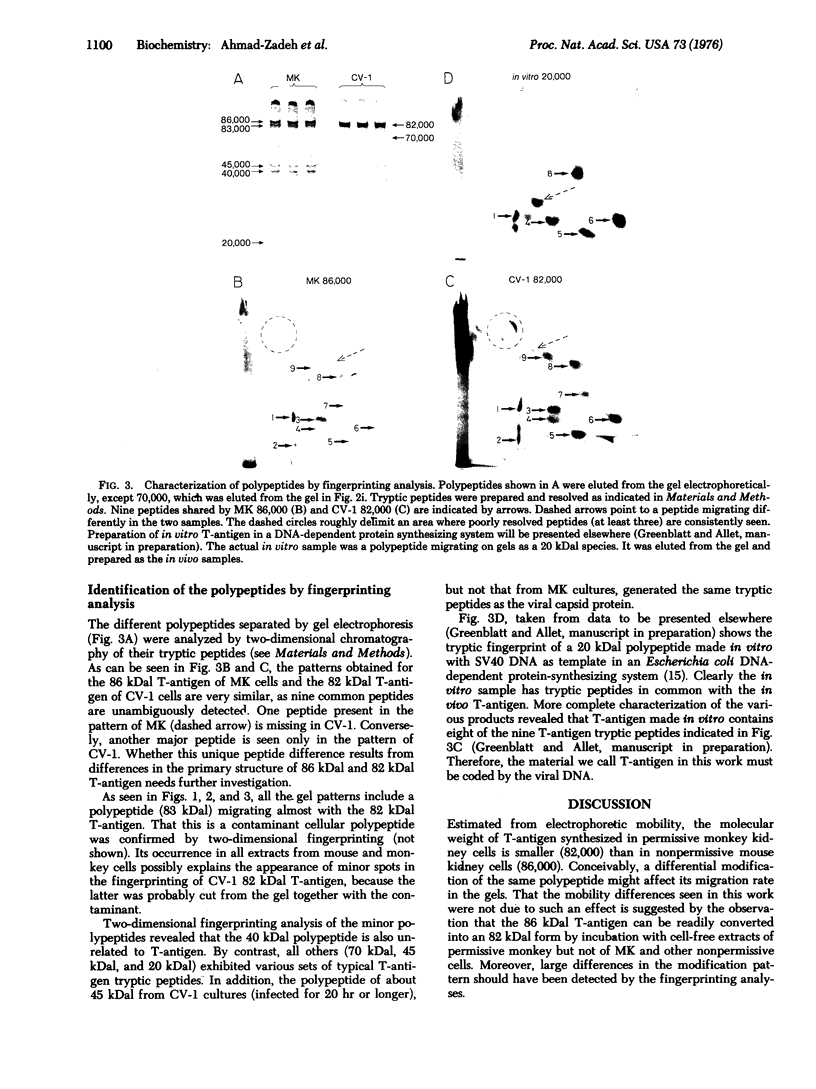
Abstract
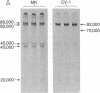
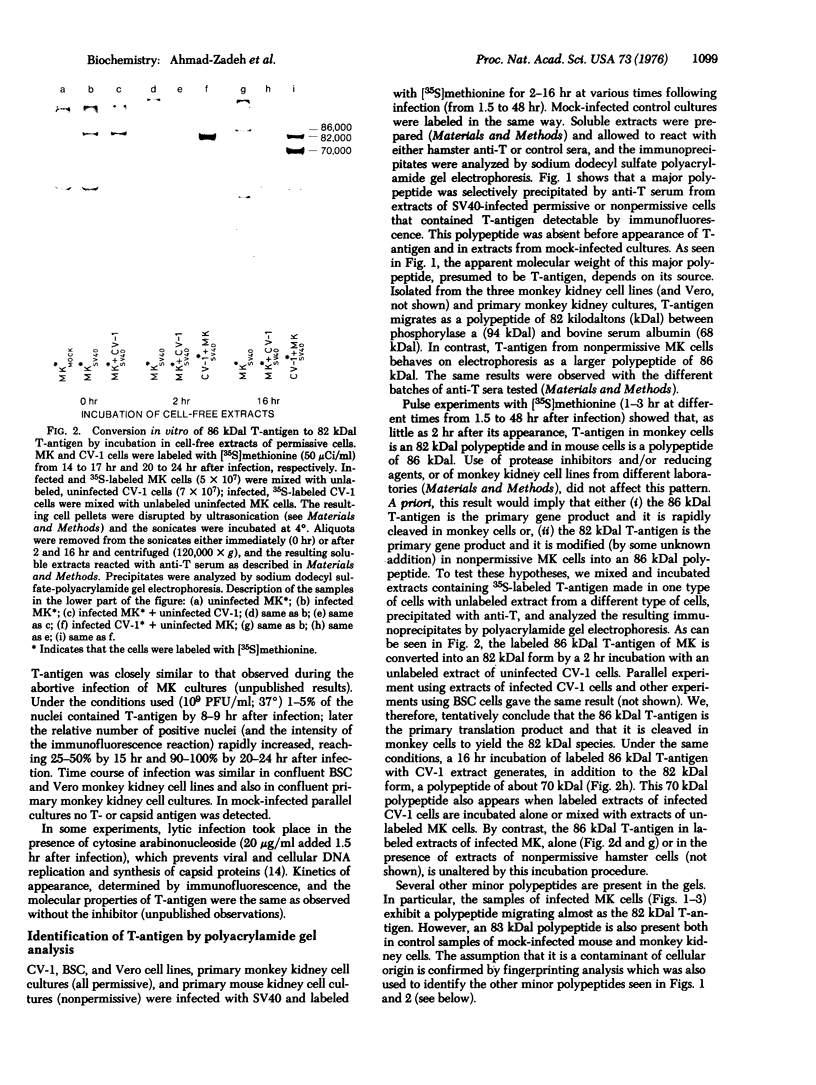
Simian-virus-40-specific T-antigen was isolated by immunoprecipitation. From other studies we have proof that the T-antigen described in this work is coded by the viral DNA. The molecular weight estimated from electrophoretic mobility in sodium dodecyl sulfate-polyacrylamide gels of T-antigen isolated from nonpermissive mouse cells in abortive infection is 86,000 and from permissive monkey cells in lytic infection is 82,000. The 86 kilodalton T-antigen is readily converted in vitro into an 82 kilodalton form by incubation with extracts from permissive monkey cells but not with extracts from nonpermissive mouse or hamster cells. This and the results of fingerprinting analysis of tryptic peptides suggest that T-antigen may be processed in permissive cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad-Zadeh C., Piguet J. D., Colli L. Molecular weight estimation of immunoglobulin subunits of polyacrylamide gel. Immunology. 1971 Dec;21(6):1065–1071. [PMC free article] [PubMed] [Google Scholar]
- Allet B., Katagiri K. J., Gesteland R. F. Characterization of polypeptides made in vitro from bacteriophage lambda DNA. J Mol Biol. 1973 Aug 25;78(4):589–600. doi: 10.1016/0022-2836(73)90281-7. [DOI] [PubMed] [Google Scholar]
- Black P. H. The oncogenic DNA viruses: a review of in vitro transformation studies. Annu Rev Microbiol. 1968;22:391–426. doi: 10.1146/annurev.mi.22.100168.002135. [DOI] [PubMed] [Google Scholar]
- Bowers C. Y., Schally A. V., Enzmann F., Boler J., Folkers K. Porcine thyrotropin releasing hormone is (pyro)glu-his-pro(NH2). Endocrinology. 1970 May;86(5):1143–1153. doi: 10.1210/endo-86-5-1143. [DOI] [PubMed] [Google Scholar]
- Del Villano B. C., Defendi V. Characterization of the SV40 T antigen. Virology. 1973 Jan;51(1):34–46. doi: 10.1016/0042-6822(73)90363-2. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J Virol. 1973 Sep;12(3):643–652. doi: 10.1128/jvi.12.3.643-652.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- May E., Kopecka H., May P. Mapping the transcription site of the SV40-specific late 16 S mRNA. Nucleic Acids Res. 1975 Oct;2(10):1995–2005. doi: 10.1093/nar/2.10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., May P., Weil R. "Early" virus-specific RNA may contain information necessary for chromosome replication and mitosis induced by Simian Virus 40. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1654–1658. doi: 10.1073/pnas.70.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., May P., Weil R. Analysis of the events leading to SV40-induced chromosome replication and mitosis in primary mouse kidney cell cultures. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1208–1211. doi: 10.1073/pnas.68.6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Berg P., Kelly T. J., Jr, Lewis A. M., Jr Mapping of simian virus 40 early functions on the viral chromosome. J Virol. 1973 Sep;12(3):653–658. doi: 10.1128/jvi.12.3.653-658.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPP F., BUTEL J. S., FELDMAN L. A., KITAHARA T., MELNICK J. L. DIFFERENTIAL EFFECTS OF INHIBITORS ON THE STEPS LEADING TO THE FORMATION OF SV40 TUMOR AND VIRUS ANTIGENS. J Exp Med. 1965 Jun 1;121:935–944. doi: 10.1084/jem.121.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Gorecki M., Mulligan R. C., Danna K. J., Rozenblatt S., Rich A. Simian virus 40 DNA directs synthesis of authentic viral polypeptides in a linked transcription-translation cell-free system. Proc Natl Acad Sci U S A. 1975 May;72(5):1922–1926. doi: 10.1073/pnas.72.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier P., Cassingena R., Wicker R., Coppey J., Suarez H. Etude du mécanisme de l'induction chez des cellules de hamster syrien transformées par le virus SV40. I. Propriétés d'une lignée cellulaire clonale. Int J Cancer. 1967 Mar 15;2(2):117–132. doi: 10.1002/ijc.2910020207. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Watkins J. F. The SV40 rescue problem. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):355–362. doi: 10.1101/sqb.1974.039.01.046. [DOI] [PubMed] [Google Scholar]
- Weil R., Kára J. Polyoma "tumor antigen": an activator of chromosome replication? Proc Natl Acad Sci U S A. 1970 Oct;67(2):1011–1017. doi: 10.1073/pnas.67.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R., Salomon E., May E., May P. A simplifying concept in tumor virology: virus-specific "pleiotropic effectors". Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):381–395. doi: 10.1101/sqb.1974.039.01.050. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Newbold J. E. Mapping of SV40 mRNA species on the viral genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):161–164. doi: 10.1101/sqb.1974.039.01.022. [DOI] [PubMed] [Google Scholar]
- Zubay G., Chambers D. A. A DNA-directed cell-free system for beta-galactosidase synthesis; characterization of the de novo synthesized enzyme and some aspects of the regulation of synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:753–761. doi: 10.1101/sqb.1969.034.01.085. [DOI] [PubMed] [Google Scholar]