Abstract
Background
Elevated levels of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) may contribute to cardiovascular disease and are associated with obstructive sleep apnea (OSA) and obesity. The relationship between OSA and obesity in determining ICAM-1 and VCAM-1 levels, and the effect of treatment, is unclear.
Objective
Our aim was to study whether positive airway pressure (PAP) usage resulted in changes in ICAM-1 and VCAM-1 after 2 years within 309 OSA patients from the Icelandic Sleep Apnea Cohort, and determine how obesity affected such changes.
Subjects/Methods
The mean body mass index (BMI) was 32.4±5.1 kg/m2; subjects had moderate-to-severe OSA (apnea-hypopnea index = 45.0±20.2) and 79% were male. There were 177 full PAP users (≥4 hours/night and ≥20 of last 28 nights), 44 partial (<4 hours/night or <20 nights), and 88 non-users.
Results
ICAM-1 (p<0.001) and VCAM-1 (p=0.012) change was significantly different among the PAP groups. The largest ICAM-1 differences were among the most obese subjects (p<0.001). At follow-up, non-users had increased ICAM-1 compared to decreased levels in full users. All groups had increased VCAM-1, but non-users had a significantly larger increase than full users.
Conclusion
Within moderate-to-severe OSA patients, PAP usage prevents increases in adhesion molecules observed in non-users after two years. For ICAM-1, the largest effect is in the most obese subjects. As OSA and obesity commonly coexist, the usage of PAP to limit increases in adhesion molecules may decrease the rate of progression of OSA-related cardiovascular disease.
Keywords: Sleep Apnea Syndromes, Inflammation, Cell Adhesion Molecules, Continuous Positive Airway Pressure, Cardiovascular Disease
Introduction
Obstructive sleep apnea (OSA) is a leading public health problem; recent estimates suggest 34% of men and 17% of women have OSA, and 13% and 6%, respectively, have moderate-to-severe disease(1). OSA is associated with cardiovascular disease risk(2). However, the underlying biological mechanisms linking OSA and cardiovascular disease are not well understood. The incorporation of biological markers of susceptibility, such as adhesion molecules, into epidemiological studies will provide insight into these mechanisms(3).
Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are expressed on cell surfaces and found in their soluble forms in the plasma(4). Leukocyte adhesion to vascular endothelial cells and migration into the vessel wall is critical in the development of atherosclerosis, and elevated levels of adhesion molecules have been demonstrated in subjects with OSA(5–11). Positive airway pressure (PAP) therapy may reduce ICAM-1 levels in OSA patients(5, 8, 10). One study has examined VCAM-1, finding no PAP treatment effect(5). However, these studies had small samples, relatively short duration, and did not directly address the role of obesity on these relationships.
Obesity is an important risk factor for OSA(12), and their shared pathways of oxidative stress and inflammation make discerning independent roles in cardiovascular disease difficult(3). Obesity stimulates an inflammatory state, as adipose tissue has resident macrophages and is a rich source of pro-inflammatory cytokines(13). Previous studies have examined the relationship between obesity and cellular adhesion molecules (CAMs)(14–25); most observe higher ICAM-1 levels with more obesity(15, 19–23, 25) and reduced levels after weight loss(14–18, 25, 26). Associations between obesity and VCAM-1 are inconsistent(14, 15, 17–20, 23–25). Circulating ICAM-1, but not VCAM-1, is a consistent predictor of cardiovascular risk in healthy populations, while VCAM-1 predicts future cardiovascular risk within patients with pre-existing disease(27–38).
The goal of this observational study was to investigate whether PAP treatment in OSA patients resulted in changes in ICAM-1 and VCAM-1 two years after treatment initiation, and how OSA severity and obesity affect such changes. We hypothesized that the degree of change in adhesion molecules with PAP would depend on obesity, with the largest change occurring in the most obese patients.
Methods
Study subjects
OSA patients were those from the Icelandic Sleep Apnea Cohort (ISAC) with completed follow-up as of June 2011 (see Figure E1 and online supplement [pages 1–4]). We selected subjects with a sleep study before starting PAP with a recorded apnea-hypopnea index (AHI) and oxygen desaturation index (ODI), baseline abdominal MRI, fasting morning blood samples at baseline and follow-up, and PAP compliance data from smartcard download or no PAP usage. Subjects completed assessments at baseline and two years after PAP initiation (mean±SD = 2.0±0.2 years). This study was approved by the National Bioethics Committee, the Data Protection Authority of Iceland and the University of Pennsylvania Institutional Review Board. Written consent was obtained from all subjects.
PAP Usage Definitions
Objective PAP compliance data (mean hours and total nights used over the last 28 days) were available from smartcard download. Full users were defined as mean use ≥4 hours/night and ≥20 nights of use and partial users as mean use <4 hours/night and/or <20 nights. Full and partial users used PAP for 7.0±1.2 and 4.3±2.1 hours/night, respectively. Non-users reported no PAP use, returned the PAP machine within one year of initiation and did not have upper airway surgery. Sixteen non-users had a prescribed mandibular advancement device, but inclusion of these few patients did not significantly impact results.
Blood Sampling
Blood was drawn the morning after sleep from the antecubital vein of fasting untreated participants at baseline and follow-up. Enzyme-linked immunosorbent assay was used to determine the serum levels of ICAM-1 and VCAM-1 (R&D Systems, Minneapolis, MN)‥ Additional details about measurements of adhesion molecules are presented in the online supplement (page 3).
Statistical Analysis
ICAM-1 and VCAM-1 changes were calculated within subject (follow-up minus baseline). Associations between PAP and CAM change were assessed using an analysis of covariance (ANCOVA). If an overall difference (p<0.05) was found, pairwise comparisons between PAP groups were performed with a Tukey-Kramer adjustment. We also assessed whether there was evidence for a linear trend (or ‘dose effect’) across the PAP groups. To determine whether obesity modified the relationship between PAP and CAM change, patients were stratified by baseline BMI (<30, 30–35, ≥35 kg/m2) and we tested for interaction between PAP and BMI group and assessed associations within BMI groups. A complementary analysis was performed using baseline visceral abdominal fat (VAT) volume tertiles. Confounders were assessed in a forward stepwise fashion (further details in online supplement [page 7]). Final models included baseline CAM level, BMI, BMI change, and hypertension status and statin use (for VCAM-1 only) at baseline and follow-up. Models in VAT tertiles were adjusted for baseline VAT, rather than BMI. Sensitivity analyses examining the effect of additional adjustments for blood pressure and lipid values in our models were also performed. Associations between continuous variables and CAM change were assessed using Pearson correlations (r) or linear regression.
Propensity Score Sub-classification
To further control for bias due to covariate imbalance, we used sub-classification by propensity score (PS) quintiles to identify a subset of PAP full and non-users balanced with respect to key covariates at baseline, i.e., age, BMI, gender, current smoking status, participation in exercise, use of statin medication, prevalence of hypertension, cardiovascular disease, diabetes, and obstructive lung disease, Epworth Sleepiness Scale (ESS), OSA severity (AHI, ODI, SaO2 nadir and percentage time SaO2<90) and baseline ICAM-1 and VCAM-1 levels. Sub-classification was performed using an established heuristic described by Maislin & Rubin(39). Balance for included variables is shown to be similar to that expected from randomization, therefore minimizing selection bias and strengthening causal inferences. The importance of utilizing propensity scores and related methodologies within observational studies has been highlighted by the National Heart Lung and Blood Institute(40). See supplement (pages 7–8) for additional details about PS methodology.
Results
Participant Characteristics
Tables 1 and 2 present sample characteristics stratified by BMI and PAP groupings.
Table 1.
Baseline Demographic and Clinical Characteristics of BMI Groupings.
| Characteristic* | Overall (N=309) |
BMI <30 (N=111) |
BMI 30–35 (N=110) |
BMI ≥35 (N=88) |
p† |
|---|---|---|---|---|---|
| Male | 79.3% | 82.9% | 81.8% | 71.6% | 0.1066 |
| Age | 55.4±9.6 | 54.9±8.9 | 57.3±10.4 | 53.7±9.1 | 0.0298 |
| BMI (kg/m2) | 32.4±5.1 | 27.3±2.1 | 32.5±1.5 | 38.7±3.0 | <0.0001 |
| Epworth Sleepiness Score | 12.0±4.9 | 12.1±4.7 | 11.5±4.9 | 12.5±5.1 | 0.3250 |
| Statin Use | 22.0% | 20.7% | 20.9% | 25.0% | 0.7249 |
| Hypertension | 44.0% | 30.6% | 47.3% | 56.8% | 0.0007 |
| Diabetes | 5.2% | 3.6% | 3.6% | 9.2% | 0.1423 |
| Cardiovascular Disease | 14.6% | 11.7% | 15.5% | 17.2% | 0.5238 |
| Obstructive Lung Disease | 17.2% | 14.4% | 20.2% | 17.0% | 0.5254 |
| Current Smoker | 17.9% | 26.1% | 15.5% | 10.3% | 0.0114 |
| AHI (events/hr) | 45.0±20.2 | 39.1±17.3 | 47.0±19.5 | 49.8±22.9 | 0.0004 |
| ODI (events/hr) | 35.8±20.2 | 28.6±14.6 | 37.2±18.9 | 43.3±24.5 | <0.0001 |
| SaO2 Nadir | 76.7±7.8 | 79.2±5.6 | 77.2±7.0 | 73.0±9.5 | <0.0001 |
| % Time SaO2 <90% | 12.6±17.1 | 7.0±10.2 | 13.1±16.3 | 19.0±22.1 | <0.0001 |
| ICAM-1 (ng/ml) | 302.3±91.1 | 289.6±87.5 | 305.9±91.8 | 313.8±94.0 | 0.1570 |
| VCAM-1 (ng/ml) | 889.1±307.4 | 875.4±305.9 | 901.0±290.7 | 891.3±331.6 | 0.8244 |
Demographic and clinical characteristics are shown at baseline in the overall sample (n=309) and stratified by BMI. Continuous characteristics are presented as means ± standard deviations (SD) and categorical covariates as percentages;
p-value from ANOVA (continuous variables) or chi-square or exact test (categorical variables) testing whether there were differences between BMI groups; Significant differences (p<0.05) are shown in bold.
Abbreviations: BMI: body mass index; AHI: apnea hypopnea index; ODI: oxygen desaturation index; SaO2 oxygen saturation; ICAM-1 Intracellular Adhesion Molecule 1; VCAM-1 Vascular Adhesion Molecule 1
Table 2.
Baseline and Follow-Up Characteristics of PAP Usage Groupings.
| Characteristic* | Baseline | 2 Year Follow Up | ||||||
|---|---|---|---|---|---|---|---|---|
| Full User (N=177) |
Partial User (N=44) |
Non User (N=88) |
p† | Full User (N=177) |
Partial User (N=44) |
Non User (N=88) |
p† | |
| Age (years) | 55.2±10.1 | 56.5±9.0 | 55.4±8.9 | 0.7382 | 57.2±10.1 | 58.5±9.1 | 57.4±8.9 | 0.7323 |
| Male | 80.2% | 81.8% | 76.1% | 0.6708 | -- | -- | -- | -- |
| BMI (kg/m2) | 33.2±4.9 | 32.8±5.1 | 30.5±4.8 | 0.0001 | 34.0±5.0 | 32.8±5.3 | 30.8±4.9 | <0.0001 |
| Epworth Sleepiness Score |
12.7±4.9 | 12.4±4.8 | 10.5±4.7 | 0.0022 | 8.1±4.5 | 9.4±5.2 | 9.0±4.6 | 0.1615 |
| Statin Use | 22.0% | 31.8% | 17.0% | 0.1549 | 27.7% | 34.1% | 21.6% | 0.2900 |
| Hypertension | 48.6% | 59.1% | 27.3% | 0.0004 | 47.2% | 54.5% | 31.8% | 0.0183 |
| Diabetes | 6.3% | 4.5% | 3.4% | 0.6761 | 6.9% | 4.5% | 2.3% | 0.2917 |
| Cardiovascular disease |
13.1% | 15.9% | 17.0% | 0.6659 | 13.6% | 15.9% | 17.0% | 0.7501 |
| Obstructive Lung Disease |
15.8% | 6.80% | 25.3% | 0.0228 | 16.5% | 9.1% | 26.7% | 0.0304 |
| Current Smoker | 17.6% | 13.6% | 20.5% | 0.6231 | 14.7% | 18.2% | 21.6% | 0.3663 |
| Diastolic BP | 83.4±10.4 | 82.3±11.3 | 82.3±9.2 | 0.6377 | 80.2±9.5 | 80.1±10.2 | 80.6±9.1 | 0.9375 |
| Systolic BP | 133.2±13.4 | 131.6±13.6 | 130.2±12.5 | 0.2008 | 134.8±13.3 | 130.6±12.5 | 130.8±11.3 | 0.0197 |
| Total Cholesterol | 195.6±45.3 | 193.6±34.4 | 195.8±45.0 | 0.9583 | 203.1±39.1 | 195.8±37.6 | 209.4±42.8 | 0.1715 |
| LDL Cholesterol | 139.8±41.1 | 137.7±32.2 | 143.0±41.9 | 0.7433 | 139.7±36.8 | 131.8±35.8 | 145.9±40.5 | 0.1218 |
| HDL Cholesterol | 40.8±10.9 | 40.6±7.9 | 38.8±10.5 | 0.3414 | 48.5±10.7 | 49.9±12.1 | 50.6±13.6 | 0.3633 |
| Triglycerides | 172.0±86.1 | 175.0±74.3 | 160.3±59.7 | 0.4425 | 170.5±102.5 | 161.6±85.3 | 147.3±77.9 | 0.1682 |
| AHI (events/hr) | 50.8±20.3 | 44.4±19.4 | 33.6±15.3 | <0.0001 | -- | -- | -- | -- |
| ODI (events/hr) | 41.2±20.7 | 35.5±19.9 | 25.2±14.5 | <0.0001 | -- | -- | -- | -- |
| SaO2 Nadir | 75.9±7.8 | 75.1±8.4 | 79.1±7.0 | 0.0021 | -- | -- | -- | -- |
| % Time SaO2 <90% |
15.4±18.6 | 13.8±17.6 | 6.2±11.1 | 0.0001 | -- | -- | -- | -- |
| ICAM-1 (ng/ml) | 299.3±79.8 | 295.4±92.2 | 311.8±110.4 | 0.4982 | 286.9±71.0 | 292.7±84.9 | 315.9±103.0 | 0.0286 |
| VCAM-1 (ng/ml) | 861.1±320.1 | 855.0±268.6 | 962.3±289.9 | 0.0298 | 955. ±371.6 | 987.3±367.6 | 1,069.6±329.2 | 0.0535 |
Demographic and clinical characteristics are shown at baseline and at two year follow up within strata defined by PAP usage (full, partial, or non) at follow up. Continuous characteristics are presented as means ± standard deviations (SD) and categorical covariates as percentages;
p-value from ANOVA (continuous variables) or chi-square or exact test (categorical variables) testing whether there were differences among PAP usage groups; Significant differences (p<0.05) are shown in bold.
Abbreviations: BMI: body mass index; AHI: apnea hypopnea index; ODI: oxygen desaturation index; SaO2 oxygen saturation; ICAM-1 Intracellular Adhesion Molecule 1; VCAM-1 Vascular Adhesion Molecule 1
Among the BMI groups (Table 1), we observed significant differences in baseline OSA severity, with the most obese subjects having the most severe OSA. There were also differences in age. More obese patients had a higher prevalence of hypertension and lower prevalence of current smokers.
At baseline, PAP users had more severe OSA and sleepiness, were more likely to be hypertensive, and had higher BMIs than non-users (Table 2). Differences among the PAP groups at baseline were all significant at follow-up, except for differences in sleepiness, which significantly decreased in all groups (p<0.005). As expected, larger ESS decreases were observed in full versus non-users (p<0.0001). There was a difference in systolic BP at follow-up among the PAP groups (p=0.020). This result appears driven by a small increase in full users and decrease in partial users; however, there was no significant difference in 2-year systolic BP changes among the groups.
Correlations between Obesity and CAM Levels
We assessed correlations between obesity measures and baseline ICAM-1 and VCAM-1 levels (Table E1). Significant correlations were found between ICAM-1 and BMI (rho=0.18, p=0.002), subcutaneous abdominal fat (rho=0.18, p=0.002), total abdominal fat (rho=0.16, p=0.004) and hip circumference (rho=0.12, p=0.030). There was no correlation between visceral abdominal fat and ICAM-1. We observed no significant correlations between any obesity measure and baseline VCAM-1.
Mean CAM Change within PAP Usage Groups
Mean changes in ICAM-1 and VCAM-1 are shown in Figure E2 and Table E2. Overall, we observed a significant two-year decrease in ICAM-1 (p=0.011) for full PAP users and increases in VCAM-1 for all PAP groups (each p<0.015). In BMI strata, a decrease in ICAM-1 was only seen among the most obese full users (p=0.039). Significant increases in VCAM-1 were found among the most obese full users (p=0.008), moderately obese partial users (p=0.037), and both the most (p=0.026) and least (p=0.022) obese non-users. There was a significant difference in ICAM-1 change among PAP groups within the most obese subjects only (p=0.004); the decrease in full users was different from the increase in non-users (p<0.05). No associations between CAM changes and PAP usage were observed in other BMI groups or the overall population.
Association between PAP Usage Groups and CAM Changes
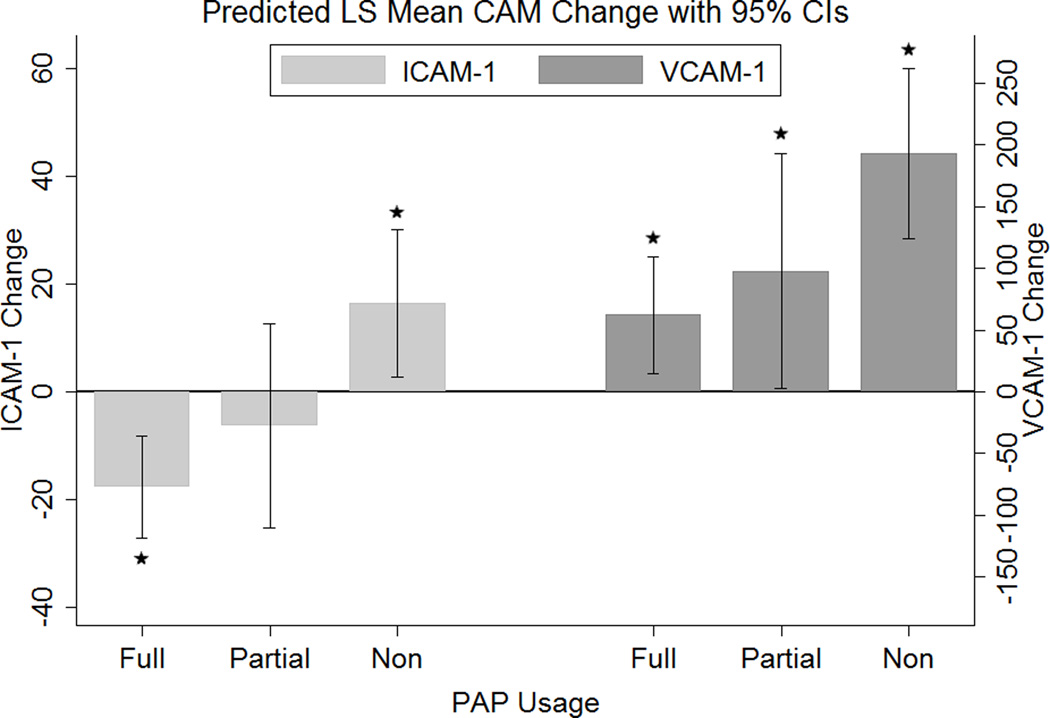
We examined whether changes in CAM levels differed based on PAP usage after adjusting for potential confounders (Figure 1, Table 3). Significant differences among PAP groups were found for changes in both ICAM-1 (p=0.001) and VCAM-1 (p=0.012). We also observed evidence for a PAP dose response across full, partial and non-users in ICAM-1 (p<0.001) and VCAM-1 (p=0.004).
Figure 1. Predicted Least Square Mean change from baseline in entire population.
The predicted least squares mean change in ICAM-1 and VCAM-1 levels for each PAP usage group are presented in the overall population, along with the associated 95% confidence intervals. Results are adjusted for baseline ICAM-1 or VCAM-1 levels, BMI, change in BMI, hypertension at baseline and follow-up, and statin use at baseline and follow-up (for VCAM-1 change only). Estimates with 95% confidence intervals not crossing 0 represent significant changes: *p<0.05. Abbreviations: LS: least squares; ICAM-1: Intracellular Adhesion Molecule 1; VCAM-1: Vascular Adhesion Molecule 1; BMI: body mass index; PAP: positive airway pressure
Table 3.
Differences in ICAM-1 and VCAM-1 change among PAP Usage Groups, overall and within each BMI Group.
| ICAM-1 Change |
|||||
|---|---|---|---|---|---|
| Full Users | Partial Users | Non-Users | p* | pTREND† | |
| Overall | −7.7±4.8§ | −6.2±9.6 | 16.5±7.0§ | 0.0005 | 0.0001 |
| BMI<30 | −6.5±8.1 | −33.6±15.4§ | 9.9±8.7 | 0.0558 | 0.2425 |
| BMI 30–35 | −20.4±6.6§ | 3.8±13.7 | −15.0±10.5 | 0.2905 | 0.4973 |
| BMI≥35 | −20.7±9.9§ | 15.7±20.5 | 73.4±19.3§ | 0.0002 | <0.0001 |
|
VCAM-1 Change |
|||||
| Full Users | Partial Users | Non-Users | p* | pTREND† | |
| Overall | 62.3±24.1§ | 97.8±48.2§ | 192.9±35.1§ | 0.0122 | 0.0035 |
| BMI<30 | 15.6±39.9 | 35.7±74.7 | 125.8±42.8§ | 0.1880 | 0.0751 |
| BMI 30–35 | 59.8±42.4 | 135.0±89.3 | 199.7±70.3§ | 0.2347 | 0.0880 |
| BMI≥35 | 122.4±45.7§ | 120.9±96.1 | 319.9±92.7§ | 0.1638 | 0.0930 |
The results of the association between PAP usage and change in adhesion molecules are presented.
p-value from ANCOVA testing the global null hypothesis of no differences between PAP usage groups and adhesion molecule change, adjusted for baseline ICAM-1 or VCAM-1 levels, BMI, change in BMI, hypertension at baseline and follow-up, and statin use at baseline and follow-up (for VCAM-1 change only). Results are shown as group specific predicted least square mean change ± standard errors;
p-value testing whether there is a linear trend, i.e. an association between CAM change and PAP usage as an ordinal variable (1=full user, 2=partial user, 3=non-user);
subgroup specific predicted mean change significantly different from zero (p<0.05); Significant differences or linear trends (p<0.05) shown in bold.
Abbreviations: ICAM-1 Intracellular Adhesion Molecule 1; VCAM-1 Vascular Adhesion Molecule 1; BMI: body mass index; PAP: positive airway pressure
When looking between PAP groups, a difference in ICAM-1 change between full and non-users (p<0.001) was found, with non-users showing a significant increase (p=0.019) compared to a decrease for full users (p<0.001) (Figure 1, Table 3). VCAM-1 significantly increased (p<0.05) in all 3 PAP usage groups. However, changes between full and non-users were significantly different (p=0.009), with non-users showing a three-fold greater two-year increase.
Role of Obesity in the Association between PAP Usage and CAM changes
The association between PAP usage and ICAM-1 change, but not VCAM-1 change, was dependent upon obesity level.
When looking at obesity defined by BMI, we observed significant interaction between PAP usage and BMI group (p<0.001) for ICAM-1, indicating the effect of PAP on ICAM-1 changes differs among BMI groups. Differences in ICAM-1 change are strongest in those with BMI≥35 (Figure E3, Table 3). Within this group, the change in ICAM-1 was significantly different between full and non-users (p<0.001), with non-users showing a significant increase (p<0.001) compared to a decrease in full users (p=0.032) (Figure E3, Table 3). We again observed a linear trend across the 3 PAP groups (p<0.0001); full users decreased, partial users had no change, and non-users increased. No differences were found among PAP groups within other BMI groups, indicating that differences in ICAM-1 change among PAP usage groups in the overall sample are driven by the most obese subjects. For VCAM-1 change, we observed no interaction or significant associations within BMI group. This lack of association may be due to reduced power resulting from the smaller sample size within BMI strata (see online supplement, page 9).
In a complementary analysis examining the PAP effect within baseline VAT tertiles, we observed similar associations (Table 4). There was significant interaction between PAP group and VAT tertile (p=0.030) on ICAM-1 change. As with BMI, the largest differences among PAP groups were seen for the top tertile (p=0.009) and there was a trend across the 3 PAP groups (p=0.004). In this group with the most visceral fat, full users had decreased ICAM-1 levels (p=0.049) and non-users increased (p=0.014); this difference between full and non-users was significant (p=0.006). In contrast to results in BMI strata, we observed a significant difference among (p=0.023) and trend across (p=0.036) the 3 PAP groups in the lowest tertile of visceral fat. However, after correction for multiple comparisons, none of the within group changes were statistically significant and there were no significant between group differences. For VCAM-1 change, we did not observe a significant interaction between PAP group and VAT tertile, again suggesting the effect of PAP was not moderated by obesity; the only significant result within VAT tertiles was a linear trend across the 3 PAP groups (p=0.016) in patients with the most visceral fat.
Table 4.
Differences in ICAM-1 and VCAM-1 change among PAP Usage Groups, overall and within Visceral Abdominal Fat Tertiles
| ICAM-1 Change |
|||||
|---|---|---|---|---|---|
| Full Users | Partial Users | Non-Users | p* | pTREND† | |
| Overall | −16.4 ± 4.8§ | −6.5 ± 9.7 | 14.1 ± 7.0§ | 0.0023 | 0.0006 |
| Visceral Ab. Fat T1 | −13.2 ± 8.0 | −33.0 ± 18.0 | 16.9 ± 9.5 | 0.0226 | 0.0361 |
| Visceral Ab. Fat T2 | −10.7 ± 8.4 | 10.3 ± 16.0 | −19.5 ± 12.0 | 0.3225 | 0.6836 |
| Visceral Ab. Fat T3 | −16.3 ± 8.2§ | −7.5 ± 16.2 | 39.1 ± 15.6§ | 0.0089 | 0.0036 |
|
VCAM-1 Change |
|||||
| Full Users | Partial Users | Non-Users | p* | pTREND† | |
| Overall | 66.0 ± 24.0§ | 91.9 ± 48.6 | 188.5 ± 35.2§ | 0.0198 | 0.0065 |
| Visceral Ab. Fat T1 | 29.2 ± 39.3 | −57.9 ± 87.2 | 150.0 ± 46.3§ | 0.0655 | 0.0837 |
| Visceral Ab. Fat T2 | 86.9 ± 48.8 | 109.7 ± 92.6 | 170.4 ± 71.3§ | 0.6618 | 0.3725 |
| Visceral Ab. Fat T3 | 77.5 ± 40.5 | 185.1 ± 80.4§ | 284.0 ± 78.6§ | 0.0568 | 0.0163 |
The results of the association between PAP usage and change in adhesion molecules are presented.
p-value from ANCOVA testing the global null hypothesis of no differences between PAP usage groups and adhesion molecule change, adjusted for baseline ICAM-1 or VCAM-1 levels, Visceral Ab. Fat Volume, change in BMI, hypertension at baseline and follow-up, and statin use at baseline and follow-up (for VCAM-1 change only). Results are shown as group specific predicted least square mean change ± standard errors;
p-value testing whether there is a linear trend, i.e. an association between CAM change and PAP usage as an ordinal variable (1=full user, 2=partial user, 3=non-user);
Subgroup specific predicted mean change significantly different from zero (p<0.05); Significant differences or linear trends (p<0.05) shown in bold.
Abbreviations: ICAM-1 Intracellular Adhesion Molecule 1; VCAM-1 Vascular Adhesion Molecule 1; BMI: body mass index; Ab.: Abdominal; PAP: positive airway pressure
Relationship between Hours and Nights of PAP Use and CAM Changes
Within full and partial users (N=221) with a BMI≥35 kg/m2, each additional hour of mean PAP use associated with an 8.9 ng/mL decrease in ICAM-1 (p=0.018) and each additional night of use was associated with a 2.5 ng/mL decrease in ICAM-1 (p=0.036). We did not observe significant associations with ICAM-1 in VAT tertiles, and no significant associations with hours or nights of PAP use and VCAM-1 change were found.
Effect of Baseline OSA Severity on Association between PAP Usage and CAM Changes
We assessed the impact of OSA severity adjustment in our models. We observed no correlations between OSA severity measures and baseline ICAM-1 or VCAM-1 levels. Adjustment for severity of OSA did not change the association between PAP use and ICAM-1 change described above. For VCAM-1 change, differences among PAP groups in the overall population became non-significant after adjustments for AHI (p=0.181) and ODI (p=0.060), but the overall trend remained similar.
Association between OSA Severity and CAM Changes among PAP Non-users
To address whether OSA severity influenced CAM increases if left untreated, we examined associations between baseline OSA severity and ICAM-1 or VCAM-1 change among PAP non-users (Table E3). ODI (p=0.023), SaO2 nadir (p=0.021) and percent time SaO2<90 (p=0.033) were associated with ICAM-1 change within non-users with BMI≥35. More severe baseline OSA was associated with larger increases in ICAM-1 in these subjects. We observed no associations between baseline OSA severity and VCAM-1 change in non-users.
Effect of Blood Pressure and Lipid Adjustment
In additional sensitivity analyses, we assessed the impact of controlling for continuous measures of blood pressure and lipid values in place of categorical outcomes. Controlling for blood pressure instead of hypertension status had no effect on the results (data not shown). Additional inclusion of lipids did not impact the results for ICAM-1; differences among the 3 groups in VCAM-1 change became borderline non-significant (p=0.059), although there remained a significant linear trend across the three PAP groups (data not shown).
Propensity Score Matched Sample Sub-Analysis
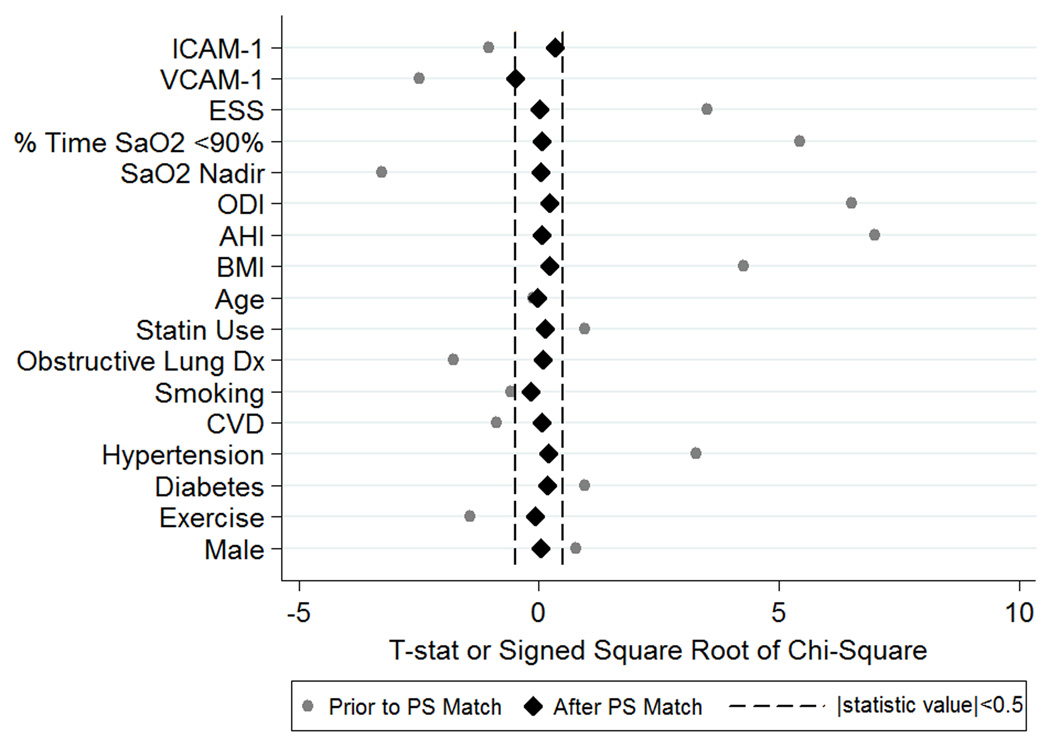
Of 177 full and 88 non-users in our observational sample, we identified 90 (50.8%) full and 62 (70.5%) non-users balanced within PS subclass for the included covariates (see Methods). Table E4 compares participants included and excluded from the sample. The resulting covariate balance is illustrated in Figure 2 (a “Love Plot”(41)). While there were significant differences (p<0.05) in 8 of the included covariates in the observational sample, after adjusting for PS subclass, there were no differences between full and non-users in the reduced PS designed sample (all p>0.63).
Figure 2. ‘Love Plot’ illustrating the covariate balance created in the propensity score matched sample.
The test statistics (t-statistics for continuous variables and signed square root of the chi-square statistics for categorical variables) comparing covariates between full and non PAP users are shown both in the original sample and after PS matching and quintile adjustment. While there were significant differences in covariates between full and non-users in the original sample, after PS matching all statistics are close to zero, indicating balance between full and non-users for all relevant covariates. Abbreviations: ICAM-1: Intracellular Adhesion Molecule 1; VCAM-1: Vascular Adhesion Molecule 1; ESS: Epworth Sleepiness Scale; ODI: Oxygen Desaturation Index; AHI: Apnea-Hypopnea Index; BMI: body mass index; CVD: cardiovascular disease.
The estimated differences between PAP groups were similar in magnitude in the propensity score and overall observational samples, indicating that results in primary analyses were not driven by imbalance in included covariates. Within the PS designed sample, full users had a decrease in ICAM-1 of −14.1 ng/mL (SE=7.3, p=0.054) compared to a significant increase in ICAM-1 of 20.1 ng/mL (SE=8.9, p=0.026) in non-users. This difference in change scores (34.2 ng/mL, p=0.005) was identical to that seen in our observational sample. For VCAM-1, full users in the PS sample had an increase of 71.9 ng/mL (SE=36.4, p=0.050), compared to an increase of 174.8 ng/mL (SE=44.6, p<0.001) in non-users. This difference in VCAM-1 increases (102.9 ng/mL) was similar to that seen in the observational sample (130.6 ng/mL), but it was no longer statistically significant in the smaller PS sample (p=0.087).
Discussion
In patients with moderate-to-severe OSA, a beneficial effect of PAP on adhesion molecules is observed in non-users over two years. For ICAM-1, full usage decreased levels, with the largest effect occurring in the most obese subjects. For VCAM-1, increased levels over 2 years were observed for all PAP groups, but non-users had much larger increases than full users. Although the VCAM-1 association appeared independent of obesity, larger increases in non-users were again seen in the most obese. Results in the observational sample were supported by a complementary analysis using propensity score methodologies. The finding that ICAM-1 is decreased after 2 years among the most obese full users is noteworthy, suggesting PAP may help to reduce future OSA-related cardiovascular risk, particularly in the most obese.
Role of Obesity on CAM Levels
We observed correlations between baseline ICAM-1 and BMI, subcutaneous and total abdominal fat, but not visceral abdominal fat. Correlations with abdominal fat become non-significant after adjustment for BMI, similar to a previous publication from the Framingham cohort(21). Overall, our study suggests that total fat measures are more significantly associated with ICAM-1 levels than visceral fat alone in a moderate-to-severe OSA population. Despite correlation between baseline ICAM-1 and VCAM-1 (r=0.43, p<0.0001), we found no significant correlations between any obesity measure and VCAM-1.
The correlation between ICAM-1 and obesity, but not VCAM-1 and obesity, is not unexpected. Previous studies have explored relationships between CAMs and both obesity(15, 16, 19, 21–25) and weight loss(14–19, 24–26). The majority of these studies (15, 19–23, 25, 26) found significantly higher ICAM-1 levels in more obese patients. Some studies have observed higher VCAM-1 associated with more obesity(15, 25), but others found no associations(19, 20, 23, 24), even where associations with ICAM-1 were observed in the same study. Similarly, most studies(14–18, 25, 26) observe significant decreases in ICAM-1 following weight loss, while two observed no change following bariatric surgery(19, 24). Studies have found decreases(15, 17, 25), no changes(14, 19), and increases(18, 24) in VCAM-1 levels following weight loss.
Role of Baseline OSA Severity on CAM Levels
We observed no association between OSA severity and CAM levels at baseline. However, more severe OSA was associated with larger ICAM-1 increases within non-users with BMI≥35. Therefore, the use of PAP to limit harmful increases in ICAM-1 appears particularly essential in the most severe OSA patients with the highest BMI levels.
As recently reviewed(33), previous studies have observed correlations between OSA severity and pre-treatment levels of ICAM-1 and/or VCAM-1(5, 6, 8, 9, 11). The majority of these studies, however, included patients without OSA in correlation analyses. Since OSA patients were previously shown to have higher levels than controls, positive associations found when combining the two groups are not surprising. It is unclear whether these studies would find significant correlations with OSA severity in analyses restricted to cases. One study did observe a significant association (p=0.04) between ICAM-1 levels and AHI in OSA patients(5). However, no correlations were seen with other severity measurements, including hypoxia severity(5). In our study, it was likely more difficult to observe associations at baseline since all patients had moderate-to-severe OSA. Our observation of increased ICAM-1 and VCAM-1 in PAP non-users over time is consistent with findings that untreated OSA patients have higher levels compared to controls(5–11).
PAP Treatment and CAM Levels in OSA
Few studies with small samples have evaluated the effect of PAP on CAM levels in OSA, one in Europeans(10) and two in Asians(5, 8). Each had relatively non-obese OSA patients, and none compared treated and untreated OSA patients over time. Our ICAM-1 results support previous studies(5, 8, 10), which found PAP usage resulted in decreased ICAM-1. However, our results indicate this change is moderated by obesity, which was not explored in prior studies. Evidence for obesity moderating the relationship between PAP usage and change in ICAM-1 levels was seen both in BMI groups and visceral fat tertiles. However, BMI was significantly correlated with ICAM-1 levels, while VAT was not. Moreover, associations between ICAM-1 changes and both hours and nights of PAP usage in full and partial users were only seen in the BMI≥35kg/m2; there were no significant relationships in the top tertile of VAT. These results suggest the effect of PAP will be strongest in patients with the highest levels of total fat, not simply in those with the most visceral fat.
While we observed an association between VCAM-1 increases and PAP usage, only one previous study examined VCAM-1, observing no association with PAP(5). Although PAP usage did not lead to decreased VCAM-1 in our cohort, as it did with ICAM-1, we observed a similar trend between both CAMs and the three PAP groups. Specifically, the smallest changes (or decreases) were seen in full users, moderate or no changes in partial users, and the largest increases in non-users. Potentially, Chin and colleagues(5) may have found no association between PAP usage and VCAM-1 due to small sample size and/or limited follow-up time (1–6 months). This merits further investigation.
CAM levels in Cardiovascular Disease
Both ICAM-1 and VCAM-1 are studied in cardiovascular literature because of their role in atherosclerosis(15, 27–38). Higher levels of ICAM-1, but not VCAM-1, are a consistent predictor of cardiovascular risk within initially healthy populations(27, 30, 31, 33–37). However, higher VCAM-1 predicts future cardiovascular risk within patients suffering from pre-existing disease(27–29, 32, 33, 38). One potential explanation for this may be differences in expression: ICAM-1 is constitutively expressed by a variety of cells, while VCAM-1 is not expressed in baseline conditions, but induced by pro-atherosclerotic conditions(27, 28, 33).
Increased CAM levels likely increase risk for future cardiovascular events. Previous studies typically assess CAM levels at baseline and examine differences between those who do and do not develop cardiovascular events over several years of follow-up(30, 31, 35–37). Therefore, we would expect the increased cellular adhesion molecules in PAP non-users to impact long-term cardiovascular risk, while blocking these increases with PAP therapy will reduce this increased risk. This is congruent with previous literature, which shows untreated severe OSA increases risk of cardiovascular events over long-term follow-up in men(42) and women(43). Previous studies describe different thresholds for identified risk levels of ICAM-1 and VCAM-1, making direct comparisons challenging. For example, ICAM-1 thresholds for increased risk of future myocardial infarction in men range from 260 ng/ml(35) to 625 ng/ml(30). In our study, no men exceeded the higher cut-point. We observe a larger proportion of non-users with ICAM-1>260 compared to full users at follow-up (70% vs. 60%, p=0.15), despite similar proportions at baseline (63% vs. 66%, p=0.62), suggesting that PAP may protect against future MI. More research is needed to determine specific thresholds for risk stratification within OSA populations.
Propensity Score Matched Sub-analysis
When examining differences in CAM change between full and non-users in our propensity score matched sample with balanced covariate distributions, we observed similar relationships as in the observational study. There was a significant difference in ICAM-1 change; full users had decreased levels compared to significant increases in non-users. While differences in VCAM-1 change were not statistically significant, we again observed a larger increase among non-users compared to full users. This result suggests that differences in CAM changes between full and non-users in primary analyses were not driven by covariate imbalance and that a causal relationship likely exists between PAP adherence and cellular adhesion molecule changes. Overall, the observation that ICAM-1 and VCAM-1 changes are beneficially effected by PAP adherence supports the hypothesis that pathways involving adhesion molecules may be one mechanism for the increased cardiovascular risk in the context of OSA. In contrast to this potential pathway, similar analyses within a larger subset of the ISAC population found no relationship between changes in fasting lipid levels and PAP adherence (44). Thus, adhesion molecules pathways are an interesting focal point for future research attempting to link OSA and cardiovascular risk in humans.
Limitations and Strengths
While our sample represents the gender distribution of Icelandic OSA patients, there was a relative lack of females, limiting generalizability. Although the Icelandic population is of European descent, future studies involving other ethnicities will be important for increasing generalizability. The ISAC cohort lacks patients with mild OSA. Thus, we cannot exclude the possibility of an effect of OSA severity on biomarker levels from mild -to-moderate disease, as has been observed for prothrombotic biomarkers(45).
We did not conduct follow-up sleep studies in the full cohort. However, changes in OSA severity over a two-year period are small, and are associated with weight changes(46, 47). Thus, controlling for baseline BMI and BMI change in analyses limits potential bias introduced by unmeasured changes in OSA severity. This bias was also addressed in the propensity score subsample. One potential limitation of propensity score methodologies is the inability to control for unobserved variables, which are theoretically controlled for in the context of a randomized trial. Thus, we cannot exclude the possibility that results were affected by currently unknown or unmeasured confounders. To address this limitation, we included as many relevant variables as possible as unmeasured variables are controlled for to the extent that they are correlated with variables included in the model.
We do not view the lack of non-OSA obese controls as a major limitation. Rather, we had a substantial proportion of OSA patients not using PAP, providing a “control” population for our primary analyses. A proportion of non-users were prescribed a mandibular advancement device (n=16); removing these subjects did not change our results (data not shown).
This study has several strengths. The relatively large sample size makes this the largest study to date on PAP treatment and CAM levels in OSA. ISAC includes patients with relevant OSA comorbidities, making it more representative of “real-world” patient populations and limiting potential biases resulting from studying only otherwise “healthy” patients. The availability of objective PAP adherence data is more accurate than self-report. In-depth phenotyping allowed us to assess a wide variety of potential confounders. Finally, to minimize bias in non-randomized PAP group comparisons, we employed propensity score methodologies to evaluate the relationship between PAP adherence and CAM change. Results from this complementary analysis were compatible with results from the overall study.
Conclusion
Our results indicate that in a moderate-to-severe OSA population, adequate PAP usage limits significant increases in both ICAM-1 and VCAM-1 levels observed in PAP non-users after two years. For ICAM-1, the benefit of PAP depends on obesity, with the largest effect seen in the most obese patients. As obesity and OSA often coexist, limiting these increases with PAP usage may help to decrease the rate of progression of OSA-related cardiovascular disease.
Supplementary Material
Acknowledgements
The authors are grateful to Sigrun Gudmundsdottir, Lovisa Gudmundsdottir, Magdalena Osk Sigurgunnarsdottir, Kristján Andri Kristjánsson, Bethany Staley, Matthew Thorne-Fitzgerald, Robert Hachadoorian, Ted Mifflin, and the other staff at the Sleep Centers of Landspitali University Hospital and the University of Pennsylvania who helped assemble and analyze the data. We are grateful to Daniel C. Barrett with help in manuscript preparation.
Funding Sources
The research was supported by NIH grants: HL072067 for “A Family Linkage Study of Obstructive Sleep Apnea”; HL094307 for “Endophenotypes of Sleep Apnea and Role of Obesity”; HL07973 for “Training in Sleep and Sleep Disorders”; 1K99NR014675-01 “Mechanisms of Sleepiness Symptoms in Obstructive Sleep Apnea and Cardiovascular Disease”; the Eimskip Fund of the University of Iceland and the Landspitali University Hospital Research Fund.
Footnotes
Disclosure statement: ESA reports personal consulting fees from Nox Medical outside the scope of the submitted work. The authors declare no competing interests with regard to the submitted work. AIP is the holder of an endowed chair, funds for which were provided by Respironics.
Supplementary information is available at the journal's website.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Progress in Cardiovascular Diseases. 2009;51(5):434–451. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32(4):447–470. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballantyne CM, Entman ML. Soluble adhesion molecules and the search for biomarkers for atherosclerosis. Circulation. 2002;106(7):766–767. doi: 10.1161/01.cir.0000028397.68936.12. [DOI] [PubMed] [Google Scholar]
- 5.Chin K, Nakamura T, Shimizu K, Mishima M, Nakamura T, Miyasaka M, et al. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. The American Journal of Medicine. 2000;109(7):562–567. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 6.El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121(5):1541–1547. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- 7.Ohga E, Nagase T, Tomita T, Teramoto S, Matsuse T, Katayama H, et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. Journal of applied physiology (Bethesda, Md: 1985) 1999;87(1):10–14. doi: 10.1152/jappl.1999.87.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. Journal of applied physiology (Bethesda, Md: 1985) 2003;94(1):179–184. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 9.Ursavas A, Karadag M, Rodoplu E, Yilmaztepe A, Oral HB, Gozu RO. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration; international review of thoracic diseases. 2007;74(5):525–532. doi: 10.1159/000097770. [DOI] [PubMed] [Google Scholar]
- 10.Zamarron C, Riveiro A, Gude F. Circulating levels of vascular endothelial markers in obstructive sleep apnoea syndrome. Effects of nasal continuous positive airway pressure. Archives of medical science : AMS. 2011;7(6):1023–1028. doi: 10.5114/aoms.2011.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamarron-Sanz C, Ricoy-Galbaldon J, Gude-Sampedro F, Riveiro-Riveiro A. Plasma levels of vascular endothelial markers in obstructive sleep apnea. Archives of Medical Research. 2006;37(4):552–555. doi: 10.1016/j.arcmed.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 13.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 14.Clifton PM, Keogh JB, Foster PR, Noakes M. Effect of weight loss on inflammatory and endothelial markers and FMD using two low-fat diets. International journal of obesity. 2005;29(12):1445–1451. doi: 10.1038/sj.ijo.0803039. [DOI] [PubMed] [Google Scholar]
- 15.Ferri C, Desideri G, Valenti M, Bellini C, Pasin M, Santucci A, et al. Early upregulation of endothelial adhesion molecules in obese hypertensive men. Hypertension. 1999;34(4 Pt 1):568–573. doi: 10.1161/01.hyp.34.4.568. [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Ohshima A, Inoue M, Ohto N, Nakasuga K, Kaji Y, et al. Weight reduction decreases soluble cellular adhesion molecules in obese women. Clinical and experimental pharmacology & physiology. 2002;29(5–6):399–404. doi: 10.1046/j.1440-1681.2002.03672.x. [DOI] [PubMed] [Google Scholar]
- 17.Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. The British journal of nutrition. 2007;98(4):852–859. doi: 10.1017/S0007114507747815. [DOI] [PubMed] [Google Scholar]
- 18.Keogh JB, Brinkworth GD, Noakes M, Belobrajdic DP, Buckley JD, Clifton PM. Effects of weight loss from a very-low-carbohydrate diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am J Clin Nutr. 2008;87(3):567–576. doi: 10.1093/ajcn/87.3.567. [DOI] [PubMed] [Google Scholar]
- 19.Konukoglu D, Uzun H, Firtina S, Cigdem Arica P, Kocael A, Taskin M. Plasma adhesion and inflammation markers: asymmetrical dimethyl-L-arginine and secretory phospholipase A2 concentrations before and after laparoscopic gastric banding in morbidly obese patients. Obesity surgery. 2007;17(5):672–678. doi: 10.1007/s11695-007-9113-3. [DOI] [PubMed] [Google Scholar]
- 20.Leinonen E, Hurt-Camejo E, Wiklund O, Hulten LM, Hiukka A, Taskinen MR. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003;166(2):387–394. doi: 10.1016/s0021-9150(02)00371-4. [DOI] [PubMed] [Google Scholar]
- 21.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 22.Rohde LE, Hennekens CH, Ridker PM. Cross-sectional study of soluble intercellular adhesion molecule-1 and cardiovascular risk factors in apparently healthy men. Arterioscler Thromb Vasc Biol. 1999;19(7):1595–1599. doi: 10.1161/01.atv.19.7.1595. [DOI] [PubMed] [Google Scholar]
- 23.Targher G, Bonadonna RC, Alberiche M, Zenere MB, Muggeo M, Bonora E. Relation between soluble adhesion molecules and insulin sensitivity in type 2 diabetic individuals: role of adipose tissue. Diabetes care. 2001;24(11):1961–1966. doi: 10.2337/diacare.24.11.1961. [DOI] [PubMed] [Google Scholar]
- 24.Vazquez LA, Pazos F, Berrazueta JR, Fernandez-Escalante C, Garcia-Unzueta MT, Freijanes J, et al. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab. 2005;90(1):316–322. doi: 10.1210/jc.2003-032059. [DOI] [PubMed] [Google Scholar]
- 25.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105(7):804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 26.Pontiroli AE, Pizzocri P, Koprivec D, Vedani P, Marchi M, Arcelloni C, et al. Body weight and glucose metabolism have a different effect on circulating levels of ICAM-1, E-selectin, and endothelin-1 in humans. European journal of endocrinology / European Federation of Endocrine Societies. 2004;150(2):195–200. doi: 10.1530/eje.0.1500195. [DOI] [PubMed] [Google Scholar]
- 27.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 28.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104(12):1336–1342. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 29.Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, et al. Increased levels of soluble vascular cell adhesion molecule 1 are associated with risk of cardiovascular mortality in type 2 diabetes: the Hoorn study. Diabetes. 2000;49(3):485–491. doi: 10.2337/diabetes.49.3.485. [DOI] [PubMed] [Google Scholar]
- 30.Luc G, Arveiler D, Evans A, Amouyel P, Ferrieres J, Bard JM, et al. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis. 2003;170(1):169–176. doi: 10.1016/s0021-9150(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 31.Malik I, Danesh J, Whincup P, Bhatia V, Papacosta O, Walker M, et al. Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta-analysis. Lancet. 2001;358(9286):971–976. doi: 10.1016/S0140-6736(01)06104-9. [DOI] [PubMed] [Google Scholar]
- 32.Mulvihill NT, Foley JB, Murphy RT, Curtin R, Crean PA, Walsh M. Risk stratification in unstable angina and non-Q wave myocardial infarction using soluble cell adhesion molecules. Heart. 2001;85(6):623–627. doi: 10.1136/heart.85.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pak VM, Grandner MA, Pack AI. Circulating Adhesion Molecules in Sleep Apnea and Cardiovascular Disease. Sleep Medicine Reviews. 2013 doi: 10.1016/j.smrv.2013.01.002. Epub ahead of print(Journal Article). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. The American Journal of Medicine. 1999;107(1):85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt C, Hulthe J, Fagerberg B. Baseline ICAM-1 and VCAM-1 are increased in initially healthy middle-aged men who develop cardiovascular disease during 6.6 years of follow-up. Angiology. 2009;60(1):108–114. doi: 10.1177/0003319708316899. [DOI] [PubMed] [Google Scholar]
- 37.Shai I, Pischon T, Hu FB, Ascherio A, Rifai N, Rimm EB. Soluble intercellular adhesion molecules, soluble vascular cell adhesion molecules, and risk of coronary heart disease. Obesity (Silver Spring, Md) 2006;14(11):2099–2106. doi: 10.1038/oby.2006.245. [DOI] [PubMed] [Google Scholar]
- 38.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 39.Maislin G, Rubin DB. Design of Non-Randomized Medical Device Trials Based on Sub-Classification Using Propensity Score Quintiles, Topic Contributed Session on Medical Devices. Proceedings of the Joint Statistical Meetings. 2010:2182–2196. [Google Scholar]
- 40.Lieu TA, Au D, Krishnan JA, Moss M, Selker H, Harabin A, et al. Comparative effectiveness research in lung diseases and sleep disorders: recommendations from the National Heart, Lung, and Blood Institute workshop. Am J Respir Crit Care Med. 2011;184(7):848–856. doi: 10.1164/rccm.201104-0634WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27(12):1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 43.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Annals of internal medicine. 2012;156(2):115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 44.Keenan BT, Maislin G, Sunwoo BY, Arnardottir ES, Jackson N, Olafsson I, et al. Obstructive sleep apnoea treatment and fasting lipids: a comparative effectiveness study. The European respiratory journal. 2014 doi: 10.1183/09031936.00043614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehra R, Xu F, Babineau DC, Tracy RP, Jenny NS, Patel SR, et al. Sleep-disordered breathing and prothrombotic biomarkers: cross-sectional results of the Cleveland Family Study. American journal of respiratory and critical care medicine. 2010;182(6):826–833. doi: 10.1164/rccm.201001-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuna ST, Reboussin DM, Borradaile KE, Sanders MH, Millman RP, Zammit G, et al. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36(5):641–649. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA : the journal of the American Medical Association. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.