Abstract
Conventional immunostaining methods consume large quantities of expensive antibodies and are limited in terms of the number of antigens that can be detected from a single sample. In order to achieve multiplexed immunostaining, we micropatterning antibodies using aqueous two-phase systems formed from polyethylene glycol (PEG) and dextran. Multiple antigens can be detected on a single fixed sample by incorporating antibodies within dextran solutions, which are then patterned by micropipetting at specific sites on the sample in a solution of PEG. The antibodies are retained within the dextran phase due to biomolecular partitioning, allowing multiple protein markers to be visualized simultaneously by way of chromogenic, chemiluminescent or immunofluorescent detection. This aqueous two-phase system-mediated antibody micropatterning approach allows antibody dilutions to be easily optimized, reduces the consumption of expensive primary antibodies and can prevent antibody cross-reactions, since the antibodies are retained at separate sites within the dextran microdroplets.
Keywords: Aqueous Two-Phase System, Antibody micropatterning, Immunohistochemistry, Multiplex immunostaining
1 Introduction
Immunostaining, one of the most frequently used techniques in the biomedical sciences, is typically performed by incubating fixed cells or tissue sections in solutions containing primary antibodies that recognize specific antigens. Labeled secondary antibodies that recognize the primary antibodies are then used to indirectly visualize the antigens. For decades, this strategy has provided valuable information about protein expression and localization to biologists and clinicians [1, 2]. However, conventional immunostaining methods consume large quantities of expensive antibodies. In addition, the number of antigens that can be detected on one sample is often limited by the number of available detection channels (typically four or less for immunofluorescence and only one for chromogenic and chemiluminescent detection). Furthermore, when multiple primary antibodies are used together in solution the results can be confounded by higher background signals and antibody cross-reactions.
Because of these limitations, there has been increasing demand for more efficient multiplexed immunostaining methods. Quantum dots [3] and other advanced imaging and probing methods [4] can increase the number of antigens detected by fluorescence, but either require specific combinations of antibodies optimized to prevent cross-reactivity or iterative inactivation of the fluorescent probes. Antigen transfer methods, such as the layered peptide array [5], offer another promising approach to multiplexed immunostaining. However, these methods require sequential transfer of antigens to multiple substrates and may not be suitable for imaging subcellular localization of proteins. Microfluidic methods [6, 7] can be used to deliver small volumes of reagents to precise regions of a sample; however, they require specialized expertise and equipment, making them cumbersome to implement in laboratories and clinics.
We present an approach that takes advantage of the phase separation of polyethylene glycol (PEG) and dextran [8] solutions to enable micropatterning of antibodies directly on cell cultures and tissue samples using easily-accessed tools, such as micropipettors. We previously demonstrated that dextran -micropatterning can confine a variety of reagents, including DNA [9], enzymes [10] and antibodies [11–12] for biotechnological applications ranging from gene delivery to multiplexed ELISA. The aqueous two-phase system-mediated antibody micropatterning procedure for multiplexed immunostaining follows a workflow similar to other standard immunostaining procedures, with the exception that the primary antibodies are applied in dextran microdroplets to samples immersed in PEG (Figure 1A). This simple strategy for applying the primary antibodies allows multiple antigens to be detected on a single sample, while consuming very small antibody quantities (less than 2 µL of diluted antibody per spot). It also prevents antibody cross-reactions, because biomolecular partitioning of the antibodies to dextran keeps the antibodies spatially separated.
Figure 1. Multiplexed immunostaining of cell monolayers.
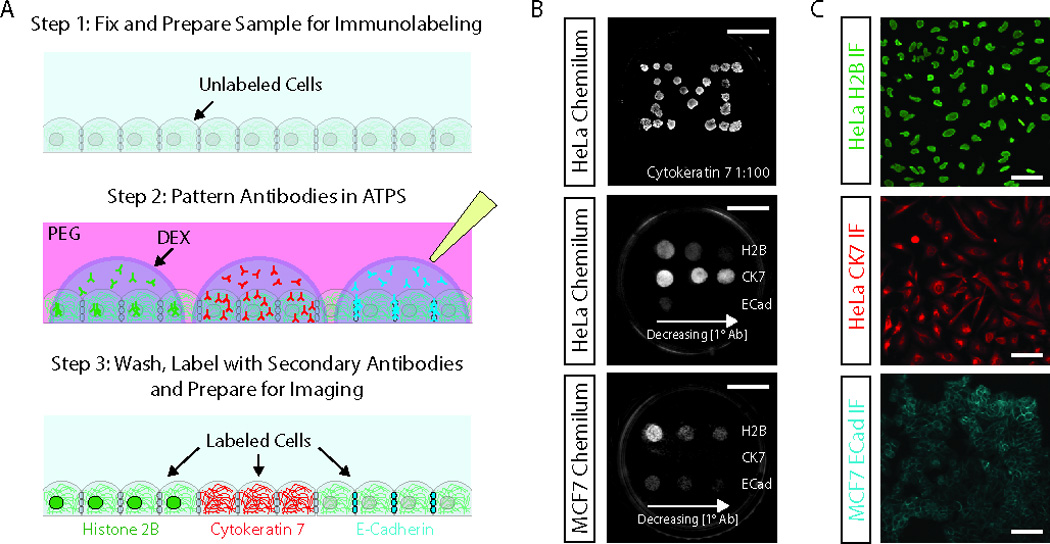
(A) Aqueous two-phase system-mediated multiplexed immunostaining uses ATPSs composed of PEG and dextran to micropattern primary antibody solutions on the surface of the sample. Apart from the primary antibody incubation step, we follow standard immunostaining procedures. (B) Chemiluminescence detection of cytokeratin 7 (CK7), histone 2B (H2B) and E-cadherin (ECad) in antibody-micropatterned HeLa and MCF7 cell monolayers. The top image shows a HeLa monolayer immunostained in a “Michigan M” pattern using 23 dextran droplets containing 1:100 anti-CK7 antibody. The middle and bottom images show cell type-specific staining for H2B (control), CK7 and Ecad in HeLa cells and MCF7 cells, respectively. From left to right the antibody dilution were 1:100, 1:400 and 1:800 for the anti-H2B antibody, 1:100, 1:600 and 1:1000 for the anti-CK7 antibody and 1:100, 1:600 and 1:1500 for the anti-ECad antibody. The spacing between the primary antibody spots can be estimated from the scale bars, which are ~10 mm. (C) Immunofluorescence detection of antigens at dextran /antibody micropatterned spots for H2B (top, 1:1000 dilution), CK7 (middle, 1:100 dilution) and ECad (bottom, 1:400 dilution). Scale bars are ~50 µm.
2 Materials and methods
2.1 HeLa and MCF7 cells culture
HeLa cells and MCF7 cells (ATCC: HTB-22; Lot: 5105358) were obtained from collaborators at University of Michigan and cultured in a humidified incubator at 37 °C under 5% CO2 in DMEM supplemented with 10% FBS and 1% Penicillin-Streptomycin-Glutamine. Near-confluent cell culture monolayers were produced by seeding 200,000 cells on 35 mm Petri dishes. The dishes were fixed 24 hours later in ice-cold methanol for 5 minutes.
2.2 Dorsal root ganglion (DRG) explant samples
Individual dorsal root ganglia were harvested from E7-E10 chicken embryos undergoing normal development in eggs purchased from Michigan State University Poultry Farm. The ganglia were dissected from the dorsal spinal cord in HBSS containing 1% anti-anti solution. The ganglia were then seeded on poly-D-Lysine-coated 35 mm polystyrene dishes in DMEM containing 10% FBS, NGF (100 ng/mL) and 1% anti-anti solution. The explant cultures were maintained for an additional 7 days with half of the medium replaced every other day. At the end of the culture period, the explants were fixed in 4% paraformaldehyde for 10 minutes.
2.3 Aorta sections
Sprague-Dawley rats (1–2 month old males) were euthanized by CO2 inhalation followed by bilateral thoracotomy. The abdominal aortas were immediately dissected and fixed in formalin overnight. All procedures involving animals were approved by the University of Michigan University Committee on Use and Care of Animals. The aortas were embedded in paraffin and sectioned by the University of Michigan Histology Core. The sections were deparaffinized by sequential 2 minute rinses in xylene (twice), 1:1 xylene:ethanol, 100% ethanol (twice), 95% ethanol, 70% ethanol, 50% ethanol and distilled water. Antigen recovery was performed by incubating the deparaffinized sections in citrate buffer (pH 6.0) containing 0.05% Triton X-100 at ~100 °C for 20 minutes.
2.4 Aqueous two-phase systems (ATPSs)
Solutions of 10% polyethylene glycol (PEG, MW 35,000 g/mol; Sigma, St. Louis, MO) containing 0.1% bovine serum albumin (96% purity; Sigma, St. Louis, MO) and 10% dextran (MW 500,000 g/mol; Pharmacosmos, Holbaek, Denmark) were used to form aqueous two-phase systems (ATPSs). The PEG solutions were applied to the samples at volumes sufficient to completely cover the samples (usually 2 mL for 35 mm Petri dishes or up to 20 mL for 75x25 mm glass slide contained within larger vessels). The antibodies were diluted in the dextran solutions and applied to the samples by micropipetting, either using handheld micropipettes or a previously described pneumatic dispensing system [10, 13], in volumes of 0.1 to 2 µL.
2.5 Immunostaining procedures
For immunofluorescence detection, the samples were blocked immediately after fixation for 1 hour in 10% normal goat serum. After blocking, the samples were completely covered with PEG and the primary antibodies were applied by micropipetting dextran /antibody droplets (0.1 to 2 µL in volume) onto the surface of the samples. The samples were then incubated overnight at 4 °C. The next day, the PEG and dextran solutions were thoroughly washed away through three rapidly applied washes and two 5-minute washes in PBS. The appropriate secondary antibodies were then bath applied to the samples for 2 hours at room temperature in the dark. Finally, three 5-minute washes in PBS were performed and the samples were mounted for imaging. The following primary antibodies were used at various dilutions: rabbit-anti-histone 2B, mouse-anti-cytokeratin 7, mouse-anti-CDH1 (E-cadherin), mouse-anti-Tuj1, mouse-anti-α-smooth muscle actin and FITC-conjugated rabbit-anti-rat IgG (all from Sigma, St. Louis, MO). Alexa-594-goat-anti-mouse IgG, Alexa-594-goat-anti-rabbit IgG and Alexa-488-goat-anti-mouse IgG secondary antibodies (all from Life Technologies, Carlsbad, CA) were used at 1:500 dilutions. For the DRG explants, FITC-WGA (10 µg/mL in dextran; Life Technologies, Carlsbad, CA) was visualized after incubation for 2 hours at room temperature in the dark, followed by washing in PBS as described above. TRITC- dextran (10 µg/mL in dextran; Life Technologies, Carlsbad, CA) was sometimes used to visualize the dextran droplets before washing.
For chromogenic and chemiluminescent detection, the samples were first blocked for endogenous peroxidase activity for 1 hour in 1% H2O2 and then blocked with 10% normal goat serum for 1 hour. The primary antibodies were applied and the samples were thoroughly washed as described above. Biotinylated (or in some cases HRP-conjugated) secondary antibodies were then bath applied for 2 hours at room temperature. The samples were then washed three times in PBS, before streptavidin-HRP (R&D Systems, Minneapolis, MN) was applied for 45 minutes. Finally, the samples were washed again and the chromogenic and chemiluminescent signals were developed using diaminobenzidine (Life Technologies, Carlsbad, CA) and SuperSignal Femto reagent (Thermo Scientific, Waltham, MA), respectively. It is important to note that it would also be possible to detect antigens using biotin-conjugated primary antibodies. Although, we did not test this in the present study, our previous studies using ELISAs demonstrate that direct detection of antigens by way of chemiluminescence is possible using ATPSs. The following antibodies were used: rabbit-anti-histone 2B (Sigma, St. Louis, MO), mouse-anti-cytokeratin 7 (Sigma, St. Louis, MO) and mouse-anti-CDH1 (E-cadherin) (Sigma, St. Louis, MO), biotin-goat-anti-mouse (Life Technologies, Carlsbad, CA) and HRP-goat-anti-rabbit (Santa Cruz Biotechnology, Dallas, TX).
2.6 Imaging and microscopy
A Nikon TE300 microscope was used for brightfield and fluorescence imaging. A Fluorchem M Western reader was used for chemiluminescence detection. Images of samples developed with diaminobenzidine were acquired using a handheld digital camera.
3 Results
Aqueous two-phase system-mediated multiplexed immunostaining can be used to pattern primary antibodies on cells in a variety of patterns, as evidenced by the formation of an immunostained “Michigan M” on a HeLa cell monolayer (Figure 1B, top). Using a gel loading micropipette tip attached to a pneumatic dispensing pump we were able to generate droplets of dextran in PEG ranging from 0.8 µL for a 0.005 sec air pulse to 4 µL for a 0.0175 sec air pulse. We previously demonstrated that a capillary needle can be used to produce much smaller droplets as small as ~10 pL. Using handheld micropipettes it is possible to produce droplets as small as 100 nL. In all of these dispensing systems, the maximum droplet volume is determined by the maximum amount of dextran solution that can be held in the dispensing tip, although generally, smaller droplets (~1 nL to 2 µL range) are preferred for micropatterning applications. We previously demonstrated that dextran droplets remain stable, without substantial changes to their size or shape, during incubation [9, 11].
We used HeLa and MCF7 cell monolayers to demonstrate that aqueous two-phase system-mediated antibody micropatterning strategy enables rapid and cost-efficient optimization of primary antibody concentrations (Figure 1B, middle and bottom). We tested three concentrations of three antibodies raised against histone 2B (H2B, nuclear localization), cytokeratin 7 (CK7, cytoskeletal localization) and E-cadherin (ECad, cell membrane localization). It is known that HeLa cells express CK7, but not ECad; whereas MCF7 cells express ECad, but not CK7. Both cell types express H2B. As expected, we observed a decrease in chemiluminescence with decreasing concentrations of all three antibodies (quantification shown in Figure S1).
Also as expected, HeLa cells stained intensely for CK7, with faint ECad staining only at the highest concentration of ECad antibody. This low level of signal at the 1:100 dilution of anti-ECad antibody was likely due to non-specific binding, since proper cell-cell junction localization of ECad signal was not observed for HeLa cells. In contrast, the MCF7 cells did not stain for CK7 at any of the antibody concentrations, but clearly stained for ECad. Based on this experiment, one can select the most appropriate antibody dilutions for further experiments. The appropriate subcellular localizations of these three markers are shown in the fluorescence images in Figure 1C. In addition, we demonstrated that the aqueous two-phase system-mediated antibody micropatterning strategy is appropriate for use with the most common modes of detection including chromogenic detection (Figure S2), chemiluminescent detection (Figure 1B) and immunofluorescence (Figure 1C). We also noted that the signal decreased at shorter incubation times, as tested using chromogenic detection of CK7 (Figure S3).
We next demonstrated that aqueous two-phase system-mediated antibody micropatterning approach can be used to stain more complex samples such as dorsal root ganglion (DRG) explants (Figure 2A) and rat abdominal aorta sections (Figure 2B). For even more precise patterning, we delivered the dextran droplets using a previously described capillary needle pipetting system [10, 13]. We were able to deliver dextran droplets selectively to the DRG axon terminals, as indicated by the localization of TRITC- dextran to the axons of Tuj1-stained DRG explants. We demonstrated biochemical staining with FITC-wheat germ agglutinin by selectively staining the axons (Figure 2A, third column) and ganglia (Figure 2A, fourth column) of the explants. Finally, we demonstrated immunohistochemical staining of fixed sections of rat abdominal aorta using two markers: α-smooth muscle actin (SMA) and FITC-anti-rat. As expected, the SMA antibody primarily labeled the tunica media of the aorta cross-sections, while the anti-rat antibody labeled the tunica intima, media and adventitia, as well as the thrombus in the lumen.
Figure 2. Multiplexed immunostaining of tissue explants and histological sections.
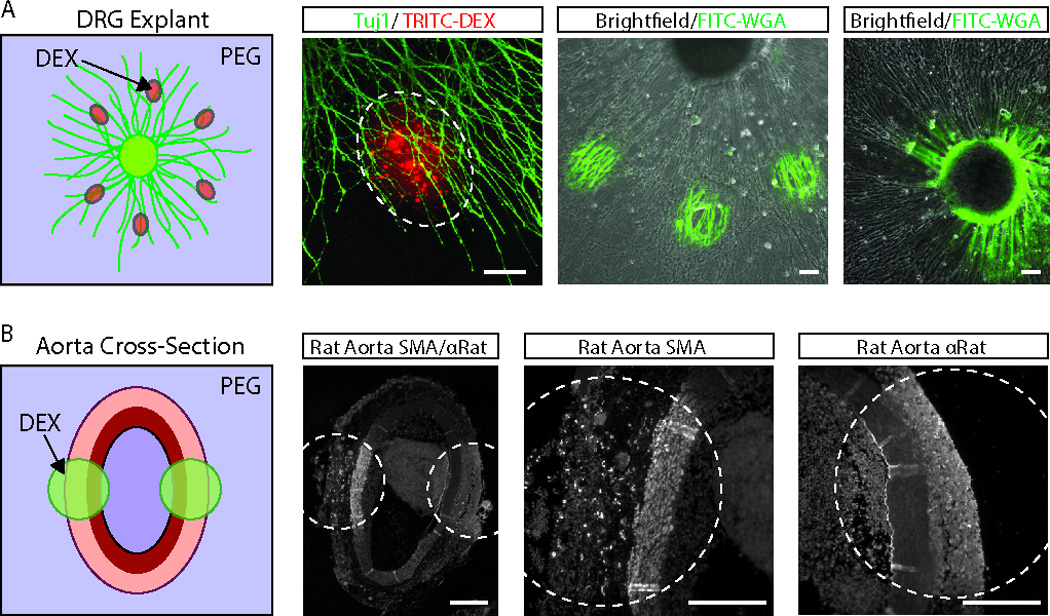
(A) DRG explants from chick embryos were selected to demonstrate the potential of this technique for multiplexed immunostaining of complex samples. The micropatterning system was initially tested by localizing TRITC- dextran -containing dextran microdroplets to the axons of Tuj1-labeled DRG explants (left-two images). We next tested the ability to micropattern biochemical stains, such as FITC-wheat germ agglutinin (FITC-WGA). FITC-WGA staining could be used to selectively label both the axons and ganglia of the DRG explants (right-two images). Scale bars are ~100 µm. (B) Multiplexed immunostaining of histological section was demonstrated on paraffinized cross-sections of rat abdominal aortas using anti-α-smooth muscle actin (SMA) and anti-rat antibodies. Scale bars are ~200 µm.
4. Discussion
We demonstrate the strength of the aqueous two-phase system-mediated antibody micropatterning system for multiplexed labeling of cell cultures, explants and tissue sections. In comparison to conventional immunostaining protocols that require milliliter volumes of antibody solutions to cover each sample, the aqueous two-phase system-mediated antibody micropatterning system vastly reduces the amount of reagents required, using diluted antibody volumes of 0.1 to 2 µL. In addition, the aqueous two-phase system-mediated antibody micropatterning technique allows multiple antigens to be probed within the same sample and read out in a single channel. In contrast to other methods for multiplexed immunostaining [3–7], the aqueous two-phase system-mediated antibody micropatterning method does not require any specialized equipment or non-standard reagents. In fact, it follows a workflow identical to the standard workflow for immunostaining, with the exception of the ATPS micropatterning step.
Aqueous two-phase system-mediated antibody micropatterning provides several benefits to researchers and clinicians. First, there is no optical cross-talk or antibody cross-reactivity that can confound interpretation of the results because the antibodies are retained at separate addressable sites on the sample. Second, the results can be read using a single channel, facilitating more rapid data collection and analysis using a variety of detection modalities. Finally, in select cases where tissue sections or cells are in short supply, aqueous two-phase system-mediated antibody micropatterning can facilitate more judicious sample usage. Because the aqueous two-phase system-mediated antibody micropatterning technique adopts standard immunostaining work flows and easily accessed reagents and tools, we expect it to be easily adopted by biomedical researchers and clinics.
Supplementary Material
Acknowledgments
The authors would like to acknowledge funding from the Coulter Foundation Grant, NIH (CA 170198) and a generous gift from the Beyster Foundation. JBW thanks the NSF (DGE 0718128; ID: 2010101926) for a pre-doctoral fellowship. J.P.F. designed and performed experiments and wrote the paper, M.T. performed experiments and assisted with writing the methods sections, J.B.W. performed the chromogenic detection experiments and assisted with writing the main text, A.T.A assisted with experiments and S.T. supervised the project.
Abbreviations
- PEG
Polyethylene Glycol
- ATPS
Aqueous Two-Phase System
- H2B
Histone 2B
- Ecad
E-Cadherin
- CK7
Cytokeratin 7
- DRG
Dorsal Root Ganglion
- SMA
Smooth Muscle Actin
- ELISA
Enzyme-linked Immunosorbent Assay
- FBS
Fetal Bovine Serum
- DMEM
Dulbecco’s Modified Eagle’s Medium
- HBSS
Hank’s Balanced Salt Solution
- FITC-WGA
Fluorescein Isothiocyanate-Wheat Germ Agglutinin
- TRITC
Tetramethylrhodamine Isothiocyanate
- HRP
Horse Radish Peroxidase
Footnotes
Conflicts of interest: J.B.W and S.T. own stock in PHASIQ, Inc. a company working on related technologies.
References
- 1.Brandtzaeg P. The increasing power of immunohistochemistry and immunocytochemistry. J Immunol Methods. 1998;216(1–2):49–67. doi: 10.1016/s0022-1759(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 2.Matos LL, Trufelli DC, de Matos MG, da Silva Pinhal MA. Immunohistochemistry as an Important Tool in Biomarkers Detection and Clinical Practice. Biomarker Insights. 2010;5:9–20. doi: 10.4137/bmi.s2185. (1841-BMI-Immunohistochemistry-as-an-Important-Tool-in-Biomarkers-Detection-and-.pdf): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing Y, Chaudry Q, Shen C, Kong KY, et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc. 2007;2(5):1152–1165. doi: 10.1038/nprot.2007.107. [DOI] [PubMed] [Google Scholar]
- 4.Gerdes MJ, Sevinsky CJ, Sood A, Adak S, et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A. 2013;110(29):11982–11987. doi: 10.1073/pnas.1300136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gannot G, Tangrea MA, Erickson HS, Pinto PA, et al. Layered peptide array for multiplex immunohistochemistry. J Mol Diagn. 2007;9(3):297–304. doi: 10.2353/jmoldx.2007.060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovchik RD, Kaigala GV, Georgiadis M, Delamarche E. Micro-immunohistochemistry using a microfluidic probe. Lab Chip. 2012;12(6):1040–1043. doi: 10.1039/c2lc21016a. [DOI] [PubMed] [Google Scholar]
- 7.Kim MS, Kim T, Kong SY, Kwon T, et al. Breast Cancer Diagnosis Using a Microfluidic Multiplexed Immunohistochemistry Platform. PLoS One. 2010;5(5):e10441. doi: 10.1371/journal.pone.0010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albertsson PÅ. Partition of cell particles and macromolecules: separation and purification of biomolecules, cell organelles, membranes, and cells in aqueous polymer two-phase systems and their use in biochemical analysis and biotechnology. 3rd ed. New York: Wiley; 1986. p. 346. [Google Scholar]
- 9.Tavana H, Jovic A, Mosadegh B, Lee QY, et al. Nanolitre liquid patterning in aqueous environments for spatially defined reagent delivery to mammalian cells. Nat Mater. 2009;8(9):736–741. doi: 10.1038/nmat2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frampton JP, Shi H, Kao A, Parent JM, et al. Delivery of Proteases in Aqueous Two-Phase Systems Enables Direct Purification of Stem Cell Colonies from Feeder Cell Co-Cultures for Differentiation into Functional Cardiomyocytes. Adv Healthc Mater. 2013;2(11):1440–1444. doi: 10.1002/adhm.201300049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frampton JP, White JB, Simon AB, Tsuei M, et al. Aqueous two-phase system patterning of detection antibody solutions for cross-reaction-free multiplexed ELISA. Scientific Reports. 2014;4 doi: 10.1038/srep04878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon AB, Frampton JP, Huang NT, Kurabayashi K, et al. Aqueous two-phase systems enable multiplexing of homogeneous immunoassays. Technology. 2014 doi: 10.1142/S2339547814500150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frampton JP, Fang ZZ, Simon AB, Chen D, et al. Aqueous Two-Phase System Patterning of Microbubbles: Localized Induction of Apoptosis in Sonoporated Cells. Advanced Functional Materials. 2013;23(27):3420–3431. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


