Highlights
-
•
Assessed recognition memory in infant monkeys with neonatal perirhinal lesions using the visual paired comparison task.
-
•
Performance was assessed at 4 developmental ages.
-
•
Novelty preference deteriorated with age after neonatal perirhinal lesions.
-
•
Presence of functional sparing.
-
•
Memory deficits after perirhinal lesions occurred earlier than after hippocampal lesions.
Keywords: Incidental recognition, Medial temporal lobe, Macaca mulatta, Visual-paired-comparison, Novelty preference, Functional plasticity
Abstract
To investigate the role of the perirhinal cortex on the development of recognition measured by the visual paired-comparison (VPC) task, infant monkeys with neonatal perirhinal lesions and sham-operated controls were tested at 1.5, 6, 18, and 48 months of age on the VPC task with color stimuli and intermixed delays of 10 s, 30 s, 60 s, and 120 s. Monkeys with neonatal perirhinal lesions showed an increase in novelty preference between 1.5 and 6 months of age similar to controls, although at these two ages, performance remained significantly poorer than that of control animals. With age, performance in animals with neonatal perirhinal lesions deteriorated as compared to that of controls. In contrast to the lack of novelty preference in monkeys with perirhinal lesions acquired in adulthood, novelty preference in the neonatally operated animals remained above chance at all delays and all ages. The data suggest that, although incidental recognition memory processes can be supported by the perirhinal cortex in early infancy, other temporal cortical areas may support these processes in the absence of a functional perirhinal cortex early in development. The neural substrates mediating incidental recognition memory processes appear to be more widespread in early infancy than in adulthood.
1. Introduction
The study of the neural substrates responsible for recognition memory has received increased attention in the last two decades as a result of recent theoretical considerations of the role of the medial temporal lobe (MTL) structures in memory. There is general agreement that, within the MTL, the hippocampus acts in concert with the parahippocampal and perirhinal cortex to support recognition memory. In this view, the hippocampus associates (or binds) contextual information from the parahippocampal cortex with object representations from the perirhinal (PRh) cortex, and encodes and maintains relationships among stimuli (Davachi, 2006, Diana et al., 2007, Eichenbaum et al., 2007, Montaldi and Mayes, 2010, Sutherland and Rudy, 1989). In addition to its role in building representations of objects, the role of PRh in object recognition memory has received growing support from studies in several species including rodents, monkeys and humans (for review see Bachevalier et al., 2002, Brown and Aggleton, 2001, Eichenbaum et al., 2007, Murray et al., 2007, Squire et al., 2007, Wan et al., 1999, Winters et al., 2008). In adult monkeys, selective lesions of the perirhinal cortex either alone, or in conjunction with the entorhinal cortex or parahippocampal cortex severely impair performance on object recognition memory tasks, including delayed nonmatching-to-sample and delayed matching-to-sample (Baxter and Murray, 2001, Buffalo et al., 1999, Buffalo et al., 2000, Gaffan and Murray, 1992, Hadfield et al., 2003, Meunier et al., 1993, Nemanic et al., 2004, Zola-Morgan et al., 1989) as well as the visual paired comparison (VPC) task (Nemanic et al., 2004). In the VPC task, a task known to measure incidental recognition memory processes, the memory deficit emerges at very short delays of only a few seconds and contrasts with the recognition memory impairment seen only at delays longer than 60 s in the same task after selective hippocampal lesions in adults. Similarly, in humans, damage to the medial temporal lobe, which included the perirhinal cortex, impaired performance on a yes/no recognition memory task at delays greater than 6 s, as compared to the impairment seen at longer delays (25 s) in patients with damage limited to the hippocampal formation (Buffalo et al., 1999). In addition, damage to the temporal lobe that included the perirhinal cortex but spared the hippocampus, impaired the ability of human subjects to make familiarity judgments in the remember/know paradigm (Bowles et al., 2007). Finally, neuroimaging studies in humans (Danckert et al., 2007, Pihlajamaki et al., 2004, Ramsøy et al., 2009) have shown that the perirhinal cortex plays a role in processing and encoding novel object information. Although the contribution of the perirhinal cortex to recognition memory processes in adults is well established, its contribution to the early developing recognition memory abilities that have been demonstrated in both humans (Diamond, 1995, Fagan, 1970, Pascalis and de Schonen, 1994) and monkeys (Bachevalier et al., 1993, Gunderson and Sackett, 1984, Zeamer et al., 2009, Zeamer et al., 2010) remains to be investigated.
We recently tested the normal development of object recognition memory in monkeys using the visual paired comparison (VPC) task (Zeamer et al., 2010) as well as that of infant monkeys that had received selective hippocampal lesions. Infant monkeys received the VPC task at four ages during development (1.5, 6, 18 and 48 months) using delays varying from 10 to 120 s and pictures of color stimuli. At the youngest age of 1.5 months, normal infant monkeys showed novelty preference at all delays and this preference became more robust by 6 months of age. However, at 18 months of age, even when novelty preference remained well above chance level, a delay-dependent effect emerged, such that novelty preference was stronger at the shortest than at the longest delays. In addition, monkeys with neonatal neurotoxic hippocampal lesions performed as well as controls at 1.5 and 6 months of age, and, by 18 months of age, also showed a delay-dependent effect. However, the decrease in novelty preference across the delays was steeper in animals with neonatal hippocampal lesions than in control animals, such that, at the longest delay of 120 s, novelty preference of animals with neonatal hippocampal lesions was significantly weaker than that of controls. This pattern of findings suggested that, in the absence of a functional hippocampus, the intact recognition memory seen at the earlier ages of 1.5 and 6 months in the hippocampal-operated monkeys could be supported by medial temporal cortical areas. One likely candidate is the perirhinal cortex given mounting evidence implicating this temporal cortical area in recognition memory in adults (see above).
Thus, in the present study, we investigated whether the perirhinal cortex could support incidental recognition memory processes in early infancy. Infant monkeys were given neonatal neurotoxic perirhinal lesions and tested in the VPC recognition task at the same time points (1.5, 6, 18 and 48 months) and using the same delays (10–120 s) as in our previous study with neonatal hippocampal lesions (Zeamer et al., 2010, Zeamer and Bachevalier, 2013). We predicted that if the perirhinal cortex mediates recognition memory processes measured with the VPC task at 1.5 and 6 months of age, animals with neonatal perirhinal lesions should be impaired on the task at all ages and with short as well as long delays. Preliminary reports of the findings were published in abstract form (Zeamer and Bachevalier, 2009).
2. Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas at Houston where this study began and by Emory University where it was completed. All rearing and behavioral testing procedures were kept constant between the two institutions.
2.1. Subjects
The subjects were sixteen full-term infant rhesus monkeys (Macaca mulatta) of both sexes born from multiparous females at the MD Anderson Cancer Center Science Park (Bastrop, TX) and the Yerkes National Primate Research Center (YNPRC) breeding colony (Atlanta, GA), and brought to our primate nursery between 1 and 2 days of age. Between 10 and 12 days postnatally, eight animals underwent a sham operation and two were kept unoperated and were used as controls (Group Neo-C, 5 males, 5 females). The remaining six received MRI-guided neurotoxic injections in the perirhinal cortex (Group Neo-PRh, 3 males, 3 females). At both institutions, all monkeys were reared using similar procedures by the same experimenters and included social interactions with age-matched peers and human caregivers as described previously (see Goursaud and Bachevalier, 2007).
2.2. Surgical procedures
2.2.1. Magnetic resonance imaging (MRI) scans and determination of injection coordinates
All animals received MRI scans immediately prior to surgery. The scans for Group Neo-PRh were used to determine the precise stereotaxic coordinates for injection of ibotenic acid (Nemanic et al., 2002) and the scans for Group Neo-C were used to assess brain maturation using T1 structural images (Payne et al., 2010). The subjects were anesthetized with ketamine hydrochloride and xylazine (10 mg/kg of 7:3 ketamine hydrochloride, 100 mg/ml, and xylazine, 20 mg/ml, i.m.), and intubated to inhale isoflurane gas (1.0–3.0%, v/v, to effect) and maintain adequate anesthesia throughout the procedure. Two sets of coronal images were taken using a 5-cm surface coil. The first set was used to calculate the neurotoxin injection sites in the perirhinal cortex (3D T1-weighted fast spoiled gradient (FSPGR)-echo sequence, TE = 2.6 ms, TR = 10.2 ms, 25° flip angle, contiguous 1 mm sections, 12 cm FOV, 256 × 256 matrix). The second set, used to measure edema caused by cell death from the neurotoxin, (Fluid Attenuated Inversion Recovery (FLAIR) sequence, TE = 140 ms, TR = 10,000 ms, inversion time (TI) = 2200 ms, contiguous 3 mm sections, 12 cm FOV, 256 × 256 matrix) were acquired in three series, offset by 1 mm posteriorly. Approximately one week after surgery, animals in Group Neo-PRh underwent a second series of scans in order to evaluate lesion extent using methods previously described by Nemanic and colleagues (2002).
2.2.2. Surgery
All surgical procedures were performed under deep anesthesia using aseptic techniques. The animal was maintained on isoflurane gas (1.0–2.0%, v/v, to effect) throughout surgery, and an IV drip containing dextrose and 0.45% sodium chloride was used to maintain normal hydration. To prevent hypothermia, a heating pad was placed underneath the animal, and the veterinary staff monitored all vital signs until the animal fully recovered from anesthesia.
Once the scalp was shaved and the skin disinfected with Nolvasan, a long lasting local anesthetic (Marcaine 25%, 1.5 ml) was injected along the midline incision (from occiput to a mid-point on orbital ridges), and the galea was then retracted. Using an electric drill, craniotomies (1 cm wide × 2.5 cm long) were made bilaterally and Bone wax (Ethicon, Inc., Somerville, NJ; 2.5 g size) was used to prevent excessive bleeding. The dura was then opened to allow a Hamilton syringe, held by Kopf electrode manipulators (David Kopf Instruments, Tujunga, CA), to be lowered simultaneously into each hemisphere. Three injection sites were chosen at 2 mm intervals along the length of the perirhinal cortex (Brodman areas 35 and 36), bilaterally, and 0.4 μl ibotenic acid (Biosearch Technologies, Novato, CA, 10 mg/ml in PBS, pH 7.4) was injected at each site at a rate of 0.4 μl/min. The dura, galea and skin were then sutured and the animal was removed from isoflurane and kept under constant supervision until fully recovered. Starting 12 h prior to surgery and lasting 7 days after, subjects received dexamethazone sodium phosphate (0.4 mg/kg, i.m.) to reduce edema and cefazolin (25 mg/kg, i.m.) to prevent infection. For pain relief, acetaminophen (10 mg/kg, p.o.) was given four times a day for 3 days following surgery.
Sham operated controls underwent the same procedures, however once the dura was cut, no needle was lowered.
2.2.3. Post-surgical MRI scans and MRI-based lesion evaluation
No histological evaluations were available because all animals are still currently participating on other cognitive experiments at this time. Previously described procedures to measure the extent of damage were used (Málková et al., 2001, Nemanic et al., 2002). Areas of hypersignal, representing the edema caused by cell death, were visually identified on the one-week post-surgical FLAIR images and then plotted onto matching coronal drawings of a normal monkey brain. Image J® was used to measure the surface area (in pixels2) of damage to the left and right perirhinal cortex, as well as unintended damage to adjacent structures. The percentage of volume damage was calculated by dividing the volume of damage to the perirhinal cortex by the volume of that structure in a normal monkey of the same age. Similar procedures were used to evaluate any additional damage to adjacent structures.
3. Behavioral testing
3.1. Behavioral apparatus
Subjects were held by an experimenter at 1.5 and 6 months of age, and were seated in a custom plexiglass primate chair at 18 and 48 months. Although the animal caregiver holding the infants at 1.5 and 6 months could see the stimuli presented on the TV monitor, he/she was unaware of the task demands and the responses that were required for the infants, thus minimizing any infants’ positioning bias toward the novel stimuli on each trial. The subjects viewed stimuli on a 19 in. display monitor from approximately 40 cm away. The experimenter controlled the presentation of the stimuli on a Dell laptop connected to the monitor. A video camera (Sony Digital8 TRV-140) was mounted on the top of the monitor such that the eyes of the animal were clearly visible on a TV screen next to the experiment to monitor the animal's looking behavior at all times. The camera output was fed through a time-date generator and connected to a VCR (JVC, USA, Wayne, NJ) so that the eye movements were recorded for later coding. To reduce outside noise, a white noise generator was used.
3.2. Pre-training
Infants tested at 1.5 months of age were first acclimated to the testing room by watching Disney cartoons for three days prior to testing, for an increasing period of time each day (15, 20 and 25 min). At 6 months of age, they received only one 20-min acclimation the day before testing began. At 18 months, animals were first trained to enter the primate chair, which took from 2 to 5 days per animal before testing began. Finally, at 48 months of age, once acclimated to the chair, they received one 20-min acclimation day in the testing room. Subjects were neither food nor water deprived during testing, and occasionally received a treat during the inter-trial interval on test day.
3.3. VPC task
All monkeys were tested as infants (1.5 and 6 months) and as juveniles (18 months) on the visual paired comparison task (VPC), which takes advantage of the subject's natural tendency to look at the novel object without earning a reward. Stimuli were color pictures of variegated objects from multiple categories, selected from a pool of 800,000 clipart images (Nova Art Explosion 800,000 Clip Art), and chosen to be as different as possible, varying in color, shape and texture. Each trial consisted of a Familiarization Phase and two Retention Tests (Zeamer et al., 2010). In the Familiarization Phase, animals fixated on a novel stimulus in the center of the screen until they reached 30 s of cumulative looking time. This was followed by a variable delay of 10 s, 30 s, 60 s or 120 s, and then two retention tests, separated by a 5 s delay, in which the now familiar image was paired with a novel image. Each retention test stayed on the screen for 5 s after the animal initially looked. Also, the left/right position of the two images was reversed during the retention tests, and the left/right position of the novel image varied pseudo-randomly on the first Retention Test. Each trial was separated by a 30 s inter-trial interval (ITI) during which the screen remained black. Delays were intermixed within daily sessions and animals received a total of 10 trials at each delay at each age for a total of 50 trials. At all ages, animals received 5 trials per testing day.
All monkeys were re-tested in the task upon reaching adulthood (48 months) using novel color stimuli and the same delays of 10–120 s. In addition, a shorter delay of 1 s was given to four monkeys in Group Neo-C (Neo-C-6 to Neo-C-8) and all monkeys of Group Neo-PRh. This additional delay was intermixed within the 4 other delays. All task procedures remained the same.
4. Data analysis
The measure of recognition memory was the animal's preference to look longer at the novel image during the retention tests. This preference score was determined using frame-by-frame analysis of eye movements to calculate the amount of time the animal spent looking toward the familiar and the novel stimuli using procedures already published (see details in Pascalis and Bachevalier, 1999, Zeamer et al., 2010). Two observers independently scored the trials (inter-observer reliability: Pearson r = 0.931). Each observer was blind to the right/left position of the novel image for each trial. From each trial, three parameters were used: (1) the total amount of time the animal spent looking toward the novel image [(time looking at novel/total time looking) × 100)], (2) the total Familiarization Phase, which is the length of time it takes the animal to accumulate 30 s of looking behavior toward the sample stimulus, and (3) the Total Retention Time, which is the total amount of time the animal looks at either image during the two retention tests. Trials for which the Total Retention Time was less than 1 s were excluded from the analyses but this occurred only on 7 of the total 2640 trials.
5. Statistical analysis
We first compared performance of animals of Group Neo-C (n = 6) prepared at UT-Houston with the two sham-operated monkeys and two unoperated controls added at the YNPRC, to assess whether any small changes in our experimental procedures affected performance of the animals on the VPC task. Given the comparable performance of these two groups of control animals, they were combined into a single sham-operated group (Group Neo-C) for further analyses. Thus, statistical comparisons will be made between Group Neo-C (n = 10) and Group Neo-PRh (n = 6). We also investigated whether the factor Sex influenced performance on the task using a MANOVA (Group × Sex × Age × Delay) with repeated measures for the last two factors. The factor Sex did not reach significance either as a main effect [F(1, 12) = 0.167, ns] or as interactions with other factors (all ps > .05) and was not included in any of the statistical analyses reported below.
Statistical analyses used a General Linear Model ANOVA with a between subject comparison for the effect of Group (Neo-C, Neo-PRh) and within subject comparisons using repeated measures for the effects of Age (1.5, 6, 18 and 48 months) and Delay (10, 30, 60 and 120 s). When sphericity was not assumed, a Huynh–Feldt correction was used. Post Hoc Tukey tests were conducted when group differences reached significance, and one-sample t-tests were used to evaluate group differences from chance. Additionally, planned comparisons were performed between the control group and the experimental group, using a one-sided planned comparison (Pedhazur, 1982). These statistical analyses were carried out on three parameters of the VPC task (see Section 4), i.e. the total Familiarization Phase and the Total Retention Time, which measure viewing behaviors, and novelty preference, which measures recognition memory. Also, given that at the later age of 48 months four animals of Group Neo-C and all animals in Group Neo-PRh were tested with a shorter delay of 1 s intermixed within the 4 other delays, we also compared performance of these two groups using the 5 delays.
In addition, Pearson correlations were used to determine whether extent of perirhinal lesions or unintended damage to adjacent areas (see Table 1) may have affected performance on the task at any age and any delay.
Table 1.
Percent of intended and unintended damage.
| Subjects | PRh |
ERh |
TE |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% | |
| Neo-PRh-1 | 89.76 | 76.91 | 83.34 | 69.04 | 28.51 | 2.28 | 15.39 | 0.65 | 4.53 | 9.70 | 7.11 | 0.44 |
| Neo-PRh-2 | 68.16 | 70.58 | 69.37 | 48.11 | 17.72 | 20.65 | 19.19 | 3.66 | 0.14 | 0.06 | 0.10 | 0.00 |
| Neo-PRh-3 | 65.45 | 81.02 | 73.23 | 53.02 | 7.72 | 3.12 | 5.42 | 0.24 | 0.26 | 3.39 | 1.82 | 0.01 |
| Neo-PRh-4 | 59.40 | 74.73 | 67.06 | 44.39 | 11.55 | 17.84 | 14.69 | 2.06 | 0.72 | 2.62 | 1.67 | 0.02 |
| Neo-PRh-5 | 75.90 | 66.81 | 71.35 | 50.71 | 38.60 | 29.86 | 34.23 | 11.53 | 0.72 | 0.41 | 0.57 | 0.00 |
| Neo-PRh-6 | 74.12 | 80.31 | 77.22 | 59.53 | 25.34 | 43.64 | 34.49 | 11.06 | 0.37 | 2.93 | 1.65 | 0.01 |
| Avg | 72.13 | 75.06 | 73.60 | 54.13 | 21.57 | 19.57 | 20.57 | 4.87 | 1.12 | 3.19 | 2.15 | 0.08 |
| Subjects | TH/TF |
AMY |
HF |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% | |
| Neo-PRh-1 | 0.00 | 0.00 | 0.00 | 0.00 | 8.24 | 10.86 | 9.55 | 0.89 | 0.13 | 2.39 | 1.26 | 0.00 |
| Neo-PRh-2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.76 | 1.38 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Neo-PRh-3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.27 | 0.14 | 0.00 |
| Neo-PRh-4 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Neo-PRh-5 | 7.02 | 3.93 | 5.47 | 0.28 | 0.00 | 0.00 | 0.00 | 0.00 | 3.37 | 0.00 | 1.68 | 0.00 |
| Neo-PRh-6 | 0.00 | 0.00 | 0.00 | 0.00 | 3.78 | 4.17 | 3.97 | 0.16 | 3.22 | 0.32 | 1.77 | 0.01 |
| Avg | 1.17 | 0.66 | 0.91 | 0.05 | 2.00 | 2.96 | 2.48 | 0.18 | 1.12 | 0.50 | 0.81 | 0.00 |
Percent damage to the perirhinal cortex for the six subjects in Group Neo-PRh as estimated from pre- and post-surgery coronal MR FLAIR images: L%, percent damage to the left hemisphere; R%, percent damage to the right hemisphere; X%, average damage to both hemispheres; W%, weighted average damage to both hemispheres (W% = (L% × R%)/100); Avg, average for the entire group; ERh, entorhinal cortex; AMY, amygdala; HF, hippocampal formation; TE, visual cortex, and TH/TF, cytoarchitectonic fields of the parahippocampal gyrus as defined by von Bonin and Bailey (1947).
Finally, to compare the effects of neonatal PRh lesions to those of neonatal hippocampal lesions on VPC recognition memory, novelty preference of animals in Groups Neo-C and Neo-PRh was compared to that reported in animals that had received neonatal hippocampal lesions (Group Neo-Hibo) and were tested in the same way (Zeamer et al., 2010). MANOVAs were used for these comparisons with Groups (3) as the between subject factor, and age (4) and delay (4) as repeated within subject factor.
6. Results
6.1. Evaluation of perirhinal cortex lesion
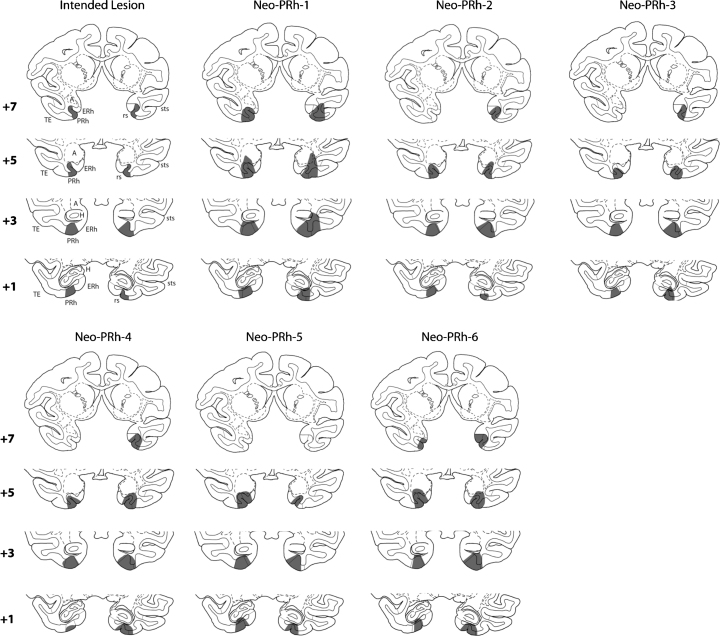
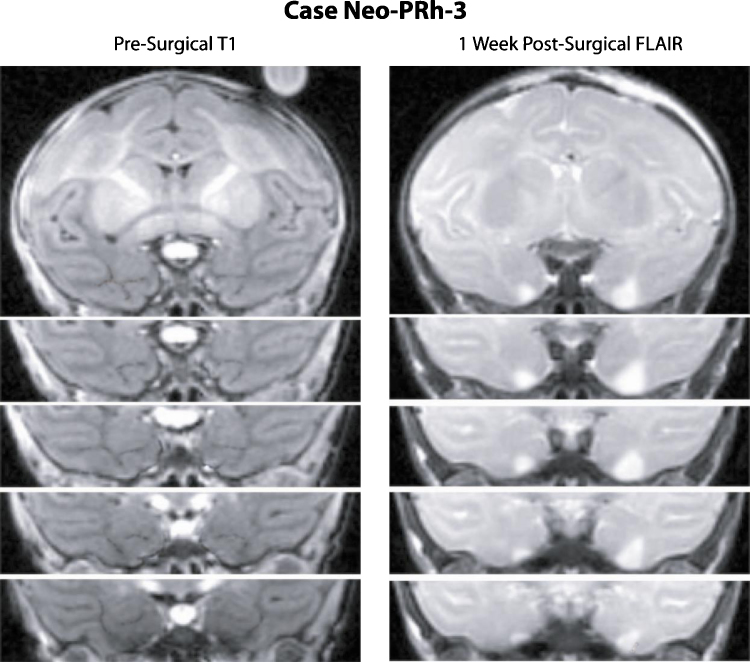
All cases received extensive bilateral PRh lesions, varying from 67.06% to 83.34% (average: 73.6%; Table 1). In addition, all cases had unintended bilateral damage to the entorhinal cortex (ERh), ranging from 5.42% to 34.49% (average: 20.57) and to area TE, ranging from 0.10% to 7.11% (average: 2.15%). Three cases, Neo-PRh-1, -2, and -6 had a small amount of damage to the amygdala (average: 2.48%) and four cases, Neo-PRh-1, -3, -5 and -6 had a minute amount of damage to the anterior hippocampus (average: 0.81%). Fig. 1 illustrates the extent of hypersignals seen on the post-surgical FLAIR for each case, plotted on matched coronal sections through a normal infant brain. A representative MR FLAIR image (Case Neo-PRh-3) is shown on Fig. 2.
Fig. 1.
Coronal sections through the hippocampal formation of a macaque brain. Left column depicts in gray the intended perirhinal lesion as reconstructed onto four anterior–posterior (top to bottom) levels. The numerals to the left of each coronal section indicate the distance in millimeters from the interaural plane. The remaining six columns depict the extent of lesion for each case in Group Neo-PRh, as estimated from 1 week post-surgical FLAIR MR images and reconstructed onto sections of a two-weeks-old macaque brain. Abbreviations: A, amygdala; ERh, entorhinal cortex; PRh, perirhinal cortex; sts, superior temporal sulcus; rs, rhinal sulcus; TE, cytoarchitectonic field as described by von Bonin and Bailey (1947).
Fig. 2.
Coronal pre-surgical T1 MR images (left column) and post-surgical FLAIR MR images (right column) through the perirhinal cortex of Case Neo-PRh-3. The white areas on the FLAIR images depict edema caused by cell damage due to the injection of ibotenic acid.
6.2. Viewing patterns
Comparisons between animals with neonatal perirhinal lesions and the sham-operated controls revealed no effects of group or delay on Total Familiarization Time or Total Retention Time. However, the effect of age was significant for Total Familiarization Time [FHuynh-Feldt(1.59, 22.3) = 17.43, p < 0.001] with both Groups Neo-C and Neo-PRh taking less time to accumulate the 30-s viewing to the stimulus at 1.5 months (1.5 vs 1.25 min, respectively) and 6 months (2.31 vs 1.06 min, respectively) than at 18 months (2.84 vs 1.96 min; ps < .008) and at 48 months (4.67 vs 5.09 min; ps < .001). A similar effect of age was detected for Total Retention Time [FHuynh-Feldt(2.16, 30.24) = 20.86, p < 0.000) with both groups looking slightly longer at the two stimuli during the retention tests at 1.5 months (7.3 vs 7.59 s, respectively) and 6 months (7.49 and 8.32 s, respectively) than at 18 months (5.99 vs 6.55 s; ps < .002) and at 48 months (5.16 vs 4.23 s; ps < .000).
6.3. Novelty preference
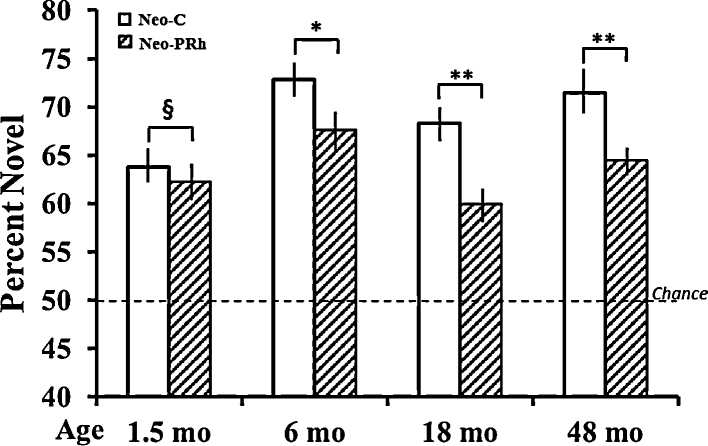
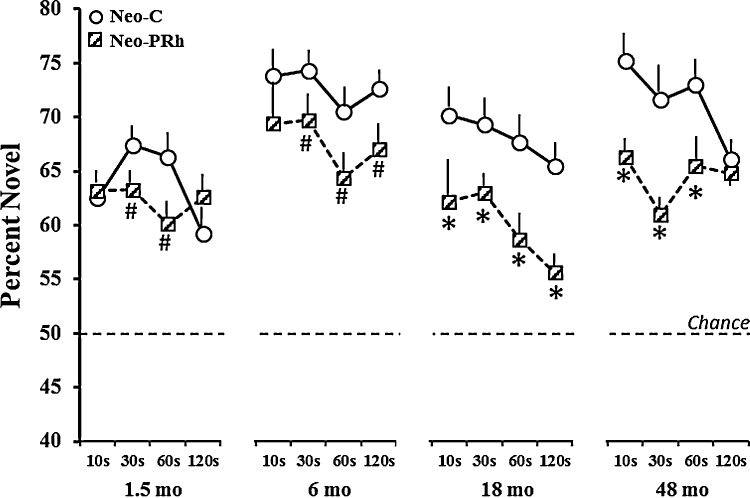
Group differences emerged for novelty preference [F(1, 14) = 11.46, p = .004], and the Group × Age interaction was also significant [FHuynh-Feldt(3, 42) = 3.09, p < .04). As shown in Fig. 3, this interaction indicated that, as for control animals, novelty preference in animals with Neo-PRh lesions became more robust from 1.5 months to 6 months (ps < .01 for both), although it worsened at later ages. Despite these changes, performance of Neo-PRh animals remained significantly better than chance at all ages and all delays (all ps < .05). Separate analyses of variance at each age (Group × Delays; see Fig. 4) revealed a significant Group by Delay interaction at 1.5 months [FHuynh-Feldt(3, 42) = 3.03, p < .05], with Group Neo-PRh obtaining scores that tended to be lower than those of Group Neo-C only at the delays of 30 s and 60 s (ps < .07). At the later ages, novelty preference in Group Neo-PRh remained significantly lower than that of Group Neo-C at all delays [Group effect: F(1, 14) = 4.3, p = 05; F(1, 14) = 9.8, p < .01; and F(1, 14) = 9.62, p < .01, for 6, 18 and 48 months, respectively). However, at these later ages, the Group × Delay interaction did not reach significance. Separate planned comparisons at all 3 ages indicated that at 6 months, Group Neo-PRh did not differ from Neo-C at the shortest delay but had scores lower at the remaining 3 delays (all ps < .06), and, at both 18 and 48 months, novelty preference scores for Group Neo-PRh were significantly different from those of Group Neo-C at all delays, except at delay of 120 s at 48 months. It is also interesting to note that, although Group Neo-C showed a delay-dependent decrease in novelty preference at both 18 and 48 months, for Group Neo-PRH this pattern was observed at 18 months but not at 48 months when their performance was similar at all delays.
Fig. 3.
Mean percent of time (±SEM) spent viewing the novel stimuli averaged over delays for Group Neo-C (white bars) and Group Neo-PRh (hatched bars) at each age. Chance is at 50%. Symbols: ** indicates group difference at p < 0.01; * indicates group difference at p < 0.05; § indicates Group × Delay, p = .04.
Fig. 4.
Mean percent of time (±SEM) spent viewing the novel stimuli for Group Neo-C (open circles, solid line) and Group Neo-PRh (hatched squares, dashed lines) across each delay at each age. Chance is at 50%. Symbols: * indicates group difference at p < 0.05; #p < .06.
Given that at 48 months, all perirhinal-operated animals and four of the controls were also tested with a shorter delay of 1 s, we also compared the two groups across the 5 delays (1 s, 10 s, 30 s, 60 s, and 120 s). Again, the group effect was significant [F(1, 8) = 7.98, p = .02] but the group × delay interaction was not [F(4, 32) = 1.59, ns]. Planned comparisons at each delay indicated no group differences at both the shortest delay of 1 s (Neo-C: 72.7 ± 3.2; Neo-PRh: 65.1 ± 2.03; t(8) = 1.78, ns) and the longest delay of 120 s (Neo-C: 66.06 ± 2.7; Neo-PRh: 64.8 ± 1.6; t(8) = 0.42, ns), but did differ significantly at the intermediate delays of 10–60 s (all p < .05).
For animals with Neo-PRh lesions, extent of the perirhinal lesions did not correlate with performance, except at 1.5 months at 10 s delay (p = .03) and at 48 months at 60 s delay (p = .03). Furthermore, no correlations between performance and unintended damage to adjacent structures reached significance at any age and any delay (all ps > .05).
6.4. Comparison with neonatal hippocampal lesions
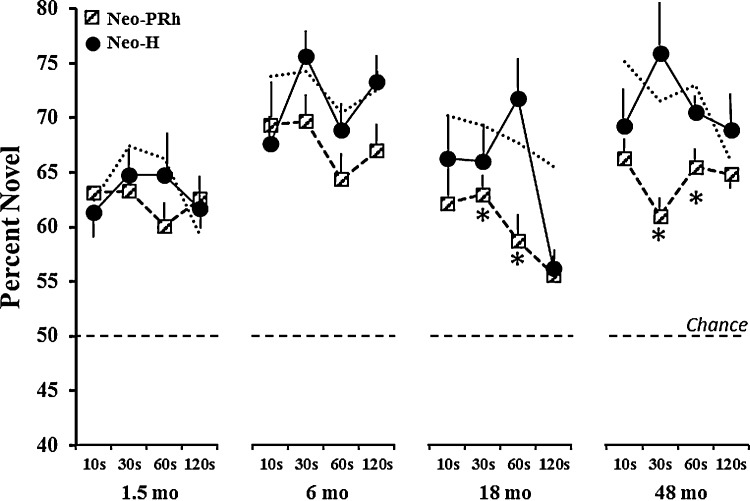
Novelty preference from animals with neonatal lesions of the hippocampus (Neo-Hibo) tested under the same conditions (Zeamer et al., 2010) was used for comparison with novelty preference of animals with Neo-PRh lesions (see Fig. 5). There was an overall effect of age [F(3, 27) = 13.29, p < 0.001], with both groups showing stronger novelty preference from 1 to 6 months of age (ps < .05). In addition, there was a significant Group effect [F(1, 9) = 8.50, p < .02] and a significant Group × Age × Delay interaction [F(9, 81) = 2.19, p = .03). This triple interaction indicates that the two groups had patterns of performance that differed across delays and across ages (see Fig. 5). Further analysis within each age revealed that at 1.5 and 6 months, the two groups did not differ, although only Group Neo-PRh differed from controls (see Fig. 4). However, at 18 months, Group Neo-PRh showed weaker novelty preference than Group Neo-Hibo [Group × Delays interaction: F(3, 27) = 4.75, p < 0.01]. Importantly, at this juvenile age, Group Neo-PRh differed from Group Neo-Hibo at the 30 s and 60 s delays (ps < .05), but not at the longest delay of 120 s when both groups were impaired as compared to controls. Finally, upon reaching adulthood, although Group Neo-Hibo performed similarly to controls, Group Neo-PRh had novelty preference scores lower than those of Group Neo-Hibo at all delays, but the shortest delay of 10 s and the longest delay of 120 s [Group effect: F(1, 9) = 8.63, p < .02].
Fig. 5.
Mean percent of time (±SEM) spent viewing the novel stimuli for Group Neo-PRh (hatched squares, dashed line) and Group Neo-H-ibo (filled circles, solid line) across each delay at each age. Chance is at 50%. Symbols: * indicates group difference at p < 0.05 and thin dashed line illustrates data from Group Neo-C for comparison.
7. Discussion
Although the contribution of the perirhinal cortex to recognition memory processes has been well documented in adults (for review see Graham et al., 2010, Winters et al., 2008), the present data demonstrate for the first time that this medial temporal cortical area is critical for the normal development of recognition memory in primates. Lesions of the perirhinal cortex in infant monkeys impacted recognition memory at the earliest age of 1.5 months and this impairment became more severe as the animals reached adolescence and adulthood. Interestingly, despite this novelty preference deficit, animals with Neo-PRh lesions maintained a delay-dependent performance similar to the controls at 18 months. By contrast, upon reaching adulthood, this delay-dependent effect was absent since their novelty preference remained constant across delays. Furthermore, the recognition memory impairment found after neonatal perirhinal lesions was less in magnitude than that reported when the same lesions are incurred in adulthood (Buffalo et al., 1999, Nemanic et al., 2004), suggesting the presence of significant functional sparing. Finally, the present findings also demonstrated that the recognition memory deficit following the neonatal perirhinal lesions occurred at younger ages and at shorter delays than the recognition memory deficit found after neonatal hippocampal lesions, indicating that, in the absence of a functional hippocampus in early infancy (1.5 and more specifically at 6 months), the perirhinal cortex could support recognition memory even when long delays are used.
7.1. Recognition memory and neonatal perirhinal cortex lesion
Neonatal lesions of the perirhinal cortex resulted in significant decreases in novelty preference that became more profound with maturation. Thus, at 1.5 months, Group Neo-PRh differed slightly, but significantly, from Group Neo-C at delays of 30 s and 60 s. By 6 months of age their performance increased as it did in controls, but at this young age novelty preference became significantly weaker at all delays, except the shortest delay of 10 s. As adolescents at 18 months, novelty preference in animals with Neo-PRh lesions worsened and showed a pattern of steady decline from the shorter (10 s) to the longer (120 s) delays (see Fig. 4). Finally, as adults at 48 months, performance of animals with Neo-PRh lesions differed from that of controls at all delays but the shortest (1 s) and longest (120 s) delays.
The cause of this decrease in novelty preference cannot be associated with perceptual deficits, ascribed to animals with adult-onset perirhinal lesions (see for reviews Graham et al., 2010, Winters et al., 2008), given that the objects were easily discriminable, and that for short delays, Neo-PRh animals obtained scores similar to controls. In addition, the decrease in performance cannot simply due to lack of motivation to look at the images on the screen since there were no significant differences between Groups Neo-PRh and Neo-C in the amount of time taken to accumulate the 30 s familiarization time or to look at the two stimuli in the retention tests. Similarly, these recognition memory deficits of Neo-PRh animals cannot be associated with the mild unintended damage to the entorhinal cortex observed in some of the animals (range: 5.4–34.5%, Table 1), given that the correlations between subjects’ novelty preference scores and amount of entorhinal cortex damage did not reach significance. Therefore, the findings suggest that the perirhinal cortex is critical to support incidental memory processes early in development. This conclusion is consistent with anatomical development of the medial temporal cortical areas indicating that at birth the perirhinal cortex can be clearly identified cytoarchitecturally and displays adult-like chemo-anatomical characteristics (Berger and Alvarez, 1994).
7.2. Early-onset vs late-onset perirhinal lesions
Despite significant recognition memory deficits, novelty preference in animals with neonatal perirhinal lesions was not totally abolished as the animals obtained scores significantly above chance at all ages and all delays. These findings sharply contrast with the lack of novelty preference found in monkeys with adult-onset perirhinal lesions at delays as short as 10 s (Buffalo et al., 1999, Nemanic et al., 2004). One possible explanation for these divergent outcomes could be related to differences in the extent of the perirhinal lesions. Yet, the extent of perirhinal lesions in the adult animals of the two previous studies averaged 87% (Nemanic et al., 2004) and 74% (Buffalo et al., 1999), and was comparable to that found in animals with neonatal perirhinal lesions (average: 74%). Further, there were no correlations between novelty preference scores and lesion size, suggesting that the weaker performance exhibited by monkeys with neonatal perirhinal lesions as compared to controls cannot simply be attributed to a difference in lesion size. Consequently, neonatal perirhinal lesions appear to have resulted in a significant functional sparing indicating that the neural substrate mediating incidental recognition memory processes seems more widespread in early infancy than in adulthood. This sparing of incidental recognition memory is likely linked to immaturity of the medial temporal structures at the time of the neonatal perirhinal lesions (Alvarado and Bachevalier, 2000, Jabès et al., 2010, Jabès et al., 2011, Lavenex et al., 2007). Although several of these medial temporal lobe structures could support incidental recognition memory in the absence of a functional perirhinal cortex, there exists evidence for the presence of an early development of direct connections between entorhinal cortex and CA1 fields of the hippocampus (Berger et al., 1993, Berger and Alvarez, 1994, Jabès et al., 2010, Jabès et al., 2011, Lavenex and Banta-Lavenex, 2013, Amaral et al., 2014), which was intact in these subjects and which could by-pass the traditional trisynaptic hippocampal circuit (entorhinal-dentate-CA3-CA2-CA1-subiculum) and support incidental recognition memory abilities. Another explanation for this functional sparing could be related to the significant refinement of projections from ventral visual areas, such as area TEO, to medial temporal-lobe structures. Our earlier anatomical work has demonstrated that visual inputs from area TEO to the perirhinal and parahippocampal cortex are considerably widespread in infancy and refined their distribution with maturation (Webster et al., 1991). Furthermore, considerable reorganization of these connections as well as maintenance of some of these transient connections occurs following neonatal damage to anterior temporal cortical areas, such as area TE (Webster et al., 1991).
7.3. Comparison with neonatal hippocampal lesions
As with the comparison to sham-operated controls, animals with neonatal perirhinal cortex lesions showed weaker preference for novelty than animals with neonatal hippocampal lesions at both 6, 18 and 48 months of age when tested with the same stimuli and under the same testing conditions. Because there were no differences in rearing or testing conditions between the two groups, all differences in preference for novelty can be attributed to the specific structures involved in the neonatal lesions. There were two important differences between the outcomes following the two types of lesions. First, the neonatal perirhinal lesions affected performance as early as 1.5 months of age and more importantly at 6 months indicating that at these earlier ages the perirhinal cortex, and presumably other medial temporal cortical areas, may support incidental recognition memory processes. Second, although the neonatal hippocampal lesions impacted recognition memory only at the longest delay, the neonatal perirhinal cortex lesions altered performance at shorter delays. This finding provides further support to a growing consensus that of all the medial temporal lobe structures, the perirhinal cortex is the cortical area the most critical for object recognition memory (for review see Bachevalier et al., 2002, Brown and Aggleton, 2001, Eichenbaum et al., 2007, Graham et al., 2010, Murray et al., 2007, Squire et al., 2007, Winters et al., 2008). The current findings further support such a distinction between the contributions of these two medial temporal lobe structures and extend this distinction to the development of recognition memory processes.
7.4. Relationship to developmental amnesia in humans
Patients suffering from developmental amnesia due to early hypoxic hippocampal damage show relatively intact recognition memory as assessed by Yes/No recognition tasks (see for review De Haan et al., 2006, Adlam et al., 2009). Yet, when tested in the VPC task in early adulthood, they display a significant loss of novelty preference indicative of a substantial deficit in incidental recognition memory (Munoz et al., 2011). When comparing the pattern of novelty preference deficits in these human cases to that described in monkeys with neonatal perirhinal lesions (this study) or neonatal hippocampal lesions (Zeamer and Bachevalier, 2013), it appears that the delay-dependent loss of novelty preference (intact performance at the short delays but memory loss at the long delays) in developmental amnesiacs shares greater similarity with the delay-dependent loss of novelty preference in animals with neonatal hippocampal lesions than the loss of novelty preference even at short delays in the case of animals with perirhinal lesions. Thus, the developmental data in monkeys with selective neonatal lesions suggest that the recognition memory loss in cases of developmental amnesia appears to be related to damage to the hippocampus rather than damage to the perirhinal cortex and is consistent with the absence of changes in the perirhinal cortex reported in these human cases (Vargha-Khadem et al., 2003).
Conflict of interest statement
None of the contributing authors have any conflicts of interest in the conduct or reporting of this research.
Acknowledgements
This work was supported by the National Institute of Mental Health (MH-58846), and the National Center for Research Resources P51RR165, currently supported by the Office of Research Infrastructure Programs/ODP51OD11132. We thank the veterinary and animal husbandry staff at the Yerkes National Primate Research Center for expert animal care, the imaging core facility for the care and handling of animals during the MR imaging procedures, and Bronwyn Briseno, Rebecca Grizzard, Amy Mahan, and Anthony Gazy for assistance with the testing of the animals and scoring the videotapes. We also thank Maria Alvarado for helpful comments on an earlier version of this manuscript.
Footnotes
Available online 21 July 2014
Contributor Information
Alyson Zeamer, Email: alyson.zeamer@utsa.edu.
Jocelyne Bachevalier, Email: jbachev@emory.edu.
References
- Adlam A.-L.R., Malloy M., Mishkin M., Vargah-Khadem F. Dissociation between recognition and recall in developmental amnesia. Neuropsychologia. 2009;47:2207–2210. doi: 10.1016/j.neuropsychologia.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado M.C., Bachevalier J. Revisiting the maturation of medial temporal lobe memory functions in primates. Learn. Mem. 2000;7:244–256. doi: 10.1101/lm.35100. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Kondo H., Lavenex P. An analysis of entorhinal cortex projections to the dentate gyrus, hippocampus, and subiculum of the neonatal macaque monkeys. J. Comp. Neurol. 2014;522:485–505. doi: 10.1002/cne.23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J., Brickson M., Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., Nemanic S., Alvarado M.C. The medial temporal lobe structures and object recognition memory in nonhuman primates. In: Squire L.R., Schacter D.L., editors. Neuropsychology of Memory. Guilford Press; New York: 2002. pp. 326–338. [Google Scholar]
- Baxter M.G., Murray E.A. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Berger B., Alvarez C. Neurochemical development of the hippocampal region in the fetal rhesus monkey II. Immunocytochemistry of peptides, calcium-binding proteins, DARPP-32, and monoamine innervation in the entorhinal cortex by the end of gestation. Hippocampus. 1994;4:84–114. doi: 10.1002/hipo.450040111. [DOI] [PubMed] [Google Scholar]
- Berger B., Alvarez C., Goldman-Rakic P.S. Neurochemical development of the hippocampal region in the fetal rhesus monkey I. Early appearance of peptides, calcium-binding proteins, DARPP-32 and the monoamine innervation in the entorhinal cortex during the first half of gestation (E47 to 90) Hippocampus. 1993;3:279–305. doi: 10.1002/hipo.450030305. [DOI] [PubMed] [Google Scholar]
- Bowles B., Crupi C., Mirsattari S.M., Pigott S.E., Parrent A.G., Pruessner J.C. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.W., Aggleton J.P. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buffalo E.A., Ramus S.J., Clark R.E., Teng E., Squire L.R., Zola S.M. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn. Mem. 1999;6:572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo E.A., Ramus S.J., Squire L.R., Zola S.M. Perception and recognition memory in monkeys following lesions of area TE and perirhinal cortex. Learn. Mem. 2000;7:375–382. doi: 10.1101/lm.32100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan M., Mishkin M., Baldeweg T., Vargha-Khadem F. Human memory development and its dysfunction after early hippocampal injury. TINS. 2006;29:374–381. doi: 10.1016/j.tins.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Danckert S.L., Gati J.S., Menon R.S., Kohler S. Perirhinal and hippocampal contributions to visual recognition memory can be distinguished from those of occipito-temporal structures based on conscious awareness of prior occurrence. Hippocampus. 2007;17:1081–1092. doi: 10.1002/hipo.20347. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context, and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Diamond A. Evidence of robust recognition memory early in life even when assessed by reaching behavior. J. Exp. Child Psychol. 1995;59:419–456. doi: 10.1006/jecp.1995.1020. [DOI] [PubMed] [Google Scholar]
- Diana R.A., Yonelinas A.P., Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Yonelinas A.P., Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J.F., III Memory in the infant. J. Exp. Child Psychol. 1970;9:217–226. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- Gaffan D., Murray E.A. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertribal intervals and fail at matching to sample despite double sample presentations. Behav. Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Goursaud A.P., Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala and orbital frontal cortex. Behav. Brain Res. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Graham K.S., Barense M.D., Lee A.C.H. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Gunderson V.M., Sackett G.P. Development of pattern recognition in infant pigtailed macaques (Macaca nemestrina) Dev. Psychol. 1984;20:418–426. [Google Scholar]
- Hadfield W.S., Baxter M.G., Murray E.A. Effects of combined and separate removals of rostral dorsal superior temporal sulcus cortex and perirhinal cortex on visual recognition memory in rhesus monkeys. J. Neurophysiol. 2003;90:2419–2427. doi: 10.1152/jn.00290.2003. [DOI] [PubMed] [Google Scholar]
- Jabès A., Lavenex P.B., Amaral D.G., Lavenex P. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J. Comp. Neurol. 2010;519:1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabès A., Lavenex P.B., Amaral D.G., Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur. J. Neurosci. 2011;31:273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P., Banta-Lavenex P.B. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav. Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lavenex P., Lavenex P.B., Amaral D.G. Postnatal development of the primate hippocampal formation. Dev. Neurobiol. 2007;29:179–192. doi: 10.1159/000096222. [DOI] [PubMed] [Google Scholar]
- Málková L., Lex C.K., Mishkin M., Saunders R.C. MRI-based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Meunier M., Bachevalier J., Mishkin M., Murray E.A. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J. Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D., Mayes A.R. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Munoz M., Chadwick M., Perez-Hernandez E., Vargha-Khadem F., Mishkin M. Novelty preference in patients with developmental amnesia. Hippocampus. 2011;21:1268–1276. doi: 10.1002/hipo.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E.A., Bussey T.J., Saksida L.M. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu. Rev. Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Nemanic S., Alvarado M.C., Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J. Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S., Alvarado M.C., Price R.E., Jackson E.F., Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J. Neurosci. Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Pascalis O., Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9:609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pascalis O., de Schonen S. Recognition memory in 3- to 4-day-old human neonates. Neuroreport. 1994;5:1721–1724. doi: 10.1097/00001756-199409080-00008. [DOI] [PubMed] [Google Scholar]
- Payne C., Machado C.J., Bliwise N., Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric MRI study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedhazur E.J. second ed. Holt, Rinehart and Winston; New York: 1982. Multiple Regression in Behavioral Research: Explanation and Prediction. [Google Scholar]
- Pihlajamaki M., Tanila H., Kononen M., Hanninen T., Hamalainen A., Soininen H. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur. J. Neurosci. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Ramsøy T.Z., Liptrot M.G., Skimmage A., Lund T.E., Sidaros K., Christensen M. Regional activation of the human medial temporal lobe during intentional encoding of objects and positions. Neuroimage. 2009;47:1863–1872. doi: 10.1016/j.neuroimage.2009.03.082. [DOI] [PubMed] [Google Scholar]
- Squire L.R., Wixted J.T., Clark R.E. Recognition memory and the medial temporal lobe: a new perspective. Nat. Rev. Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R.J., Rudy J.W. Configural association theory: the role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- Vargha-Khadem F., Salmond C.H., Watkins K.E., Friston K.J., Gadian D.G., Mishkin M. Developmental amnesia: effects of age at injury. PNAS. 2003;100:10055–10060. doi: 10.1073/pnas.1233756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonin G., Bailey P. University of Illinois Press; Urbana, IL: 1947. The Neocortex of Macaca mulatta. [Google Scholar]
- Wan H., Aggleton J.P., Brown M.W. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J. Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M.J., Ungerleider L.G., Bachevalier J. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. J. Neurosci. 1991;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters B.D., Saksida L.M., Bussey T.J. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci. Biobehav. Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Zeamer A.E., Bachevalier J. 39th Annual Meeting Society for Neuroscience Abstracts. 2009. Neonatal lesions of the perirhinal cortex alter the development of object recognition memory abilities in monkeys. 98.6. [Google Scholar]
- Zeamer A., Bachevalier J. Long-term effects of neonatal hippocampal lesions on novelty preference in monkeys. Hippocampus. 2013;23:745–750. doi: 10.1002/hipo.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A.E., Alvarado M.C., Bachevalier J. Development of medial temporal lobe memory processes in non-human primates. In: Blumberg M., Freeman J., Robinson S., editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford University Press; New-York: 2009. pp. 607–616. [Google Scholar]
- Zeamer A.E., Heuer E., Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus monkeys with and without neonatal hippocampal lesions. J. Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L.R., Amaral D.G., Suzuki W.A. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J. Neurosci. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]