a Synergy2 plate reader (BioTek). The turbidity in each well was normalized to control wells lacking the antifungal compound but with an equivalent amount of DMSO solvent (the 40 µM compound dilution contained 1% DMSO). Relative turbidity measurements at 4 days (Histoplasma) or 3 days (Cryptococcus) were used for linear regression analysis and MIC50 determination. The minimal inhibitory concentration preventing visible growth of fungi (MIC) was determined by inspection of plates and visual assessment of growth or no-growth. Compounds with an MIC50 < 3.5 µM in Histoplasma yeasts were also evaluated in Cryptococcus yeasts. All compounds with an initial MIC50 < 4.5 µM were evaluated in triplicate.
4.2.2. Evaluation of mammalian-cell toxicity
Cytotoxicity was evaluated using the human hepatocyte cell line HepG2 (ATCC HB-8065) for selected compounds with an MIC50 ≤ 1.6 µM in Histoplasma yeasts. For mammalian cell culture, cells were maintained at 37ºC under 5% CO2/95% air in Minimum Essential Medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM L-glutamine. Mammalian cells were seeded into wells of a 96-well microtiter plate at 1×104 cells per well and allowed to adhere overnight. Antifungal compounds were subsequently added to cells in a concentration range from 80 µM to 0.3 µM. After 72 hours, HepG2 cells were quantified by measuring cell mass by crystal violet staining.35 Briefly, the growth medium was removed and cells washed once with phosphate-buffered saline (PBS). Cells were fixed in 3% paraformaldehyde, and stained with 0.1% crystal violet. Residual stain was removed by washing cells repeatedly with water and the retained crystal violet was solubilized in 10% acetic acid for 20 minutes with agitation. The retained dye was quantified by absorbance at 590 nm and normalized to untreated samples. Assays were performed on three replicates. Results were compared to control wells that lacked aminothiazole but contained an equivalent amount of DMSO as mammalian cell growth was decreased at solvent concentrations above 2%.
Acknowledgements
We thank The Ohio State University (OSU) Medical Center Clinical Microbiology Laboratory and Tamara Doering for providing fungal strains for testing. The project described was supported by Award Number Grant 8UL1TR000090-05 from the National Center For Advancing Translational Sciences and, in part, NIH (NIAID) grant R21AI109437. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health. This work was supported by funds provided by the OSU Public Health Preparedness for Infectious Diseases (PHPID) program (phpid.osu.edu) and by the OSU College of Pharmacy.
Abbreviations
- ATCC
American type culture collection
- DMAP
4-dimethylaminopyridine
- DMC
dichloromethane
- DMF
dimethylformamide
- DMSO
dimethyl sulfoxide
- EDAC
1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide
- FIC
fractional inhibitory concentration
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HMM
Histoplasma-macrophage media
- HR-ESI
high resolution–electrospray ionization
- MEM
minimum essential medium
- MIC50
minimal concentration that inhibits 50% of fungal growth
- MIC
minimal inhibitory concentration
- MW
molecular weight
- PBS
phosphate-buffered saline
- TFA
trifluoroacedic acid
- TFAA
trifluoroacedic anhydride
- THF
tetrahydrofurane
- SI
selectivity index
- SAR
structure-activity-relationship
- YPD
yeast extract peptone dextrose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Brown GD, Denning DW, Levitz SM. Science. 2012;336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]; (b) Pfaller MA, Diekema DJ. Crit. Rev. Microbiol. 2010;36:1. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 2.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. AIDS. 2009;23:525. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 3.(a) Galanis E, Hoang L, Kibsey P, Morshed M, Phillips P. Can. J. Infect. Dis. Med. Microbiol. 2009;20:23. doi: 10.1155/2009/719659. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. Clin. Infect. Dis. 2006;42:822. doi: 10.1086/500405. [DOI] [PubMed] [Google Scholar]
- 4.Fera MT, La Camera E, De Sarro A. Expert. Rev. Anti Infect. Ther. 2009;7:981. doi: 10.1586/eri.09.67. [DOI] [PubMed] [Google Scholar]
- 5.Maertens J. Eur. J. Haematol. 2007;78:275. doi: 10.1111/j.1600-0609.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Messer SA, Jones RN, Moet GJ, Kirby JT, Castanheira M. J. Clin. Microbiol. 2010;48:2984. doi: 10.1128/JCM.00328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Maligie MA, Selitrennikoff CP. Antimicrob. Agent. Chemother. 2005;49:2851. doi: 10.1128/AAC.49.7.2851-2856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hage CA, Connolly P, Horan D, Durkin M, Smedema M, Zarnowski R, Smith P, Wheat LJ. Antimicrob. Agents Chemother. 2011;55:4447. doi: 10.1128/AAC.01681-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards JA, Kemski MM, Rappleye CA. Antimicrob. Agents Chemother. 2013;57:4349. doi: 10.1128/AAC.00459-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards JA, Zemska O, Rappleye CA. Infect. Immun. 2011;79:3348. doi: 10.1128/IAI.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Kashyap SJ, Garg VK, Sharma PK, Kumar N, Dudhe R, Gupta JK. Med. Chem. Res. 2012;21:2123. [Google Scholar]; (b) Siddiqui N, Arshad MF, Ahsan W, Alam MS. Int. J. Pharm. Sci. Drug Res. 2009;1:136. [Google Scholar]
- 10.Gonzalez Cabrera D, Douelle F, Feng T-S, Nchinda AT, Younis Y, White KL, Wu Q, Ryan E, Burrows JN, Waterson D, Witty MJ, Wittlin S, Charman SA, Chibale K. J. Med. Chem. 2011;54:7713. doi: 10.1021/jm201108k. [DOI] [PubMed] [Google Scholar]
- 11.(a) Borelli C, Schaller M, Niewerth M, Nocker K, Baasner B, Berg D, Tiemann R, Tietjen K, Fugmann B, Lang-Fugmann S, Korting HC. Chemother. 2008;54:245. doi: 10.1159/000142334. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Narayana B, Vijaya Raj KK, Ashalatha BV, Kumari NS, Sarojini BK. Eur. J. Med. Chem. 2004;39:867. doi: 10.1016/j.ejmech.2004.06.003. [DOI] [PubMed] [Google Scholar]; (c) De Logu A, Saddi M, Cardia MC, Borgna R, Sanna C, Saddi B, Maccioni EJ. Antimicrob. Chemother. 2005;55:692. doi: 10.1093/jac/dki084. [DOI] [PubMed] [Google Scholar]
- 12.Alwan SM. Molecules. 2012;17:1025. doi: 10.3390/molecules17011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Balas Q, Anthony NG, Al-Jaidi B, Alnimr A, Abbott G, Brown AK, Taylor RC, Besra GS, McHugh TD, Gillespie SH, Johnston BF, Mackay SP, Coxon GD. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005617. no pp given. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayhoub AS, Khaliq M, Kuhn RJ, Cushman M. J. Med. Chem. 2011;54:1704. doi: 10.1021/jm1013538. [DOI] [PubMed] [Google Scholar]
- 15.Krasavin M, Karapetian R, Konstantinov I, Gezentsvey Y, Bukhryakov K, Godovykh E, Soldatkina O, Lavrovsky Y, Sosnov AV, Gakh AA. Arch. Pharm. 2009;342:420. doi: 10.1002/ardp.200800201. [DOI] [PubMed] [Google Scholar]
- 16.Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, Pang S, Shen DR, Fang Q, de Fex HF, McIntyre KW, Shuster DJ, Gillooly KM, Behnia K, Schieven GL, Wityak J, Barrish JC. J. Med. Chem. 2006;49:6819. doi: 10.1021/jm060727j. [DOI] [PubMed] [Google Scholar]
- 17.Gallardo-Godoy A, Gever J, Fife KL, Silber BM, Prusiner SB, Renslo AR. J. Med. Chem. 2011;54:1010. doi: 10.1021/jm101250y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daugan AC-M, Dean AW, Fillmore MC. 2009. WO 2009/150196 A1.
- 19.(a) Florjancic AS, Dart MJ, Ryther KB, Perez-Medrano A, Carroll WA, Patel MV, Tietje KR, Li T, Kolasa T, Gallagher ME, Peddi S, Frost JM, Nelson DW. 2010. US 2010/0093814 A1.; (b) Barda DA, Burkholder TP, Clayton JR, Hao Y, Heath PC, Henry JR, Knobeloch JM, Mendel D, McLean JA, Remick DM, Rempala ME, Wang Z-Q, Yip YYM, Zhong B. 2006. WO 2006/091671 A1.
- 20.Zheng S, Zhong Q, Jiang Q, Mottamal M, Zhang Q, Zhu N, Burow ME, Worthylake RA, Wang G. ACS Med. Chem. Lett. 2013;4:191. doi: 10.1021/ml300322n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu X-L, Li G, Wu G, Zhu J, Zhou L, Chen P-L, Chamberlin AR, Lee W-H. J. Med. Chem. 2009;52:1757. doi: 10.1021/jm8015969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Espinel-Ingroff A, Fothergill A, Fuller J, Johnson E, Pelaez T, Turnidge J. Antimicrob. Agents Chemother. 2011;55:2855. doi: 10.1128/AAC.01730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Diagn. Microbiol. Infect. Dis. 2011;69:45. doi: 10.1016/j.diagmicrobio.2010.08.013. [DOI] [PubMed] [Google Scholar]; (c) Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS, Testing C. S. f. A. Drug Resist. Updat. 2011;14:164. doi: 10.1016/j.drup.2011.01.004. [DOI] [PubMed] [Google Scholar]; (d) Wheat J, Marichal P, Vanden Bossche H, Le Monte A, Connolly P. Antimicrob. Agents Chemother. 1997;41:410. doi: 10.1128/aac.41.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Callahan HL, Portal AC, Devereaux R, Grogl M. Antimicrob. Agents Chemother. 1997;41:818. doi: 10.1128/aac.41.4.818. Gomes, T. C.; de Andrade Junior, H. F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lescano SA, Amato-Neto V. Rev. Soc. Bras. Med. Trop. 2012;45:485. doi: 10.1590/s0037-86822012000400014. [DOI] [PubMed] [Google Scholar]; (c) Merschjohann K, Sporer F, Steverding D, Wink M. Planta Med. 2001;67:623. doi: 10.1055/s-2001-17351. [DOI] [PubMed] [Google Scholar]
- 24.Odds FCJ. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]; Garcia LS. Clinical Microbiology Procedures Handbook. 3rd American Society for Microbiology; Washington, D.C.: 2010. [Google Scholar]
- 25.Uto Y, Ogata T, Kiyotsuka Y, Miyazawa Y, Ueno Y, Kurata H, Deguchi T, Yamada M, Watanabe N, Takagi T, Wakimoto S, Okuyama R, Konishi M, Kurikawa N, Kono K, Osumi J. Bioorg. Med. Chem. Lett. 2009;19:4159. doi: 10.1016/j.bmcl.2009.05.123. [DOI] [PubMed] [Google Scholar]
- 26.Pokhodylo N, Shyyka O, Matiychuk V. Med. Chem. Res. 2014;23:2426. [Google Scholar]
- 27.Zimenkovskii BS, Kutsyk RV, Lesyk RB, Matyichuk VS, Obushak ND, Klyufinska TI. Pharm. Chem. J. 2006;40:303. [Google Scholar]
- 28.Uto Y, Ogata T, Harada J, Kiyotsuka Y, Ueno Y, Miyazawa Y, Kurata H, Deguchi T, Watanabe N, Takagi T, Wakimoto S, Okuyama R, Abe M, Kurikawa N, Kawamura S, Yamato M, Osumi J. Bioorg. Med. Chem. Lett. 2009;19:4151. doi: 10.1016/j.bmcl.2009.05.119. [DOI] [PubMed] [Google Scholar]
- 29.Biswal SS, Singh A, Rastinehad F, Shen M, Boxer MB, Zhang Y-Q, Rohde JM, Oh K, Venkannagari S. 2014. WO 2014/145642 A2.
- 30.Zhao H, Caflisch A. Bioorg. Med. Chem. Lett. 2014;24:523. doi: 10.1016/j.bmcl.2014.01.083. [DOI] [PubMed] [Google Scholar]
- 31.Ratan R, Gazaryan I, Smirnova NA. 2013. US 2013/0005666 A1.
- 32.Hargrave KD, Hess FK, Oliver JT. J. Med. Chem. 1983;26:1158. doi: 10.1021/jm00362a014. [DOI] [PubMed] [Google Scholar]
- 33.(a) Stiller J, Marques-Lopez E, Herrera RP, Frohlich R, Strohmann C, Christmann M. Org. Lett. 2011;13:70. doi: 10.1021/ol102559f. [DOI] [PubMed] [Google Scholar]; (b) Alhamadsheh MM, Palaniappan N, DasChouduri S, Reynolds KA. J. Am. Chem. Soc. 2007;129:1910. doi: 10.1021/ja068818t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worsham PL, Goldman WE. J. Med. Vet. Mycol. 1988;26:137. [PubMed] [Google Scholar]
- 35.Scragg MA, Ferreira LR. Anal. Biochem. 1991;198:80. doi: 10.1016/0003-2697(91)90509-r. [DOI] [PubMed] [Google Scholar]