Abstract
Dopamine (DA) neurons in the midbrain are crucial for motivational control of behavior. However, recent studies suggest that signals transmitted by DA neurons are heterogeneous. This may reflect a wide range of inputs to DA neurons, but which signals are provided by which brain areas is still unclear. Here we focused on the pedunculopontine tegmental nucleus (PPTg) in macaque monkeys and characterized its inputs to DA neurons. Since the PPTg projects to many brain areas, it is crucial to identify PPTg neurons that project to DA neuron areas. For this purpose we used antidromic activation technique by electrically stimulating three locations (medial, central, lateral) in the substantia nigra pars compacta (SNc). We found SNc-projecting neurons mainly in the PPTg, and some in the cuneiform nucleus (CuN). Electrical stimulation in the SNc-projecting PPTg regions induced a burst of spikes in presumed DA neurons, suggesting that the PPTg-DA(SNc) connection is excitatory. Behavioral tasks and clinical tests showed that the SNc-projecting PPTg neurons encoded reward, sensorimotor and arousal/alerting signals. Importantly, reward-related PPTg neurons tended to project to the medial and central SNc, whereas sensorimotor/arousal/alerting-related PPTg neurons tended to project to the lateral SNc. Most reward-related signals were positively biased: excitation and inhibition when a better and worse reward was expected, respectively. These PPTg neurons tended to retain the reward value signal until after a reward outcome, representing ‘value state’; this was different from DA neurons which show phasic signals representing ‘value change’. Our data, together with previous studies, suggest that PPTg neurons send positive reward-related signals mainly to the medial-central SNc where DA neurons encode motivational values and sensorimotor/arousal signals to the lateral SNc where DA neurons encode motivational salience.
Keywords: antidromic activation, reward value, salience, cuneiform nucleus, substantia nigra pars compacta, monkey
Introduction
DA neurons encode reward prediction errors and thereby guide learning to acquire better rewards (Schultz, 1998). This is the most well documented feature of DA neurons. However, neuronal recording experiments using various behavioral tasks have suggested that DA neurons have additional or different features (Brown et al., 2009; Tritsch et al., 2012), leading to a view that DA neurons are functionally heterogeneous (Roeper, 2013; Hong, 2013). Seemingly opposite to the notion that DA release in the brain underlies pleasure (Wise 1989), some DA neurons are excited by aversive stimuli (Brischoux et al., 2009; Coizet et al., 2010; Horvitz, 2000; Lammel et al., 2011). In the monkey substantia nigra pars compacta (SNc), the heterogeneity of response to aversive stimuli creates a functional gradient of DA neurons (Matsumoto and Hikosaka, 2009): DA neurons in the medial SNc are excited when a better reward is expected (positive value) and inhibited when a worse punishment is expected (negative value), suggesting that they signal motivational values; in contrast, DA neurons in the lateral SNc are excited by both a better reward and a worse punishment, suggesting that they signal motivational salience. DA neurons in the lateral SNc are also activated by high cognitive demands (Matsumoto and Takada, 2013). These results raise the possibility that DA neurons with different functional features receive inputs from different brain areas, which is partially supported by anatomical and optogenetic studies (Lammel et al., 2012; Watabe-Uchida et al., 2012). However, what information is transmitted and processed in each of the afferent connections to DA neurons is still unclear.
Perhaps the best characterized afferent connection to DA neurons is a polysynaptic circuit that originates from the border region of the globus pallidus (GPb), mediated by the lateral habenula (LHb) and the rostromedial tegmental nucleus (RMTg) (Hong and Hikosaka, 2008; Jhou et al., 2009a; Brinschwitz et al., 2010; Balcita-Pedicino et al., 2011; Hong et al., 2011). Studies on macaque monkeys suggest that the GPb-LHb-RMTg-DA circuit transmits motivational value information (most commonly reward prediction error signal) to DA neurons (Matsumoto and Hikosaka, 2007; Hong and Hikosaka, 2008; Bromberg-Martin et al., 2010; Hong et al., 2011). These results raise two questions. First, what is the synaptic mechanism underlying the excitation of DA neurons? Since the RMTg-DA connection is GABAergic inhibitory (Jhou et al., 2009b; Barrot et al., 2012), the positive value is translated from a decrease in activity in GPb-LHb-RMTg neurons to an increase in activity in DA neurons; this is a disinhibition. Is the burst activity of DA neurons reflecting a positive value due only to the disinhibition? Or, does any excitatory input contribute to the burst activity? Second, where is the source of motivational salience signal in DA neurons? Since the GPb-LHb-RMTg-DA circuit mainly aims at the medial SNc (Matsumoto and Hikosaka, 2009), it is likely that the lateral SNc receives inputs from other brain areas where information related to motivational salience is processed.
The pedunculopontine tegmental nucleus (PPTg) is a candidate that fills the missing link. This is supported by several lines of data: 1) PPTg project to the DA area (Scarnati et al., 1984; Scarnati et al., 1987; Lavoie and Parent, 1994a; Charara et al., 1996); 2) activation of PPTg evokes burst activity in DA neurons (Floresco et al., 2003); 3) PPTg neurons encode reward-related signals (Okada et al., 2009; Okada and Kobayashi, 2013) and sensorimotor signals (Kobayashi et al., 2002; Pan and Hyland, 2005); animals with a PPTg damage have difficulty in associating stimuli with rewards (Inglis et al., 2000). However, these data do not prove the hypothesis that PPTg sends reward-related and sensorimotor information to the DA area. This question is important because PPTg projects to many brain areas other than the DA area (Mena-Segovia et al., 2004) and contains several kinds of neurons using different neurotransmitters (Lavoie and Parent, 1994b; Futami et al., 1995; Wang and Morales, 2009). To test the hypothesis, it is crucial to identify neurons in the PPTg that project to the DA area. A reliable method is antidromic activation of PPTg neurons by electrical stimulation in the DA area. Furthermore, since the DA area consists of functionally heterogeneous divisions, it is much more informative if we know where in the DA area each PPTg neuron projects to. To this end, we placed three electrodes in the SNc (medial, central, lateral) chronically, and used them for antidromic stimulation and neuronal recording. We found that PPTg neurons transmit heterogeneous signals to these SNc regions with excitatory connections, forming connectivity whereby the medial SNc region tend to receive reward-related signals while the lateral SNc receives sensorimotor and arousal signal.
Materials and Methods
Two rhesus monkeys (Macaca mulatta), R (male, 10 y/o, 11 kg) and Z (male, 6 y/o, 7 kg), were used as subjects in this study. All animal care and experimental procedures were approved by the National Eye Institute and Institutional Animal Care and Use Committee and complied with the Public Health Service Policy on the humane care and use of laboratory animals.
Behavioral tests
A major goal of our study was to understand what kinds of information are transmitted from PPTg neurons to DA neurons. In order to achieve this goal, we used a variety of behavioral tasks in which the monkey’s behavior was examined in different contexts. The tasks included: (1) Reward-biased saccade task, (2) Reward-unbiased saccade task, (3) Passive auditory task. In addition, we used a variety of ‘clinical’ tests. Below, we described details of these tasks.
Reward-biased saccade task (Fig. 1A)
Figure 1.
Behavioral tasks. A. One-direction-rewarded saccade task (1DR). After an initial fixation in the center of the screen, a target appears on the left or right side and the monkey was required to make a saccade to it. A reward (juice) was delivered after the saccade to the left in one block of 24 trials and to the right in the other block of trials. These blocks were alternated. The distribution of saccade latencies in each block is shown below. B. Eight-direction visually guided saccade task. The saccade target appeared at one of the 8 locations. The amount of reward was the same among the 8 directions. C. Auditory passive listening task. It consisted of 20~35 tic sounds delivered every 0.5 sec (regular tics) and occasional odd tics. The tic sounds were sometimes (50%) followed by a beep sound or a tone with a juice.
The goal of this task was to examine reward-related properties of PPTg neurons. Specifically, saccades to one direction were followed by a juice reward, but saccades to the other direction were followed by no reward. Details of this “one direction rewarded (1DR)” task were described previously (Lauwereyns et al., 2002; Hong et al., 2011). Visual stimuli were rear-projected by a projector onto a frontoparallel screen 33 cm from the monkey’s eyes. Eye movements were monitored using a scleral search coil system (Robinson, 1963). A trial started when a small spot of light appeared on the screen, on which the monkey had to fixate. After the monkey maintained fixation on the spot for 750~1250ms, the fixation spot disappeared and another spot of light (target) appeared on either the right or left side, 10° from the fixation spot. The monkey was required to make a saccade to the target within 750 ms. Correct and incorrect saccades were signaled by a tone and a beep 200 ms after the saccade, respectively. Within a block of 24 trials, saccades to one fixed direction were rewarded with 0.3 ml of apple juice while saccades to the other direction were not rewarded. The position-reward contingency was reversed in the next block with no external instruction. Even in the unrewarded trials, the monkey had to make a correct saccade; otherwise, the same trial was repeated. In rewarded trials a liquid reward was delivered which started simultaneously with a tone stimulus.
Reward-unbiased saccade task (Fig. 1B)
The goal of this task was to examine the visual and saccade-related properties of PPTg neurons, while excluding reward-related properties. The procedure was the same as 1DR task, except that the target appeared at one of the 8 positions with the same eccentricity and that the successful trial was always followed by a reward (0.3 ml of apple juice). We changed the target eccentricity depending on the neuron’s response.
Passive auditory task (Fig. 1C)
The goal of this task was to examine complex auditory responses of PPTg neurons. Sound stimuli consisted of regular tics (interval: 0.5 s) and unexpected sounds. There were three kinds of unexpected sounds: (1) Odd tics – a tic sound was inserted between two regular tics with a probability of 10%, (2) A beep (duration: 400 ms) delivered at the end of a trial with a probability of 25%, (3) A tone (duration: 400ms) with juice delivery at the end of a trial with a probability of 25%. One trial consisted of 20–35 regular tics with or without the unexpected sounds. The inter-trial interval varied between 3~6 sec.
Clinical tests
PPTg neurons showed a variety of activity that was not elucidated by the computer-controlled behavioral tasks. To obtain some evidence for their functions, we tested each neuron’s activity in the following manners. To examine general visual properties, we presented various visual images on the screen, including stationary pictures of various objects and moving light spots. We also presented real objects in front of the monkey. To examine general auditory properties, we presented various click and tapping sounds at various positions (e.g., behind and in front of the monkey). To examine somatosensory and motor properties, we lightly touched and moved various body parts of the monkey, and examined the relationship between neuronal firing and the monkey’s spontaneous body movements.
Recording and stimulation procedures
To study whether and how signals are transmitted from PPTg neurons to DA neurons, we placed a single electrode in the PPTg and a set of three electrodes in the SNc. This required careful placement of recording/stimulation chambers, chamber-based MR imaging, and exploration of single neuronal activity in the PPTg and SNc, as described below.
Electrode access to PPTg and SNc
To minimize artifactual interactions between the PPTg and SNc electrodes, we placed two recording/stimulation chambers along the midline, the posterior chamber aiming at the PPTg and the anterior chamber aiming at the SNc. For monkey R, the PPTg chamber was placed vertically in the stereotaxic coordinates while the SNc chamber was tilted anteriorly by 20°. For monkey Z, the PPTg chamber was tilted posteriorly by 15 ° while the SNc chamber was tilted anteriorly by 15°. These recording/stimulation chambers together with a head holder were held in place with dental acrylic and secured to the skull with ceramic screws. The placement of the chambers was guided by a brain atlas (Saleem and Logothetis, 2006). After the chambers were implanted surgically, MR images were acquired along the 3D coordinates of each chamber using a 4.7T vertical scanner (Bruker BioSpec 4.7T). We used a T1 weighted modified driven-equilibrium Fourier transform (MDEFT) (Lee et al., 1995). To visualize the chamber coordinates, we filled chamber-attached grid holes with saline-gadolinium solution. The MDEFT images had a field of view 96 × 96 × 64 mm, and 0.5 mm isotropic voxel size. The MRI procedure was performed for both monkey R and Z. Based on the MR images, we performed craniotomy and, after the monkey’s recovery, performed single unit recording (see below).
Single-unit recording
We used monopolar tungsten electrodes (Frederick Haer, diameter: 0.25 mm, 0.5–2 M Ohm) for single unit recording. To position the electrode we used a grid system, which allowed recordings at every 1 mm between penetrations. The electrode was introduced into the brain through a stainless steel guide tube, which was inserted into one of the grid holes and then to the brain via the dura. We advanced the electrode using an oil-driven micromanipulator (MO-97A, Narishige). The neuronal electrical signal was amplified with a band-pass filter (200 Hz–10 kHz; BAK, Mount Airy, MD) and was collected at 40 kHz. Activity of a single neuron was isolated on-line using a custom voltage-time window discrimination software (Blip; available at www.simonhong.org). The software allowed us to set multiple inclusion and exclusion windows so that only such activity was isolated that goes through all inclusion windows but does not go through any of the exclusion windows.
Antidromic activation
Electrical stimulation at the axon terminal of a PPTg neuron would evoke an action potential (spike) that conducts antidromically to the cell soma of the PPTg neuron. We used this method to test if a PPTg neuron projects its axon to the SNc where DA neurons were located. For this purpose, we chromically implanted three bipolar platinum electrodes (Frederick Haer, diameter: 250μm, 10 kOhm) in the medial, central, and lateral portions of the SNc (Fig. 2, also see below for further comments). To detect antidromic spikes, we lowered the recording electrode toward and inside the PPTg, while electrical stimulation was applied via the three SNc electrodes one at a time at every 0.5 s. We used a biphasic square pulse (0.2ms negative followed by 0.2 ms positive, <300 μA) for the stimulation. If spikes with a fixed latency were detected, we performed a collision test by triggering the electrical stimulation by spontaneous spikes of the same neuron: if the stimulation is applied before (or immediately after) a spontaneous spike reaches the stimulation site, the antidromic spike would collide with the spontaneous spike and therefore would not conduct to the cell soma. The procedure for the antidromic activation was controlled by Blip. To minimize the stimulation-induced electrical artifact, we used an artifact remover (Artifact Zapper-1, Riverband Instrument) by which the averaged post-stimulation potential was subtracted from the current post-stimulation potential. After the identification of antidromic activation, we examined the activity of the SNc-projecting PPTg neuron using various tasks (Fig. 1). If the PPTg neuron remained stable, we simultaneously recoded its activity and the multiunit SNc activity.
Figure 2.
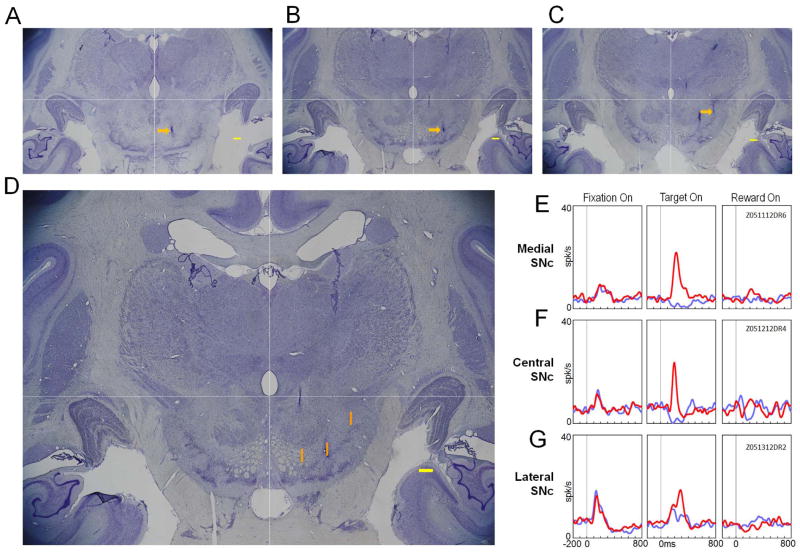
The locations of the stimulating electrodes in the SNc. The tips of the three electrodes are visualized in Nissl-stained coronal sections: medial (A), central (B) and lateral (C) portions of the SNc. These sections were slightly separated from caudal (A) to rostral (C) directions by 1 mm (A–B) and 0.4 mm (B–C). D. The three electrode positions are projected to the middle section shown in B (orange lines). The bottom of each orange line indicates the stimulation site. Scale bars in A–D indicate 1 mm. E–G: Activity of presumed DA neurons recorded at the medial, central and lateral SNc, respectively, during 1DR task. The activity is shown separately for the rewarded (red) and non-rewarded (blue) trials. It is aligned at the onsets of the fixation point, target, and reward.
Chronic implantation of stimulating electrodes
It has been noted (but not published) that the effectiveness of electrical stimulation declines if the electrode is implanted at the same position for a long time. It may be because neural tissue around the electrode tip is damaged and/or replaced with glial tissue (Griffith & Humphrey, 2006; Polikov et al., 2005). To minimize this effect, we positioned the electrodes about 3 mm above the DA neuron areas and, only during experiment, moved them down to the DA neuron areas. This method appeared to work well because DA neuron activity remained detectable even after repeated experiments in most of the electrodes. Also, the antidromic activation of PPTg neurons remained effective during repeated experiments spanning more than 2 months.
Orthodromic activation
To determine the nature of PPTg connection to DA neurons, we examined the responses of presumed DA neurons to electrical stimulation of the PPTg. After examining the activity of a PPTg neuron, we repeatedly stimulated the location of the PPTg neuron (inter-stimulus interval: 1.2–1.7 s) and recorded the multiunit activity of presumed DA neurons via the three implanted electrodes in the SNc. For stimulation we used a biphasic square pulse: a 0.2ms 100 μA negative pulse followed by a 0.2ms 20 μA positive pulse.
Data Analysis
To analyze reward-related activity, we primarily focused on the neuron’s response to the saccade target which indicated the presence or absence of the upcoming reward. The post-target response was defined as the discharge rate during 150–350 ms period after the target onset minus the background discharge rate during the 1000 ms period before the fixation point appeared. The post-reward response was defined as the average discharge rate during 150–350 ms after the onset of the tone stimulus (which was synchronized with reward onset if reward was present) minus the background discharge rate. We set the time windows such that they included major parts of the excitatory and inhibitory responses of both PPTg and DA neurons.
Using one-way ANOVA and ROC (receiver operating characteristic) we classified neurons into three groups: 1) reward-positive type, if their firing rate increased in response to an expected reward (p<0.01, ANOVA & ROC>0.5); 2) negative type, if their firing rate decreased in response to an expected reward (p<0.01, ANOVA & ROC<0.5); 3) reward unmodulated type (p>0.01, ANOVA). We further classified these reward modulated neurons into “value state” type (i.e., sustained modulation) and “value change” type (i.e., phasic modulation). A neuron was classified as value state type if it showed sustained modulation (p<0.05, Wilcoxon signed rank test) during the period from 350 ms to 750 ms after the appearance of the fixation point. A neuron was classified as value change type if it showed no sustained modulation.
Histological examination
To verify the locations of neurons in the PPTg that projected to the SNc, we made electrolytic marking lesions (12 μA, 30 sec, electrode being anode) at recording sites of some of the antidromically activated neurons in monkey Z. At the conclusion of the experiment, the animal was deeply anesthetized with pentobarbital sodium and perfused with 4% paraformaldehyde. Frozen sections were cut every 50 μm in the coronal plane. The sections were stained with cresyl violet (Nissl staining) in which the marking lesions were identified. The locations of the three stimulating electrodes were identified as three linear lesions in the SNc which were aligned in parallel (Fig. 2). The locations of the recorded neurons were reconstructed by the 3D coordinates of the recording sites relative to the marking lesions.
Results
Electrophysiological identification of PPTg neurons projecting to DA neuron area
The goal of our study was to understand what kind of information is conveyed from the PPTg to DA neurons. To this end, we first identified neurons in the PPTg that projected to DA neuron area and then examined the information carried by the PPT neurons. To test the PPTg-DA projection, we used the antidromic technique. To examine the neuronal information, we used a variety of behavioral tests. These tests are: (1) one direction rewarded (1DR) saccade task, to examine reward related properties (Fig. 1A). The monkeys made saccades more quickly (shorter latencies) to the target that appeared at the recently rewarded site than the non-rewarded site (Fig. 1A, bottom); (2) eight direction saccade task to examine eye movement related properties (Fig. 1B); (3) passive auditory task in which sounds were presented in different contexts (e.g., finger snaps, clapping, and knocking sounds), to examine sound-related properties (Fig. 1C); (4) passive visual task in which visual stimuli were presented in different contexts (e.g. sudden appearance of computer mouse on the monkey’s screen, flicker of pictures, light spot shone directly to the eye), to examine visual properties; (5) clinical examinations (e.g., touching various parts of the monkey’s body; moving the monkey’s arms, legs and trunk; examining voluntary movements), to examine somatosensory and motor properties.
To antidromically activate PPTg neurons, we chronically implanted three bipolar electrodes in the SNc (Fig. 2). Since DA neurons in the medial and lateral parts of the SNc encode different kinds of information (Matsumoto and Hikosaka, 2009), we spaced the electrodes mediolaterally. Each electrode was placed at the location where single neuronal activity characteristic of DA neurons was recorded (Fig. 2E–G, see below for more explanation). After the experiments, the locations of their electrode tips were visualized histologically as small linear lesions in three coronal sections (arrows in Fig. 2A–C). These sections were separated caudo-rostrally (A-B-C) as expected, because the electrode penetrations were tilted forward (by 15 deg) and the three sites were separated ventro-dorsally (A-B-C). Then, the three coronal sections were aligned based on MR images (as shown by white dashed lines in Fig. 2A–D), and the three lesions were projected onto the section showing the central site (orange lines in Fig. 2D). The projected lesions well corresponded to the recording coordinates of the three sites. The bottom of each lesion (orange line) is likely to indicate the location of the electrode tip; the rest of the lesion probably reflects the upward offsetting of the electrode (3 mm) after each experiment (see Methods). The reconstructed tip positions of the central and lateral electrodes were located in clusters of dark-stained cells in the SNc which characterize DA neurons (Domesick et al., 1983; Poirier et al., 1983). The medial electrode tip was located in the area ventral to the red nucleus and adjacent to the axonal tracts of the oculomotor nerve; this may be at the border between the SNc and the VTA (Poirier et al., 1983).
The neuronal activity recorded with the three electrodes was characteristic of DA neurons (Fig. 2E–G). The medial and central neurons were excited by the saccade target if it indicated an upcoming reward, but were inhibited by the target if it indicated no reward. The lateral neuron showed excitatory responses in both cases, but more strongly to the reward-predicting target. These features were consistent with a previous finding that more ventromedially located DA neurons tend to encode motivational values, whereas more dorsolaterally located DA neurons tend to encode motivational salience (Matsumoto and Hikosaka, 2009). Such DA-like neuronal activity continued to be detected after experimental sessions across many days, especially from the central electrode.
We found that a considerable portion of neurons in the PPTg and adjacent regions were activated antidromically from one of the three SNc sites. Hereafter, we refer to these antidromically activated neurons as “SNc-projecting neurons.” Many of the SNc-projecting neurons (39/50, 78%) showed responses during the behavioral tests described above. Their responses were classified into reward-related, sensorimotor, and arousal/alerting (Table 1). Below, we present example data for neurons that belonged to these groups.
Table 1.
Properties of PPTg neurons and their target locations in the SNc.
| Type | Medial | Central | Lateral | Total |
|---|---|---|---|---|
| Target | ||||
| Reward-related | 6 | 7 | 3 | 16 |
| Sensorimotor | 0 | 6 | 12 | 18 |
| Arousal/alerting | 0 | 2 | 3 | 5 |
| No response | 2 | 4 | 5 | 11 |
| Total | 8 | 19 | 23 | 50 |
Reward information transmitted from PPTg to DA neurons
Figure 3 shows two example neurons that encoded reward values (A–B and C–D). Histological examination suggested that these neurons were located in the PPTg (Fig. 9B). Both neurons were excited by the reward-predicting target and inhibited by the no-reward-predicting target (Fig. 3A and C), similarly to DA neurons (see Fig. 2E–G). However, the time courses of their responses were different: phasic (Fig. 3A) and tonic (Fig. 3C). The phasic and tonic natures were present across task-related events: after the onset of the fixation point, before and after the reward outcome (i.e., reward or no reward).
Figure 3.
Examples of reward-coding PPTg neurons projecting to the SNc. A. Phasic type. This neuron was excited by the reward-predicting target (red) and inhibited by the no-reward-predicting target (blue), and did so in a phasic manner. The same convention as in Figure 2E–G. B. Antidromic activation of the neuron in A by the stimulation at the central SNc (threshold: 60 μA, latency: 2.1 ms). The antidromic spike was blocked when the stimulation was shortly followed by a spontaneous spike (bottom, collision test). C. Tonic type. The neuron also showed reward-related activity similarly, but did so in a tonic manner. D. Antidromic activation of the neuron in C by the stimulation at the lateral SNc (threshold: 100 μA, latency: 6.2 ms).
Figure 9.
Histologically reconstructed locations of SNc-projecting neurons in and around the PPTg (monkey Z). The locations of the neurons are projected to two Nissl-stained coronal sections in the central PPTg area (A and B). Section A is rostral to section B by 0.5 mm. Inset figure indicates the image of the whole section. Indicated for each neuron are 1) information encoded by the neuron (by color) and 2) the location of the antidromic activation site in the SNc (M, C, L). A marking lesion is visible in each of section A and B (orange arrow). SC (superior colliculus), IC (inferior colliculus), CuN (cuneiform nucleus), PAG (periaqueductal gray), PPTg (pedunculopontine tegmental nucleus), scp (superior cerebellar peduncle), mlf (medial longitudinal fasciculus). Scale bars indicate 1 mm.
The phasic neuron (Fig. 3A) was activated by the stimulation of the central SNc electrode with a fixed latency of 2.1 ms (Fig. 3B, top). The antidromic nature of this response was confirmed by a collision test (Fig. 3, bottom): when a spontaneous spike of the neuron was followed by the electrical stimulation within 2.5 ms, the neuron showed no response. This indicated that this PPTg neuron projected to the central portion of the SNc. The tonic neuron (Fig. 3C) was activated antidromically by the stimulation of the lateral SNc electrode with a latency of 2.0 ms, indicating that it projected to the lateral portion of the SNc.
Among the 50 SNc-projecting neurons in the PPTg and adjacent regions, 16 neurons were related to reward and its prediction (Fig. 4). A majority of them (15/16) showed positive value coding (i.e., stronger activity in the rewarded than non-rewarded condition). The most common was the tonic-positive type (56.2%, n=9/16) (Fig. 4A, an example shown in Fig. 3C). These neurons were activated phasically and then tonically after the fixation point came on. They were further activated after the reward-predicting target came on, but were inhibited after the no-reward-predicting target came on. The predictive discrimination between reward and no reward continued until after the reward outcome. The activity of these neurons thus keeps track of the state of the predicted value (hereafter called “value state”).
Figure 4.
Activity of reward-related neurons in the PPTg area projecting to the SNc (n=16). They were classified into four types and the average activity is shown for each type: tonic positive type (A, n=9/16), phasic positive type (A, n=4/16), tonic negative type (C, n=1/16), and post-reward type (D, n=2/16). The same convention as in Figure 3.
The second most common was the phasic-positive type (25%, n=4/16) (Fig. 4B, an example shown in Fig. 3A). These neurons responded to the fixation point and the target, but phasically. Their activity returned to the original level thereafter. These neurons thus monitor the changes in the predicted value (hereafter called “value change”). According to this feature, these neurons typically encode “reward prediction error” (Schultz, 1998). For example, if the actual reward is the same as the predicted reward, these neurons would not change their activity because there is no change in the predicted value. Among the rest of the reward-related neurons, two neurons were classified as the tonic negative type (6.2%, n=1/16) (Fig. 4C). Only one neuron was activated by the reward outcome (12.5%, n=2/16) (Fig. 4D).
Notably, many neurons in the PPTg and adjacent regions showed reward-related activity, but were not activated antidromically from the SNc (Table 2). Compared with such non-antidromic neurons, the antidromic neurons (i.e., SNc-projecting neurons) were more biased to the positive type (Table 2). In addition, ‘value state’ neurons were more abundant among the antidromic neurons, whereas ‘value change’ neurons were more abundant among the non-antidromic neurons (Table 3).
Table 2.
Value preference of reward-related neurons in the PPTg/CuN that were activated (Anti(+)) and not activated (Anti(−)) antidromically by SNc stimulation.
| Anti(+) | Anti(−) | |
|---|---|---|
| Positive | 15 | 23 |
| Negative | 1 | 7 |
Positive: higher activity in the rewarded condition. Negative: higher activity in the non-rewarded condition
Table 3.
Temporal property of reward-related neurons in the PPTg/CuN that were activated (Anti(+)) and not activated (Anti(−)) antidromically by SNc stimulation.
| Anti(+) | Anti(−) | |
|---|---|---|
| Change | 4 | 24 |
| State | 10 | 6 |
Change: respond phasically to monitor a change in value. State: respond tonically to update the current state of reward value.
Sensorimotor information transmitted from PPTg to DA neurons
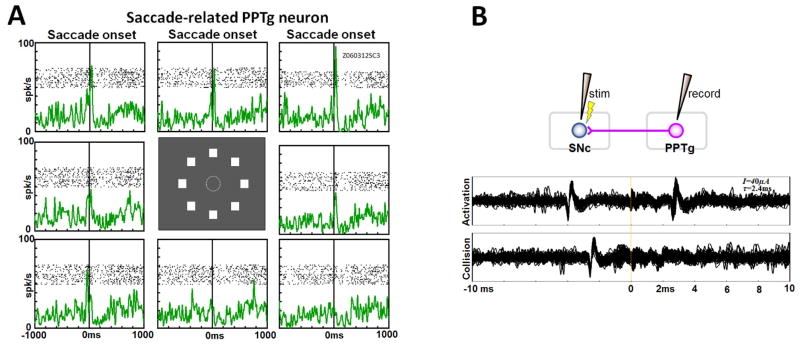
Among the 50 SNc-projecting neurons in the PPTg and adjacent regions, 18 neurons were related to sensorimotor activities (Table 1). These neurons responded to a variety of stimuli including visual, auditory, and tactile stimuli and/or changed their activity in relation to saccadic eye movements, posture changes, and self-initiated movements. Figure 5 shows an example neuron (located in the right PPTg, Fig. 9B) that showed activity related to saccades. The neuron showed a burst of spikes which started just before the initiation of the saccade to a visual stimulus. The saccadic activity was direction-dependent: stronger when the saccade was directed to the visual stimuli in the top-left directions. The saccadic activity was not modulated by the predicted reward outcome (verified with 1DR; data not shown). The neuron was antidromically activated by the stimulation of the lateral SNc electrode with a latency of 2.4 ms (Fig. 5B).
Figure 5.
An example of saccade-related PPTg neuron projecting to the SNc. A. The neuron’s activity is aligned on the onset of saccades, shown separately for the 8 directions. B. Antidromic activation of the neuron by the stimulation at the lateral SNc (threshold: 40 μA, latency: 2.4 ms).
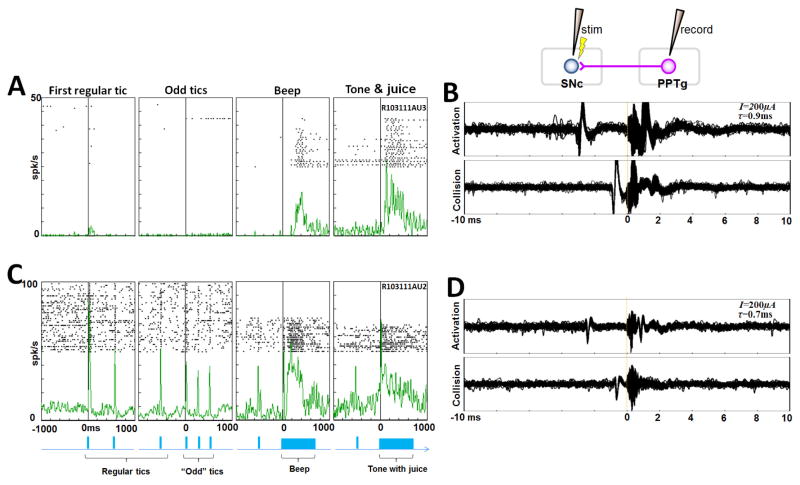
Figure 6 shows two example neurons that responded to auditory stimuli. The first neuron (Fig. 6A) was located in the PPTg (see Fig. 12, below the CuN & PPTg border, classified as visual & auditory, when unexpected). This neuron responded to a beep sound and a tone associated with juice delivery, but the responses decreased as the stimuli were repeated. The neuron was antidromically activated by the stimulation of the lateral SNc electrode with a latency of 0.9 ms (Fig. 6B). The second neuron (Fig. 6C) was found inside of the CuN above the neuron in Fig. 6A (see Fig. 12 for its location). This responded to the tic sound in addition to the beep sound and the tone. The response to the regular tics was strongest after the first tic sound and was attenuated thereafter. The neuron was antidromically activated by the stimulation of the lateral SNc electrode with a latency of 0.7 ms (Fig. 6D).
Figure 6.
Examples of sound-responsive neurons projecting to the SNc. A. This PPTg neuron responded to the beep sound and the tone with juice, but the responses habituated as the stimuli were repeated (shown by spike rasters arranged from bottom to top). B. Antidromic activation of the neuron in A by the stimulation at the lateral SNc (threshold: 200 μA, latency: 0.9 ms). C. This CuN neuron responded to all sounds with some habituation. D. Antidromic activation of the neuron in C by the stimulation at the lateral SNc (threshold: 200 μA, latency: 0.7 ms).
Figure 12.
Locations of antidromically activated PPTg neurons of monkey R. The same figure as in Figure 11 with a more detailed description of the neural types is presented. Scale bar indicates 2 mm.
We also found 5 antidromically activated PPTg neurons (~10%) that responded to various unexpected stimuli (auditory, visual, somatosensory) and self-initiated movements. These neurons often showed reward-related activities as well. These neurons were found inside of the PPTg (Fig. 9A and B, classified as arousal/alerting).
Functional and topographical patterns of PPTg-DA connections
Our data using the antidromic activation method indicate that many neurons in the PPTg and the adjacent regions projected to the SNc where DA neurons were clustered. However, it was unclear whether the PPTg neurons have synaptic contact with DA neurons and if so whether the synaptic effect was excitatory or inhibitory. To answer this question, we switched to the orthodromic stimulation method: Stimulated the location of an antidromically activated PPTg neuron and recorded from presumed DA neurons (multi-unit activity) at the antidromic stimulation site. In one example (Fig. 7) the orthodromic stimulation (single pulse, 100 μA) caused a burst activity in the presumed DA neurons at a latency of 6 ms. Qualitatively the same results were obtained at 8 sites in the PPTg. The result is consistent with the hypothesis that neurons in the PPTg and the adjacent regions have excitatory connections with DA neurons in the SNc. However, it is possible that the electrical stimulation in the PPTg also activated neuronal elements that did not belong to PPTg neurons, which needs to be examined in future experiments.
Figure 7.
Orthodromic activation of presumed DA neurons by PPTg stimulation. Electrical stimulation (single pulse, 100 μA) was applied to the site where the reward-related neuron shown in Figure 4C was recorded. The electrode, which had been used for the antidromic activation of the PPTg neuron, was used for recording multi-unit activity of DA neurons. The DA neurons were excited by the PPTg stimulation.
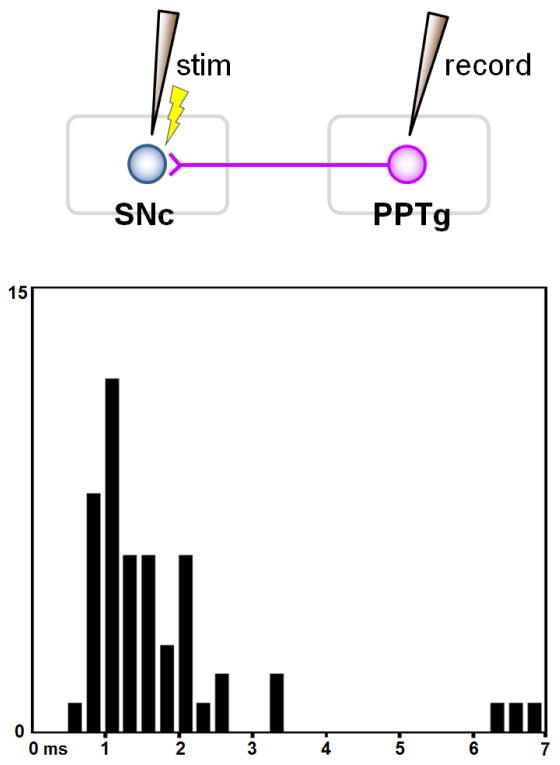
If the antidromic and orthodromic effects originate from the same neurons (i.e., SNc-projecting PPTg neurons), their latencies should be in similar ranges. As shown in Figure 8, the antidromic latency was between 0.7 and 2.5 ms for most neurons, although a small number of neurons showed much longer latencies (up to 7 ms). The range of the antidromic latencies is roughly consistent with the orthodromic latencies.
Figure 8.
Latency distribution of the antidromic activation of neurons in the PPTg area by the stimulation of the SNc.
One of our aims was to reconstruct the location of the antidromically activated neurons. The results are shown in Figures 9, 10, 11 and 12. In monkey Z, the reconstruction was done using histological sections (Fig. 9). We made marking lesions at the recording sites of some of the antidromically activated neurons, which were later identified in Nissl-stained sections (orange arrows in Fig. 9A and 9B). The locations of other antidromically activated neurons were estimated with reference to these marking lesions, and are shown at two rostro-caudal levels (Fig. 9B, 0.5 mm posterior to Fig. 9A). Most of them were inside the PPTg, but some were in the CuN or just above it. There was no clear segregation or clustering of neurons in terms of their information coding (indicated by different colors) or their SNc projection sites (indicated as M, C. L). More detailed features of individual neurons are shown in Figure 10.
Figure 10.
Locations of antidromically activated PPTg/CuN neurons of monkey Z. The same figure as in Figure 9, but more detailed descriptions of the neural types are presented. Scale bar indicates 1 mm.
Figure 11.
MRI-based reconstruction of the locations of SNc-projecting neurons in and around the PPTg (monkey R). The brain structures are reconstructed on the basis of an MR image of this monkey (inset figure) and histological sections in monkey Z (as shown in Figure 9). SC (superior colliculus), CuN (cuneiform nucleus), PAG (periaqueductal gray), PPTg (pedunculopontine tegmental nucleus), scp (superior cerebellar peduncle), mlf (medial longitudinal fasciculus), mcp (middle cerebellar peduncle). Scale bar indicates 2 mm.
For monkey R, the reconstruction was done using MR images (Fig. 11). To delineate individual nuclei, we relied on the histological sections and the corresponding MR images in monkey Z. The section in Figure 11 represents an intermediate part of the PPTg along its rostro-caudal stretch. Shown here are the locations of antidromically activated neurons that were close to this section (within 0.5 mm). Their locations were estimated to be mostly dorsal to the superior cerebellar peduncle (scp). In this monkey a majority of the neurons showed sensorimotor activities and were activated antidromically from the lateral part of the SNc. More detailed features of individual neurons are shown in Figure 12.
The antidromically activated neurons in the PPTg and CuN encoded information related to reward, sensorimotor activities, and arousal/alerting, but there was no clear indication that these functional types were differentially located. However, their terminal sites in the SNc appear to be segregated (Table 1). Reward-related neurons tended to project to the medial and central parts of the SNc. The lateral portion of the SNc received fewer number of reward-related inputs. In contrast, sensorimotor neurons tended to project to the lateral part of the SNc. A similar tendency was observed for neurons related to arousal/alerting. None of the sensorimotor or arousal/alerting-related neurons projected to the medial SNc.
Discussion
Using the electrodes in the SNc for electrical stimulation and the electrode in the PPTg for recording, we were able to identify neurons in the PPTg that projected to the SNc and examined their information coding using various behavioral tasks. By switching the SNc electrodes from stimulation to recording and the PPTg electrode from recording to stimulation, we were able to investigate the synaptic effects of PPTg neurons on SNc-DA neurons and examined the information encoded by the DA neurons. Electrophysiological methods we used were not perfect in demonstrating the detailed characteristics of the PPTg-SNc connection, but applying the methods to behavioral experiments using monkeys has rarely been done. The combination of behavioral and electrophysiological experimental procedures advanced our understanding of the function of the PPTg and its contribution to the DA system.
PPTg contributes to regional differences in DA neuron function
We found that many neurons in the PPTg and some neurons in the CuN projected to the SNc. The projection of PPTg neurons to the SNc confirms previous studies (Scarnati et al., 1987; Lavoie and Parent, 1994). To our knowledge, however, the projection of CuN neurons to the SNc has not been reported. All of the three sites in the SNc were effective in evoking antidromic spikes in PPTg/CuN neurons. However, most of the neurons were activated antidromically from only one SNc site. It was noticeable that there were many auditory PPTg neurons along the dorsolateral part of the PPTg partly interfacing the CuN and projected to the lateral part of the SNc. Other than this, there was no clear indication that neurons were located differently in the PPTg depending on their SNc target regions. These results suggest that most groups of neurons projecting to different SNc regions are intermingled within the PPTg.
Using saccade tasks (reward-biased or unbiased) and sensory stimulation, we found that the SNc-projecting PPTg/CuN neurons encoded reward, sensorimotor and arousal signals. Our data are consistent with previous studies showing PPTg/CuN activity related to reward and its expectation (Okada et al., 2009; Okada and Kobayashi, 2013), sensorimotor events (Matsumura et al., 2000; Kobayashi et al., 2002; Pan and Hyland, 2005; Thompson and Felsen, 2013), and responses to auditory stimuli (Reese et al., 1995). Our data also indicate that neurons encoding various signals were mostly intermingled in the PPTg/CuN. One exception was auditory response which was found predominantly in the lateral part of the PPTg and CuN (Fig. 10).
The above observations appear to indicate that neurons are distributed randomly in the PPTg/CuN in terms of their target regions in the SNc and their information coding. Interestingly, however, there was a correlation between their target regions and information coding: PPTg neurons encoding reward values tended to project more medially in the SNc, whereas PPTg neurons encoding sensorimotor and arousal signals tended to project more laterally (Table 1, Fig. 13).
Figure 13.
Differential PPTg/CuN inputs to DA neurons in the SNc. PPTg/CuN neurons encoding reward value signals tend to project to the medial and central parts of the SNc. PPTg/CuN neurons encoding sensorimotor and arousal/alerting signals tend to project to the lateral and central parts of the SNc.
This topological information transmission may lead to regional differences in the function of DA neurons, which actually has been shown previously (Matsumoto and Hikosaka, 2009). Thus, DA neurons in the medial SNc tend to encode motivational value (i.e., excited by rewarding stimuli and inhibited by punishing stimuli), whereas DA neurons in the lateral SNc tend to encode salience (i.e., excited by both rewarding and punishing stimuli). Our data in the present study suggest that the motivational value encoded by medial DA neurons may be caused, at least partly, by inputs from reward value-coding neurons in the PPTg.
Notably, medial DA neurons receive strong inputs also from the GPb-LHb-RMTg circuit (Matsumoto and Hikosaka, 2009) which transmits motivational value information (Hong and Hikosaka, 2008; Hong et al., 2011). A majority of neurons in this circuit encode values in the negative direction (i.e., inhibited by rewarding stimuli and excited by punishing stimuli), but since RMTg neurons are GABAergic inhibitory (Jhou et al., 2009b; Barrot et al., 2012), the recipient DA neurons encode values in the positive direction. In contrast, a majority of SNc-projecting PPTg neurons encode values positively, suggesting that the PPTg-DA connection is excitatory. Indeed, electrical stimulation of the PPTg (where neurons were activated antidromically from the SNc) induced short-latency excitations in DA neurons, confirming the results from previous physiological (Futami et al., 1995; Scarnati et al., 1987) and anatomical (Charara et al., 1996) studies.
These results suggest that medial DA neurons receive motivational value information through, at least, two circuits whose final effects are opposite - excitatory and inhibitory. For example, burst activity of DA neurons in response to unexpected rewards or reward-predicting stimuli may be caused by a disinhibition via the GPb-LHb-RMTg circuit and an excitation via the PPTg circuit; a pause of DA neuron activity in response to unexpected punishments or punishment-predicting stimuli may be caused by an inhibition via the GPb-LHb-RMTg circuit and a disfacilitation via the PPTg circuit. Such a dual action may make the value information transmission more robust.
There is a subtle but potentially important difference between the two value inputs to DA neurons. The GPb-LHb-RMTg circuit signals motivational value mainly with a phasic excitation or inhibition (representing ‘value change’ or reward prediction error) (Matsumoto and Hikosaka, 2007; Hong and Hikosaka, 2008; Hong et al., 2011), whereas the PPTg circuit signals motivational value mainly with a tonic excitation or inhibition (representing ‘value state). Our current observation, which was consistent with a previous finding (Okada and Kobayashi, 2013), was rather unexpected, because DA neurons represent value change (reward prediction error) (Schultz, 1998; Matsumoto and Hikosaka, 2007). It is unclear whether and how the value state signal is translated into the value change signal in DA neurons.
We so far have focused on the dual value inputs to medial DA neurons (Fig. 12). For lateral DA neurons, on the other hand, the PPTg appears to have a different role. The GPb-LHb-RMTg circuit has a weaker effect on lateral DA neurons which indeed tend not to encode motivational values; they tend to encode salience (Matsumoto and Hikosaka, 2009). In other words, lateral DA neurons are excited by both rewarding and punishing stimuli (instead of being inhibited by punishing stimuli). It remained unknown how lateral DA neurons acquire the salience information. Our data raise the possibility that the PPTg is a source of the salience information. Some PPTg neurons projecting to the lateral SNc were excited by unexpected sensory stimuli. Such unexpected stimuli are salient (Redgrave et al., 1999), alerting and arousing the animal, similarly to rewarding or punishing stimuli. Other PPTg neurons projecting to the lateral SNc encode sensorimotor signals. This could be related to salience, but could also suggest another feature of lateral DA neurons. A recent study showed that lateral DA neurons become active when sensory signals are to be stored as working memories for future motor actions (Matsumoto and Takada, 2013).
Our data, together with the above considerations, suggest that the PPTg supports the DA system in two ways: 1) driving and 2) topographically arranging. First, the PPTg may act as a driver of the DA system, because many of the SNc-projecting PPTg neurons responded, with a burst of firing, to reward-predicting or unexpected sensory stimuli and because their effects on DA neurons were excitatory. This is consistent with the proposal that the PPTg is a key area that contributes to the burst firing of DA neurons (Grace et al., 2007). Dysfunctions of the PPTg-DA connection would then lead to deficient burst activity of DA neurons (Pan and Hyland, 2005) and hence deficient positive reward prediction error signals in the brain. Indeed, animals with PPTg lesions have difficulty in learning and updating associations between stimuli/actions and reward outcomes (Florio et al., 1999; Inglis et al., 2000; Maclaren et al., 2013). Monkeys with PPTg lesions show abnormal posture and hypokinesia, mimicking parkinsonian symptoms (Kojima et al., 1997; Nandi et al., 2002).
Second, the PPTg may contribute to the heterogeneous functions of the DA system. In the literature, we have found little evidence supporting this hypothesis, perhaps because neurons with different signals are intermingled in the PPTg. We speculate, however, that some of the regional differences in the SNc could be related to the differential inputs from the PPTg, as discussed above. Well known among them is predominant cell loss in the lateral SNc in Parkinson’s disease (Fearnley and Lees, 1991), which could be due to intrinsic features (e.g., genetic expression) of lateral SNc-DA neurons (Duke et al., 2007). Depending on the regional extent or selectivity of the cell loss, parkinsonian symptoms vary (e.g., dementia if more cell loss in the medial SNc) (Rinne et al., 1989; Paulus and Jellinger, 1991). Such differential symptoms could be related to the differential inputs from the PPTg.
Future issues
Discussion so far has been focused on the functional contribution of the PPTg to the DA system. But, one important questions remains: How do PPTg neurons acquire the reward, sensorimotor and arousal signals? The PPTg is known to receive inputs from many brain areas (Matsumura, 2000; Mena-Segovia et al., 2004), but dominant inputs originate from basal ganglia nuclei, particularly the substantia nigra pars reticulata (SNr) (Beckstead and Frankfurter, 1982) (Noda and Oka, 1986; Takakusaki et al., 2003) and the globus pallidus internal segment (GPi) (Shink et al., 1997; Rolland et al., 2011), where neurons encode sensorimotor signals strongly modulated by expected rewards (Hikosaka et al., 2006; Joshua et al., 2009). SNr and GPi neurons have GABAergic inhibitory connections to PPTg neurons (Noda and Oka, 1986; Scarnati et al., 1987; Granata and Kitai, 1991), particularly non-cholinergic (Shink et al., 1997) or glutamatergic (Grofova and Zhou, 1998) neurons, although it is unknown whether the SNr/GPi-recipient PPTg neurons project to SNc-DA neurons. In addition, some DA neurons in the SNc or VTA innervate the PPTg (Rolland et al., 2009). These results raise the possibility that the processing of reward, sensorimotor and arousal signals occurs through mutual connections between the PPTg and the basal ganglia nuclei.
Another remaining question is about neurotransmitters used for the PPTg-SNc connection. It is well known that the PPTg contains cholinergic, glutamatergic, and GABAergic neurons (Mena-Segovia et al., 2009; Lavoie and Parent, 1994b). In our experiments DA neurons in the SNc were excited with short latencies by electrical stimulation in the PPTg. This is likely mediated by glutamate, not acetylcholine (Futami et al., 1995). However, it does not mean that individual PPTg neurons antidromically activated from the SNc use glutamate as a neurotransmitter because any slower cholinergic effects would be masked by the quicker glutamatergic effects. In fact, some PPTg neurons showed unusually long antidromic latencies (> 6 ms) and thus could be cholinergic. More data are necessary to test if such cholinergic-like neurons have distinct functional features.
Acknowledgments
We thank M. Yasuda, I. Monosov, and E. Bromberg-Martin for discussions and M. Smith, D. Parker, I. Bunea, M.K. Smith, G.Tansey, A.M. Nichols, T.W. Ruffner, J.W. McClurkin, and A.V. Hays for technical assistance. This research was supported by the Intramural Research Program at the National Institutes of Health, National Eye Institute.
References
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: Ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519(6):1143–64. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(41):14094–101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Frankfurter A. The distribution and some morphological features of substantia nigra neurons that project to the thalamus, superior colliculus and pedunculopontine nucleus in the monkey. Neuroscience. 1982;7(10):2377–88. doi: 10.1016/0306-4522(82)90202-0. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106(12):4894–9. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinschwitz K, Dittgen A, Madai VI, Lommel R, Geisler S, Veh RW. Glutamatergic axons from the lateral habenula mainly terminate on GABAergic neurons of the ventral midbrain. Neuroscience. 2010;168(2):463–76. doi: 10.1016/j.neuroscience.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Henny P, Bolam JP, Magill PJ. Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J Neurosci. 2009;29(9):2915–25. doi: 10.1523/JNEUROSCI.4423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol. 1996;364(2):254–66. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Klop EM, Redgrave P, Overton PG. The parabrachial nucleus is a critical link in the transmission of short latency nociceptive information to midbrain dopaminergic neurons. Neuroscience. 2010;168(1):263–72. doi: 10.1016/j.neuroscience.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domesick VB, Stinus L, Paskevich PA. The cytology of dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area of the rat: a light- and electron-microscopic study. Neuroscience. 1983;8(4):743–765. doi: 10.1016/0306-4522(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Duke DC, Moran LB, Pearce RK, Graeber MB. The medial and lateral substantia nigra in Parkinson’s disease: mRNA profiles associated with higher brain tissue vulnerability. Neurogenetics. 2007;8(2):83–94. doi: 10.1007/s10048-006-0077-6. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain : a journal of neurology. 1991;114 ( Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neuroscience research. 1995;21(4):331–42. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- Florio T, Capozzo A, Puglielli E, Pupillo R, Pizzuti G, Scarnati E. The function of the pedunculopontine nucleus in the preparation and execution of an externally-cued bar pressing task in the rat. Behav Brain Res. 1999;104(1–2):95–104. doi: 10.1016/s0166-4328(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in neurosciences. 2007;30(5):220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Granata AR, Kitai ST. Inhibitory substantia nigra inputs to the pedunculopontine neurons. Experimental brain research. 1991;86(3):459–66. doi: 10.1007/BF00230520. [DOI] [PubMed] [Google Scholar]
- Griffith RW, Humphrey DR. Long-term gliosis around chronically implanted platinum electrodes in the Rhesus macaque motor cortex. Neurosci Lett. 2006;406(1–2):81–6. doi: 10.1016/j.neulet.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Grofova I, Zhou M. Nigral innervation of cholinergic and glutamatergic cells in the rat mesopontine tegmentum: light and electron microscopic anterograde tracing and immunohistochemical studies. J Comp Neurol. 1998;395(3):359–79. [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95(2):567–84. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–71. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. Dopamine system: manager of neural pathways. Front Hum Neurosci. 2013;7:854. doi: 10.3389/fnhum.2013.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Olmstead MC, Robbins TW. Pedunculopontine tegmental nucleus lesions impair stimulus--reward learning in autoshaping and conditioned reinforcement paradigms. Behav Neurosci. 2000;114(2):285–94. doi: 10.1037//0735-7044.114.2.285. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61(5):786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513(6):566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Adler A, Rosin B, Vaadia E, Bergman H. Encoding of probabilistic rewarding and aversive events by pallidal and nigral neurons. J Neurophysiol. 2009;101(2):758–72. doi: 10.1152/jn.90764.2008. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Inoue Y, Yamamoto M, Isa T, Aizawa H. Contribution of pedunculopontine tegmental nucleus neurons to performance of visually guided saccade tasks in monkeys. J Neurophysiol. 2002;88(2):715–31. doi: 10.1152/jn.2002.88.2.715. [DOI] [PubMed] [Google Scholar]
- Kojima J, Yamaji Y, Matsumura M, Nambu A, Inase M, Tokuno H, Takada M, Imai H. Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralateral hemiparkinsonism in the monkey. Neurosci Lett. 1997;226(2):111–4. doi: 10.1016/s0304-3940(97)00254-1. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70(5):855–62. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491(7423):212–7. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol. 1994a;344(2):210–31. doi: 10.1002/cne.903440204. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J Comp Neurol. 1994b;344(2):190–209. doi: 10.1002/cne.903440203. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Uğurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34(3):308–12. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Maclaren DA, Wilson DI, Winn P. Updating of action-outcome associations is prevented by inactivation of the posterior pedunculopontine tegmental nucleus. Neurobiology of learning and memory. 2013;102:28–33. doi: 10.1016/j.nlm.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–5. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–41. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Takada M. Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron. 2013;79(5):1011–24. doi: 10.1016/j.neuron.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Nambu A, Yamaji Y, Watanabe K, Imai H, Inase M, Tokuno H, Takada M. Organization of somatic motor inputs from the frontal lobe to the pedunculopontine tegmental nucleus in the macaque monkey. Neuroscience. 2000;98(1):97–110. doi: 10.1016/s0306-4522(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27(10):585–8. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Micklem BR, Nair-Roberts RG, Ungless MA, Bolam JP. GABAergic neuron distribution in the pedunculopontine nucleus defines functional subterritories. The Journal of comparative neurology. 2009;515(4):397–408. doi: 10.1002/cne.22065. [DOI] [PubMed] [Google Scholar]
- Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. 2002;125(Pt 11):2418–30. doi: 10.1093/brain/awf259. [DOI] [PubMed] [Google Scholar]
- Noda T, Oka H. Distribution and morphology of tegmental neurons receiving nigral inhibitory inputs in the cat: an intracellular HRP study. The Journal of comparative neurology. 1986;244(2):254–66. doi: 10.1002/cne.902440211. [DOI] [PubMed] [Google Scholar]
- Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y. Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J Neurosci. 2009;29(15):4858–70. doi: 10.1523/JNEUROSCI.4415-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Kobayashi Y. Reward prediction-related increases and decreases in tonic neuronal activity of the pedunculopontine tegmental nucleus. Frontiers in integrative neuroscience. 2013;7:36. doi: 10.3389/fnint.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25(19):4725–32. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. Journal of neuropathology and experimental neurology. 1991;50(6):743–55. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Poirier LJ, Giguere M, Marchand R. Comparative morphology of the substantia nigra and ventral tegmental area in the monkey, cat and rat. Brain Res Bull. 1983;11(3):371–397. doi: 10.1016/0361-9230(83)90173-9. [DOI] [PubMed] [Google Scholar]
- Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends in Neurosciences. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. Auditory input to the pedunculopontine nucleus: II. Unit responses. Brain research bulletin. 1995;37(3):265–73. doi: 10.1016/0361-9230(95)00001-u. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Rummukainen J, Paljärvi L, Rinne UK. Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Annals of neurology. 1989;26(1):47–50. doi: 10.1002/ana.410260107. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–45. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends in neurosciences. 2013 doi: 10.1016/j.tins.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Rolland AS, Tande D, Herrero MT, Luquin MR, Vazquez-Claverie M, Karachi C, Hirsch EC, François C. Evidence for a dopaminergic innervation of the pedunculopontine nucleus in monkeys, and its drastic reduction after MPTP intoxication. J Neurochem. 2009;110(4):1321–9. doi: 10.1111/j.1471-4159.2009.06220.x. [DOI] [PubMed] [Google Scholar]
- Rolland AS, Karachi C, Muriel MP, Hirsch EC, François C. Internal pallidum and substantia nigra control different parts of the mesopontine reticular formation in primate. Mov Disord. 2011;26(9):1648–56. doi: 10.1002/mds.23705. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press; 2006. [Google Scholar]
- Scarnati E, Campana E, Pacitti C. Pedunculopontine-evoked excitation of substantia nigra neurons in the rat. Brain Res. 1984;304(2):351–61. doi: 10.1016/0006-8993(84)90339-1. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Proia A, Di Loreto S, Pacitti C. The reciprocal electrophysiological influence between the nucleus tegmenti pedunculopontinus and the substantia nigra in normal and decorticated rats. Brain Res. 1987;423(1–2):116–24. doi: 10.1016/0006-8993(87)90831-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Shink E, Sidibe M, Smith Y. Efferent connections of the internal globus pallidus in the squirrel monkey: II. Topography and synaptic organization of pallidal efferents to the pedunculopontine nucleus. J Comp Neurol. 1997;382(3):348–63. [PubMed] [Google Scholar]
- Takakusaki K, Habaguchi T, Ohtinata-Sugimoto J, Saitoh K, Sakamoto T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience. 2003;119(1):293–308. doi: 10.1016/s0306-4522(03)00095-2. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Felsen G. Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. Journal of neurophysiology. 2013;110(12):2817–29. doi: 10.1152/jn.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490(7419):262–6. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. The European journal of neuroscience. 2009;29(2):340–58. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74(5):858–73. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13(2–3):129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]