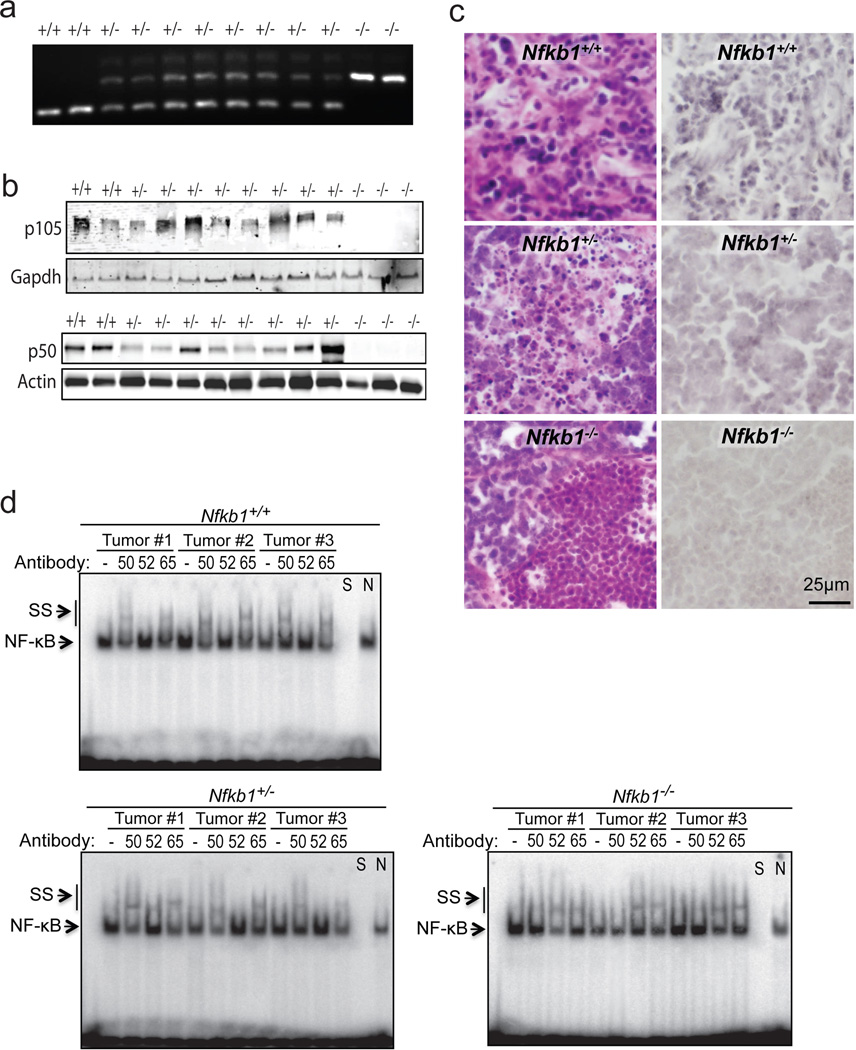
Figure 3.
Thymic tumors from Nfkb1+/− animals retain heterozygosity. (a) Tumors from Nfkb1−/−, Nfkb1+/− and Nfkb1+/+ mice were examined by pcr using genotype specific primers. (b) Immunoblot of tumor lysate from the indicated mice with anti-p105 (C-terminal) antibody (upper) and anti-p50 antibody (lower). (c) Immunohistochemical analysis, using anti-p50 antibody, of tumors harvested from Nfkb1+/+, Nfkb1+/− and Nfkb1−/− mice. Hematoxylin and eosin sections from the same tumors are also shown (left). (d) EMSA using the Ig-κB probe of tumor lysates from three separate tumors from mice of each genotype. Supershift (SS) was performed with anti-p50 (50), anti-p52 (52) or anti-p65 (65) antibody where shown. To confirm the band specificity, competition with specific (S) and nonspecific (N) DNA was performed as indicated.
Genotyping was performed using snap frozen tumor samples following thawing and lysis at 55°C for 18 hours. Nucleic acid was extracted and DNA amplified by PCR using primers described for Nfkb1−/− animals (Jackson Laboratory). Amplified DNA was run through 2 % agarose gel. For tumor protein, snap frozen samples were thawed, homogenized in 700 ml RIPA lysis buffer and incubated on ice for 5 minutes. Subsequently, samples were centrifuged (14000g × 5 minutes) and the supernatant used for immunobloting and EMSA. Immunoblots were performed with anti-p105 C-terminal (#4717, Cell Signaling Technologies), anti-p50 (sc-8414, Santa Cruz Biotechnology), anti-actin (sc-7210) or anti-gapdh (sc-137179) antibodies. EMSA was performed as previously described11 using a probe bearing the decameric Ig-κB consensus sequence (5’-GGGACTTTCC-3’). Supershift was performed by pre-incubation with anti-p50 (sc-1190x), anti-p52 (sc-298x) or anti-p65 (sc-8008) antibodies for 30 min on ice.