Abstract
Video filmed by a camera mounted on the head of a Northern Goshawk (Accipiter gentilis) was used to study how the raptor used visual guidance to pursue prey and land on perches. A combination of novel image analysis methods and numerical simulations of mathematical pursuit models was used to determine the goshawk's pursuit strategy. The goshawk flew to intercept targets by fixing the prey at a constant visual angle, using classical pursuit for stationary prey, lures or perches, and usually using constant absolute target direction (CATD) for moving prey. Visual fixation was better maintained along the horizontal than vertical direction. In some cases, we observed oscillations in the visual fix on the prey, suggesting that the goshawk used finite-feedback steering. Video filmed from the ground gave similar results. In most cases, it showed goshawks intercepting prey using a trajectory consistent with CATD, then turning rapidly to attack by classical pursuit; in a few cases, it showed them using curving non-CATD trajectories. Analysis of the prey's evasive tactics indicated that only sharp sideways turns caused the goshawk to lose visual fixation on the prey, supporting a sensory basis for the surprising frequency and effectiveness of this tactic found by previous studies. The dynamics of the prey's looming image also suggested that the goshawk used a tau-based interception strategy. We interpret these results in the context of a concise review of pursuit–evasion in biology, and conjecture that some prey deimatic ‘startle’ displays may exploit tau-based interception.
KEY WORDS: Pursuit–evasion, Predator–prey, Avian vision, Northern Goshawk, Visual guidance, Sensory ecology, Accipiter gentilis, Looming, Antipredator behavior, Startle effect
INTRODUCTION
Many problems in the study of animal behavior require an integrated biomechanical and sensory ecology approach that considers the organism's locomotor and perceptual capabilities, its sensory cues and the dynamics of its behavioral responses (Fernández-Juricic, 2012; Martin, 2012). Animal-borne video methods (Rutz and Troscianko, 2013) now make it possible to measure the visual cues received by organisms in the field, revealing new information about, for example, how they move through their environment (BBC, 2009), interact with conspecifics (Takahashi et al., 2004) and use tools (Rutz et al., 2007). Recent studies have used the stable video recorded by cameras mounted on the heads of birds (headcams) to explore the visual strategies used by falcons chasing prey (Kane and Zamani, 2014) and peafowl detecting model predators (Yorzinski and Platt, 2014). Here, we report using headcam video to explore for the first time pursuit–evasion and landing behavior in the Northern Goshawk, Accipiter gentilis (Linnaeus 1758) (hereafter, goshawk), a large diurnal raptor (Fig. 1). Biologically derived models have inspired robotic algorithms for swarming, following and collision avoidance (Mischiati and Krishnaprasad, 2012; Srinivasan, 2011), and the goshawk is of special interest in this context because it can maneuver at high speed through cluttered environments (Sebesta and Baillieul, 2012). In this study, headcam video was interpreted using new optical flow-based image analysis methods that enabled us to determine which specific visual guidance and pursuit strategies the raptor used, while video filmed from the ground provided complementary information on spatial trajectories. We also studied how effective prey-evasion tactics were at thwarting goshawk visual fixation.
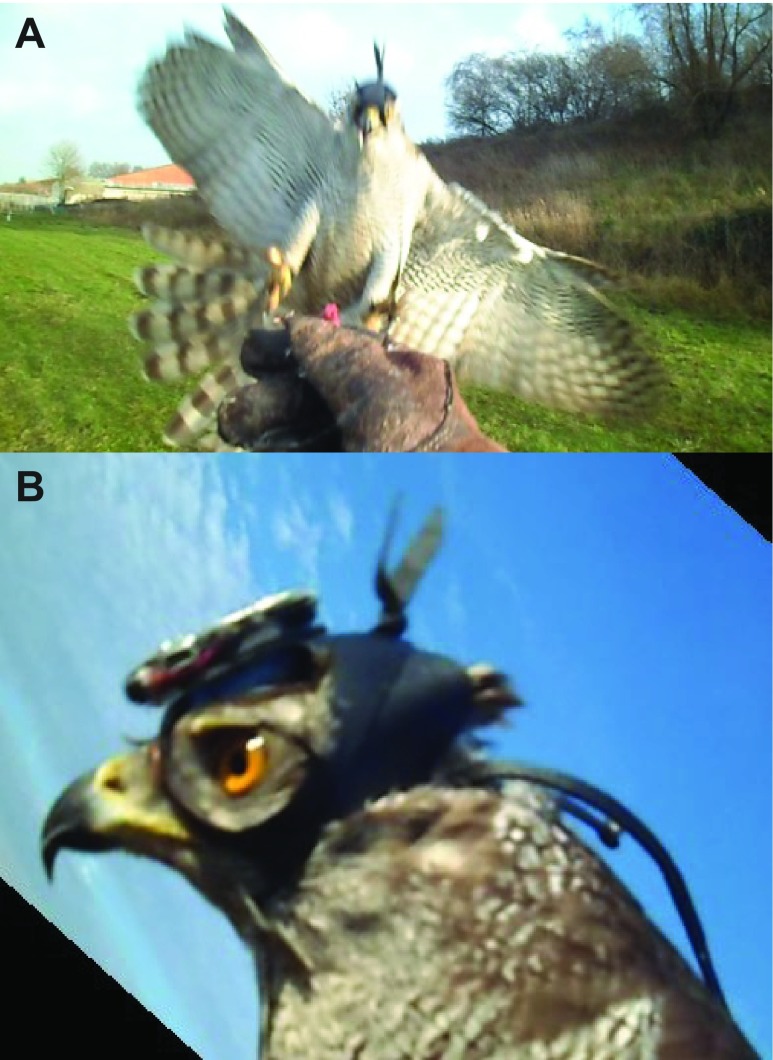
Fig. 1.
Goshawk with head-camera. (A) Northern Goshawk wearing the hood with integral video camera landing on the falconer's glove, showing how the bird pitches upward, extends its feet and sometimes rolls slightly in impact posture. [Typical goshawk wingspan 98–115 cm (Squires and Reynolds, 1997).] (B) Goshawk wearing the head-camera (headcam) showing its orientation pitched downward relative to the head axis. Image credit: Robert Musters.
Pursuit–evasion in biology
Before reviewing pursuit–evasion strategies and their appearance on headcam video, we consider the basic geometry of headcam images for a predator moving at constant velocity, vp, with its head axis along vp (Fig. 2A). The resulting optical flow field radiates outward from the predator's center of motion (Lee and Kalmus, 1980). Possible images of moving and stationary prey are also shown in this figure; the prey's position is described using horizontal and vertical camera angles (θ and χ) that map on to the goshawk visual angles. We can define an angle, γ, to characterize the orientation of the prey's projected velocity relative to the local optical flow. For γ=0 deg, the prey moves opposite to the optical flow toward the center of motion, which is equivalent to being on a collision course.
Fig. 2.
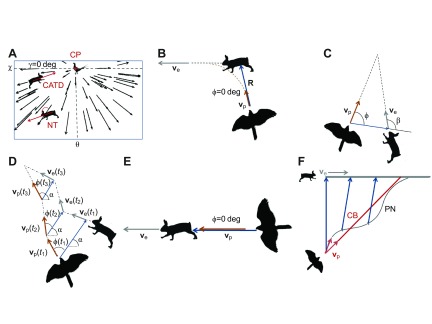
Schematic illustration of headcam images and trajectories for various pursuit–evasion strategies. (A) Schematic headcam image, showing the orientation of the horizontal and vertical camera and visual angles (θ, χ). Black arrows indicate the optical flow due to self-motion of the predator at constant velocity. The intersection of the dashed lines indicates the center of motion at (0 deg, 0 deg). An angle, γ, defines the orientation of the prey's velocity (red arrows) with respect to the local optical flow field. Labels indicate a rabbit not tracked (NT) by interception strategies (γ≠0 deg), a pheasant tracked by classical pursuit (CP) and a rabbit tracked by constant absolute target direction (CATD; γ=0 deg). Earth-frame trajectories for each pursuit strategy are depicted as follows: (B) CP; (C) the geometry for the constant bearing (CB) criterion used in CATD; (D) CATD; (E) CPE; (F) finite-feedback implementations of proportional navigation (PN) in which the predator's trajectory (gray curve) can oscillate around the optimal bearing angle trajectory (CB, red curve). The baseline vector, R, is shown as a blue arrow. ve, prey velocity; vp, constant predator velocity; ϕ, bearing angle between R and vp; β, angle between ve and R; α, the constant angle of R relative to the fixed Earth frame. D is adapted from Ghose et al. (Ghose et al., 2006); F is adapted from Shaw (Shaw, 1985).
The time-optimal pursuit strategy for stationary prey is classical pursuit (CP), in which the predator's velocity always points toward the prey (Nahin, 2012) so the prey's image remains stationary at the center of motion (Fig. 2A). However, in general CP is inefficient when the prey moves (Fig. 2B). CP has been observed for bees (Zhang et al., 1990), flies (Land, 1993; Trischler et al., 2010), beetles (Gilbert, 1997) and bats following conspecifics (Chiu et al., 2010) and chasing slow prey (Kalko and Schnitzler, 1998).
To explain interception, in which the predator moves toward the prey's estimated future location (Collett and Land, 1978; Lanchester and Mark, 1975), it is useful to define the baseline vector, R, pointing from predator to prey, the bearing angle, ϕ, between R and vp, and the angle β between prey velocity ve and R (Fig. 2C). For constant ve and |vp|≥|ve|sinβ, time-optimal interception is possible if the predator maintains its bearing angle at:
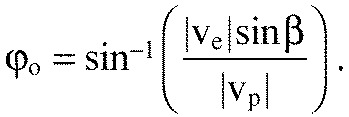 |
(1) |
This is called the constant bearing decreasing range (CB) criterion (Nahin, 2012). If the prey maneuvers infrequently, then the CB criterion can be applied during each constant velocity interval. This strategy is called constant absolute target direction (CATD) because
List of symbols and abbreviations
- a
relative goshawk–target linear acceleration (m s−2)
- CATD
constant absolute target direction
- CB
constant bearing decreasing range
- CP
classical pursuit
- CPE
classical pursuit–evasion
- DP
deviated pursuit
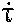
tau-dot
- f
focal length (pixels)
- I
prey image size (pixels)
- O
prey size (m)
- PN
proportional navigation
- R
baseline vector (m)
- s
on-screen trajectory arc length (pixels)
- Tp
time when goshawk assumes impact posture (s)
- v
relative goshawk–target speed (m s−1)
- ve
prey velocity (m s−1)
- vp
predator velocity (m s−1)
- Z
goshawk–target distance (m)
- Zmin
minimum Z (m)
- Zp
Z when goshawk assumes impact posture (m)
- β
baseline–prey velocity angle (deg)
- γ
prey velocity angle on image (deg)
- Δt
simulation timestep (s)
- θ
image horizontal angle (deg)
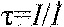
tau (s)
- ϕ
bearing angle (deg)
- ϕo
optimal bearing angle (deg)
- χ
image vertical angle (deg)
ϕ varies but R has a constant orientation in the Earth frame, as indicated by the angle α in Fig. 2D. (Ghose et al., 2006; Reddy et al., 2006). The predator can implement CATD by maneuvering to keep the prey's image at constant visual angle (determined by the instantaneous value of ϕo) for fixed predator head orientation (CATD in Fig. 2A). Deviations in ϕ from the ϕo set point serve as the predator's control signal, stimulating it to accelerate to compensate. For stationary prey (|ve|=0), Eqn 1 gives ϕo=0, and CATD reduces to CP. For classical evasion, i.e. the prey flees directly away from the predator (β=0 deg), Eqn 1 gives ϕo=0 and CATD reduces to classical pursuit–evasion (CPE) (Fig. 2E).
CATD is used by dragonflies (Combes et al., 2012; Olberg, 2012), bats (Ghose et al., 2006; Ghose et al., 2009) and humans (Fajen and Warren, 2004). CATD is a motion camouflage strategy (Justh and Krishnaprasad, 2006; Reddy et al., 2006) because the predator perceives no prey motion on its visual field and vice versa; the only motion cue is looming (retinal expansion) (Reddy et al., 2007). However, camouflage of the predator's motion on the prey's visual field may be a by-product of its sensory implementation, given that data are mixed on whether prey respond more strongly to predators on a tangential approach or collision course (Fernández-Juricic et al., 2005; Stankowich and Blumstein, 2005).
Proof of CATD can be determined from empirical data in several ways. The constant orientation of R in 3D predator and prey trajectories is a definitive test for CATD. In predator headcam video, CATD can be demonstrated if two requirements for a collision course are met: constant prey visual angle and γ=0 deg (CATD in Fig. 2A). Deviated pursuit (DP) is the more general case of constant prey visual angle for ϕ≠ϕo and γ≠ deg, so it results in curved predator trajectories even for prey that remain motionless or move at constant velocity (Shima, 2007), as observed for flies tracking fixed targets (Osorio et al., 1990).
These strategies can be implemented by feedback-based steering laws like proportional navigation (PN) (Shaw, 1985). High-gain feedback is prone to instabilities due to sensorimotor delays (Reddy et al., 2007), and bats and insects use finite feedback implementations with a time delay between evasive maneuvers and predator responses (Ghose et al., 2006; Land, 1993; Srinivasan and Zhang, 2004). Depending on the feedback constant and prey motion, the prey's velocity and visual angles on the headcam image can oscillate about a set point (Fig. 2F), or PN can result in a gradually arcing predator trajectory qualitatively similar to those seen for CP and DP.
As few studies of pursuit strategies have been conducted for birds, we surveyed the literature for evidence not interpreted in this context. A few field studies (Angell, 1969; Curio, 1976) have reported observing birds using interception to catch prey. Diving kingfishers compensate for refraction to aim directly at their prey underwater, consistent with CP, in ground (Katzir, 1994) and headcam video (EarthRangers, 2011). Gannets make V-shaped dives consistent with CP as well as underwater chases with active propulsion that likely use visual guidance, leaving bubble trails suitable for 3D tracking (Machovsky-Capuska et al., 2012). Animal-borne video of penguins pursuing krill shows the prey image close to the forward direction (Watanabe and Takahashi, 2013). Barn owls view salient objects at the center of their visual field (Ohayon et al., 2008) and fly directly toward stationary prey (Ilany and Eilam, 2008), but gaze alternately toward moving voles and a prospective interception point (Fux and Eilam, 2009).
Because falcons are bifoveate like other diurnal raptors, Tucker et al. proposed that these birds might pursue prey using DP at their deep foveal 45 deg visual angle; however, the curving trajectories they reported for stooping falcons were not compared quantitatively to models (Tucker et al., 2000). We have used headcam video to determine that falcons pursuing flying birds most often fixed the prey at a constant visual angle within the forward binocular range (Kane and Zamani, 2014); the fixation angles varied significantly between chases and changed when the prey maneuvered, consistent with CATD but not DP at a preferred foveal angle. Optical flow tracking was usually not possible because of the featureless background (the sky) and constantly maneuvering prey, so we were able to confirm CATD in only a few cases, such as that shown in Fig. 3. A recent article used high-speed 3D video to study the aerodynamics of peregrine falcons stooping toward stationary lures along the face of a tall dam (Ponitz et al., 2014). This work showed that falcons kept their heads oriented close to the forward direction and flew relatively directly toward the prey along an inclined glide path that leveled out (and in one case swerved slightly) near impact. Thus, although this study did not analyze for visual or pursuit strategies, the falcon presumably would see its prey in its upper binocular visual field until shortly before impact. By contrast, a high-speed stereometric 3D video study of cliff swallows engaged in complex tandem flights with conspecifics found no evidence that the birds used any specific visual strategies (Shelton et al., 2014).
Fig. 3.
Headcam image filmed by a falcon pursuing a crow (red tracks) that was fixed at a constant visual angle before it was intercepted by a second falcon (upper left). The falcon wearing the headcam flew at constant velocity, as shown by the constant center of motion (indicated by a distant background object tracked in blue), confirming use of CATD. Solid circles indicate initial positions. Image credit: Eddy de Mol and Francois Lorrain.
The antipredator response of prey has presumably been shaped by relevant predator pursuit strategies. For example, the evasive behavior observed for rodents under attack by barn owls has been proposed as an adaptive response to interception (Edut and Eilam, 2004). Studies of owls attacking simulated prey showed that sideways evasion was far more effective than dodging (turning toward the predator), and that both were significantly more effective than classical evasion (Ilany and Eilam, 2008; Shifferman and Eilam, 2004). Prey evasion by dodging, swerving and steep take-offs has been explained theoretically as taking advantage of limited predator maneuverability and response time (Hedenström and Rosén, 2001; Howland, 1974; van den Hout et al., 2009). However, a purely locomotive mechanism cannot explain the extremely low capture rate and high observed frequency of sideways evasion compared with dodging by rodents (Ilany and Eilam, 2008) and multiple bird species (Devereux et al., 2008; Kullberg et al., 2000; Lima, 1993; Lima and Bednekoff, 2011; Lind et al., 2002) fleeing actual or model raptors. The theoretical models also assumed limitations due to fixed wing aerodynamics, while birds achieve low-radius high-g turns using a variety of maneuvers (Ros et al., 2011; Shelton et al., 2014; Warrick et al., 2002). This suggests that sideways evasion may also function to frustrate predator visual guidance (Shifferman and Eilam, 2004).
Goshawk hunting behavior and vision
Several field studies have identified the most common tactics used by the elusive Northern Goshawk as it hunts birds and small mammals in forested and open habitats (Fox, 1995; Kenward, 2006; Kenward, 1978; Squires and Reynolds, 1997). A short duration sit- and-wait predator, the goshawk usually forages from an elevated perch and then silently swoops down upon prey. Other tactics include foraging by soaring overhead, then stooping on prey, and rapidly pursuing prey in a contour-hugging tailchase. The goshawk is also known for persistent chases through dense brush or forests. Reported hunting success rates range from 5% to >30% (Kenward, 2006; Rutz, 2006). Goshawks raised and trained for falconry were found to use the same hunting behaviors as wild birds (Fox, 1981). Goshawk speeds have been estimated at 30 m s−1 for high altitude stoops (Alerstam, 1987), and 10–15 m s−1 for level flight and gliding attacks (Kenward, 1978; Rutz, 2006; Widen, 1989).
Diurnal raptors have frontally oriented eyes with a forward binocular region [measured at 35±1 deg in other raptor species (O'Rourke et al., 2010)] and a limited range of eye motion (Jones et al., 2007) that we estimate at ≤±3 deg for the goshawk (Buck, 2013a). As a consequence, they use head motion to track salient objects (O'Rourke et al., 2010). Goshawk eyes are bifoveate (Fite and Rosenfield-Wessels, 1975) with a retinal geometry similar to that of the Red-tailed Hawk (Lord, 1956): a lower acuity shallow foveal angle of 16±1 deg and a higher acuity deep fovea angle of 31±2 deg, both defined relative to the forward head axis.
This study considers two common types of goshawk prey: Ring-necked Pheasants (Phasianus colchicus) (Giudice and Ratti, 2001), which typically escape using steep take-off angles (Tobalske and Dial, 2000), rapid acceleration to ≤18 m s−1 (Giudice and Ratti, 2001) and rapid turns; and European rabbits (Oryctolagus cuniculus) (Tislerics, 2000), which evade predators by fleeing at speeds of ≤16 m s−1 (Garland, 1983), dodging, erratic zigzag swerving (jinking) and sharp sideways turns (Driver and Humphries, 1988).
GPS has enabled studies of many aspects of bird flight, including V-formation flocking (Portugal et al., 2014) and hierarchical decision-making in flocks (Flack et al., 2013), but its current logging rate (≤10 Hz) and accuracy (≥0.25 m) cannot resolve the rapid maneuvering of a goshawk or record its prey's motion. Stereometric video is useful for filming staged encounters with lures, but goshawk hunts in the field have unpredictable locations and spatial extents greater than the current ≤19 m3 reconstruction volumes (Theriault et al., 2014). Therefore, for this study we made use of headcam and ground video of goshawks flown for falconry in the field during foraging, flying toward perches and lures, and hunting live, wild prey.
RESULTS
We recorded and analyzed 29 total headcam sequences: 10 pheasant and six rabbit pursuits, three pursuits after bird-shaped falconry lures and 10 landings on perches. Distinct pursuits were defined as a chase begun with the goshawk at rest; in three cases, the goshawk made two successive pursuits after the same prey. Eleven pursuits showed moving prey while five showed stationary pheasants that fled shortly before interception. The goshawk used the same hunting tactics as wild goshawks: three stoops begun from mid-air and 13 swoops from perches usually followed by powered flight after the prey. The prey pursuits had a mean duration of 4.3±3.2 s (range: 0.53–8.4 s). The goshawk successfully captured only one prey (a pheasant) (success rate: 6% per prey and 8% per pursuit), and came within ≤2.1 m of intercepting its prey in eight other pursuits. Supplementary material Table S1 gives further details.
Fig. 4A–C shows video images with tracks indicating prey motion for three typical pursuits after live prey. For each video, we measured the target's horizontal and vertical visual angles (θ, χ), γ (moving targets only) and image size, I, versus time. The optical flow was measured for all but one video of a moving prey (Fig. 4B) in which the background was too erratic. The Materials and methods section explains how the goshawk–target distance, Z, relative goshawk–target speed, v, and linear acceleration, a, were computed from camera geometry and I. Computer simulations of CP and CATD were used to produce plots of θ, χ and Z versus time for comparison with measured data (Fig. 5A; see Materials and methods for details of the image analysis and simulations). Plots of measured θ, χ, γ and Z versus time are shown for five pursuits in Fig. 5B–F (supplementary material Figs S1–S5 show plots of all measured data).
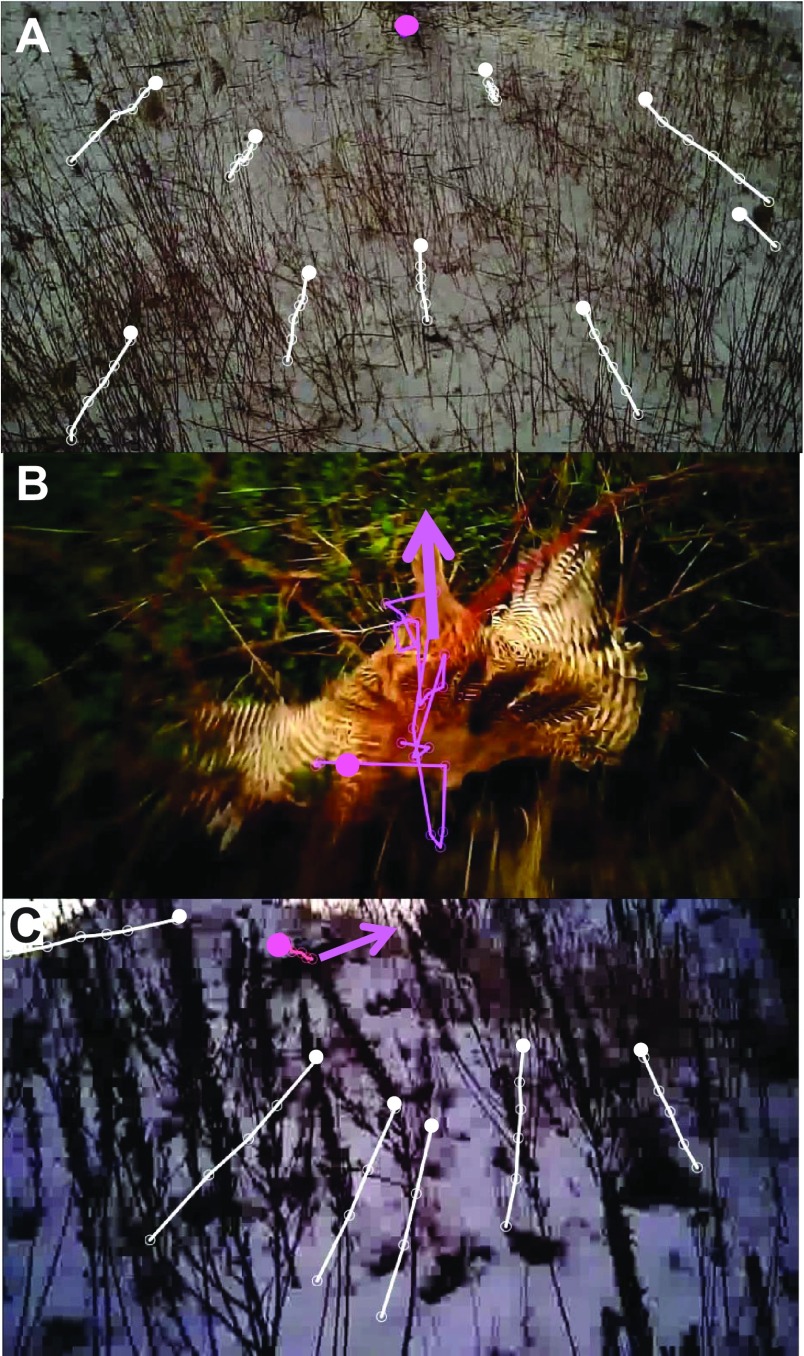
Fig. 4.
Headcam images with target tracks (magenta circles and lines) and optical flow (white lines and circles) of three pursuits. Details of pursuits corresponding to these labels are given in supplementary material Table S1. Solid circles indicate the first position in the time sequence: (A) stationary pheasant (P4a); (B) a moving pheasant tracked by CP (P7); optical flow was not tracked because the background changed too quickly; and (C) a running rabbit (R3) tracked by CATD. In B and C, magenta arrows at the prey's center of mass point along its velocity, ve, and opposite the local optical flow (γ=0 deg). Image credit: Robert Musters.
Fig. 5.
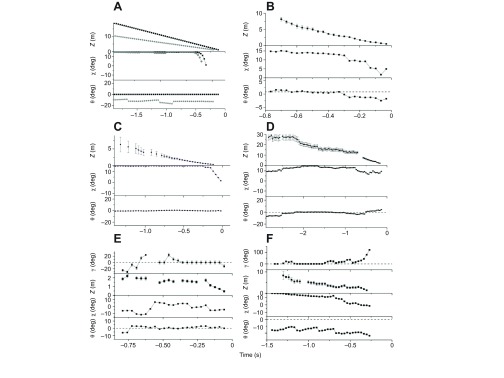
Simulated and measured headcam pursuit visual angles and prey distances. (A) Computer-simulated time series of visual angles (θ, χ) and predator–prey distances, Z, using CP (black squares and line) and CATD (gray circles and lines). Time series data from measured video tracks of pursuits and landings (labels and details given in supplementary material Table S1). (B) Pursuing a lure (LR1); (C) landing on a perch (L7); and pursuits after (D) a stationary pheasant (P4a), (E) a moving pheasant (P7) and (F) a moving rabbit (R3). Dashed lines: γ versus time: γ=0 deg; θ versus time: the average θ value at which the goshawk viewed its target before flight. Z is goshawk–target distance and γ defines the orientation of the prey's velocity on screen relative to the optical flow.
The main headcam findings are as follows. The goshawk's target was visible on camera in almost all cases before each pursuit or landing. While searching for prey and before flying to its target, the goshawk visually tracked salient objects (prey, lure, perch, etc.) in a retinal fixation area at the center of its visual field; during searches for prey, moving objects (prey, the falconer, etc.) were tracked via ~2 Hz head saccades. The retinal fixation area agreed with the center of motion when the goshawk flew toward its target starting either from mid-air or from a perch (cf. Fig. 2A and Fig. 4A).
The visual angle used by the goshawk to view prey and other targets during searching, pursuits and landings did not agree with either the shallow (16±1 deg) or deep (31±2 deg) goshawk foveal angles. Instead, the goshawk used interception when flying toward targets in 25 of 28 pursuits: it used CP when flying toward all stationary prey, lures and perches and CATD to pursue moving prey in eight of 11 pursuits. Fig. 4A is a typical video image showing that the optical flow for flight toward stationary targets (prey, lures or perches) was consistent with constant velocity motion with the prey at the center of motion. A comparison of the computer-simulated CP data for θ, χ and Z versus time in Fig. 5A with the corresponding measured values illustrates this agreement (Fig. 5B–D; supplementary material Figs S1–S3). Sample video images from pursuits of moving prey (Fig. 4C) show how headcam video provided a view of prey and background that allowed us to use a new image analysis method: by tracking both the prey and optical flow, we could measure the prey velocity angle, γ, relative to the local optical flow. This allowed us to confirm CATD, as opposed to merely constant visual angle (DP). In eight pursuits of moving prey, the goshawk viewed moving prey at γ≈0 deg and an approximately constant θ value that changed when the prey maneuvered (Fig. 5E,F; supplementary material Figs S4, S5), proving they were on a collision course and providing strong support for CATD. For moving prey, five tracks agreed with CPE (the special case for CATD when θ≈0 deg) and three with CATD for θ≠0 deg. The goshawk had to maneuver to achieve CATD, as in all cases the prey could be seen to maneuver relative to the background, e.g. in pursuing a flying, jinking pheasant, the goshawk fixed the pheasant's image at θ=1.1±1.4 deg and γ≈0 deg (CPE) in spite of the pheasant's erratic motion (Fig. 4B, Fig. 5E). While pursuing a jinking rabbit (Fig. 4C), the goshawk maintained γ≈0 deg and θ constant apart from saw-tooth oscillations with period 0.2 s equal to the approximate goshawk wingbeat period (Pennycuick et al., 1994); the measured θ versus time was in close agreement with computer simulations of CATD (cf. Fig. 5A,F). Tracking of optical flow relative to prey motion in the goshawk study also permitted us to look for evidence of feedback-based interception. In five cases these data showed evidence of one or more oscillations in θ and γ about their optimal values as the goshawk maneuvered, as expected for a feedback-based interception mechanism (Fig. 5E,F; supplementary material Fig. S4C–E, Fig. S5A,D,E).
Prey were observed to use various tactics to successfully evade interception (supplementary material Table S1). There were seven cases of prey fleeing directly away (minimum goshawk–prey distance Zmin=3±3 m), eight of sideways evasion (Zmin=9±11 m) and only one of dodging (Zmin=1.4 m). Motion of the prey could be visualized separately relative to the background, showing that prey frequently maneuvered even when the goshawk managed to visually fix the prey successfully. Rabbits were able to make a major turn in one bound (0.2–0.3 s), while pheasants could turn or take-off in ~0.2 s. These data also showed that γ diverged from 0 deg only when the prey evaded successfully by making a large sideways swerve (supplementary material Fig. S4A, Fig. S5D) or when the prey was initially stationary and used sideways evasion shortly before impact (supplementary material Fig. S3). In three cases, the goshawk maintained θ constant but not γ≈0 deg (DP) as the prey flew on a sideways trajectory (supplementary material Fig. S4B, Fig. S5B,C). Thus, sideways evasion was effective at thwarting visual fixation and this resulted in greater Zmin values on average than other evasion tactics in this small sample.
While the goshawk fixed its targets horizontally, visual fixation on the target was not maintained as closely in the vertical direction (supplementary material Fig. S3, Fig. S4C,E, Fig. S5A,E). When flying from a perch, the goshawk apparently initially flew with its head tilted downward relative to its velocity, as both the center of motion and the target were off-screen at a large positive vertical visual angle, though centered horizontally. However, the target's motion to lower vertical visual angles (χ<0 deg) just before impact is explained by the goshawk assuming impact posture, consistent with the χ versus time behavior predicted by our computer simulations (cf. Fig. 5A with 5B,C,F and supplementary material Fig. S1, Fig. S2). For lure pursuits and landings, the drop in χ was so abrupt that we were able to measure the time and distance before impact (Tp and Zp) at which the goshawk assumed impact posture (Fig. 6A); for live prey, the changes in χ were more gradual.
Fig. 6.
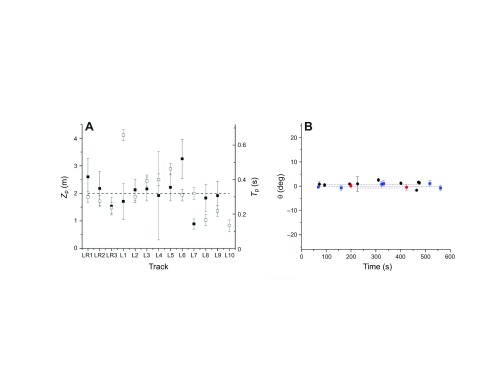
Impact posture and headcam stability data. (A) Distance from target (Zp, solid squares) and time (Tp, open squares) at which the goshawk began to assume impact posture. Dashed line: mean value of Zp and Tp. (B) Average horizontal fixation angle θ of stationary objects for three videos (different color symbols) versus time, plotted over the measured range. Dashed lines: average values for each time series.
Fig. 6B shows the fixation angle versus time for three video sequences in which the goshawk alternately observed a stationary object while perched and pursued prey vigorously, often through brush. The plots of average horizontal angle, θ versus time had slopes equal to zero within error bars, indicating that the headcam orientation was stable within measurement error. Optical flow tracking also allowed us to determine that the goshawk turned its head during forward motion only infrequently and by small amounts [cf. fig. 4C and fig. 3C in Lee and Kalmus (Lee and Kalmus, 1980)]. The target image's occasional small horizontal deviations immediately before impact were consistent with the goshawk's habit of swerving to one side before impact (Fig. 1A). In two pursuits, the goshawk initially maintained constant Z while maneuvering to attack the prey from behind (Fig. 5D; supplementary material Fig. S3).
Measured values for v and a gave goshawk groundspeed and linear acceleration for stationary targets only; we could not determine centripetal acceleration during turns. Headcam video of stationary targets was analyzed to measure the range of average goshawk groundspeeds for flights toward stationary prey (10–18 m s−1), with lower ranges for flights toward stationary lures (7–16 m s−1) and perches (5–13 m s−1). The agreement with speeds reported for wild goshawks indicated the headcam did not interfere with flight. Speed relative to moving prey ranged from 0 to 13 m s−1, consistent with previous data indicating both prey species can match the goshawk's top speed. The goshawk's Z versus time was consistent with constant relative speed in 71% (12 of 17) of flights toward stationary targets, as well as 10 out of 11 (91%) pursuits after moving prey. The last result means that while the goshawk was always maneuvered to visually fix its maneuvering prey, it managed to achieve a constant relative speed on timescales that were long compared with the video frame rate period (1/30 s). In 29% of flights toward stationary targets, the Z data agreed with constant linear deceleration before impact. Wind speed data were retrieved from a nearby weather station (Royal Netherlands Meteorological Institute), with mean values of 3.9±1.6 m s−1 and range 1.5–6.7 m s−1.
To study the pursuit trajectories of both goshawk and prey, we analyzed an archive of videos filmed using a single video camera on the ground that showed goshawks flying from the falconer's glove to pursue, attack and capture fleeing rabbits and pheasants. In 67% (58 of 86) of the videos, the goshawk's and prey's trajectories could be determined most of the time, while the remainder had poor prey visibility or camera angle. Although the prey evaded capture using the same tactics in ground and headcam video, the ground videos were selected to show successful captures so they could not be used to compute the effectiveness of different evasive tactics.
We examined the ground video for evidence of whether the goshawk and prey trajectories were consistent with different pursuit strategies, and if so, how they were implemented. In general, the goshawk initially flew to reach the prey's immediate vicinity and then converted to a tailchase while it attacked the prey, often repeatedly. Fig. 7 shows still images and on-screen trajectories for a pursuit in which the goshawk initially flew with its velocity oriented downward and out of the screen. In this case, it is unambiguous that the goshawk initially flew with its horizontal bearing on a CATD interception course with the rabbit: after take-off, the goshawk's trajectory on the image was a vertical line, it always faced forward, and its 3D trajectory intercepted that of the prey (Fig. 7A,C). The final pursuit could be identified as CPE because it took place in a plane approximately orthogonal to the camera axis, it showed that the goshawk flew slightly above and immediately behind the rabbit and the two animals moved with their velocities in approximately the same direction (Fig. 7B,C).
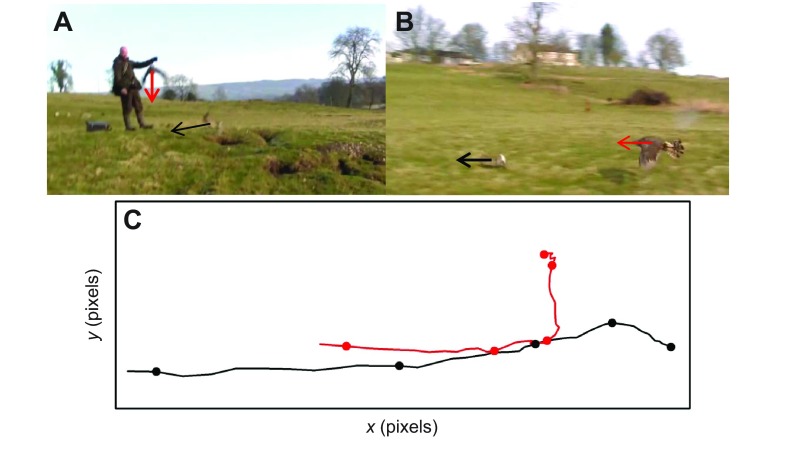
Fig. 7.
Video filmed from the ground of a goshawk pursuing and attacking a rabbit. (A) Still image shortly after the rabbit was flushed, showing the goshawk flying to intercept the rabbit, then (B) turning to pursue it via CPE. Their approximate directions are indicated by red (goshawk) and black (rabbit) arrows starting at each animal's center of mass. (C) Goshawk (red) and rabbit (black) tracks from this video. Circles show each animal's position every 0.4 s and arrows indicate their directions and starting positions. Image credit: David and Adam Burn.
Most videos were not filmed either head-on or in orthogonal view. In spite of this, we could determine considerable relevant information about each animal's motion. By measuring both the on-screen trajectory and s (arc length along the trajectory) we could determine whether the animal's motion was consistent with constant velocity, which requires both a linear on-screen trajectory and linear dependence of s versus time. From its pose, we could determine the apparent direction of its motion on-screen, whether it moved in a constant direction and when it changed direction. The combination of linear on-screen trajectory, linear s versus time and constant pose thus presented a strong argument for motion at constant velocity, as the last requirement rules out accelerated motion that only appears linear on-screen. We also could tell whether the goshawk's pose was consistent with CP (which requires it to orient toward the prey's current location) or with interception (for which it must orient toward the prey's extrapolated future position). In fact, none of the ground videos showed the goshawks using CP initially when the prey did not use direct evasion (Fig. 7A; supplementary material Fig. S6A,D). Proof of CPE could be ascertained for constant predator and prey velocities when the goshawk and prey had parallel tracks on video, their plots of s versus time had the same slope, and the goshawk flew toward the prey. Similarly, support for CATD could only be demonstrated for constant predator and prey velocities when the goshawk's bearing pointed toward the extrapolated future position of the prey on-screen and their 3D trajectories intersected at the same time.
Using these ideas, we measured tracks and arc lengths for the goshawk and prey in 20 typical pursuits with mean duration 1.5±0.8 s. In 16 of the tracked pursuits, the goshawks initially flew on a trajectory consistent with a CATD piece-wise linear interception course, sometimes correcting course when the rabbit changed direction by jinking (supplementary material Fig. S6A,C,E). In four cases, the entire chase was also CPE. In 12 cases, when it came close to intercepting the prey, the goshawk immediately turned in 0.2–0.4 s (one to two wingbeats) through ≤90 deg in order to follow the prey in a high-speed CPE tailchase ending in attack and eventual capture. In the remaining four videos, the goshawk's trajectory constantly curved as it made a banked turn into a final pursuit that approximated a tangent to the prey's motion (supplementary material Fig. S6B,D,F). Apart from ruling out CP for all four and CATD for three of the pursuits where the rabbit moved at constant velocity, we cannot further describe these pursuits, which could correspond to DP, PN or no single strategy.
Finally, we also analyzed four videos filmed from the vantage point of a stationary target, which confirmed that the goshawk flies toward its target at an approximately constant bearing angle (supplementary material Fig. S7) (see Buck, 2013b).
DISCUSSION
While searching for prey or before flying to a perch, the goshawk fixed its target at the center of its visual field, as previously found for barn owls (Fux and Eilam, 2009). It used CP when flying toward stationary prey, lures and perches, similar to results found for chickens (Moinard et al., 2005) and pigeons (Green et al., 1992) landing on perches. Like dragonflies (Combes et al., 2012) and bats (Ghose et al., 2006), the headcam data showed the goshawk maintained the prey at constant visual angle during pursuits and pursued moving prey using CATD in the majority of cases; also like bats, the goshawk maintained the criterion for CATD more closely for horizontal than for vertical visual angles. The pursuit trajectories recorded by ground video supported the frequent use of CATD by goshawks, with some pursuits using undetermined strategies. As falcons and goshawks are now classified in separate orders (McCormack et al., 2013), it is interesting to compare results from our two headcam studies (Kane and Zamani, 2014). While both species were observed to fix prey visually and to use interception, compared with the goshawk, the falcons fixed their prey visually over longer times (possibly because the falcon's prey maneuvered more gradually), did not view prey at the center of motion, and occasionally turned their heads in flight, likely to use their deep fovea. The goshawk did not foveate targets, while falcons sometimes turned their heads, apparently to foveate prey.
The prey in this study were observed to flee by sideways evasion or classical evasion plus jinking, and only rarely by dodging. When the prey fled directly or jinked, this resulted in only small variations about visual fixation that the goshawk quickly resolved via maneuvering. Even when the prey was not fixed visually, the goshawk managed to keep the prey at approximately constant horizontal visual angle. By contrast, abrupt, large-angle sideways evasion led to a large, rapid loss of visual fixation along the horizontal visual angle, even though the ground video showed goshawks were capable of executing large angle turns in a single wingbeat period. By definition, sideways evasion causes the greatest deviation in γ and horizontal visual angle, so it is relevant that the goshawk tolerated less variation in the horizontal than in the vertical visual angle during pursuits. Our headcam data also showed that the goshawk did not approach prey as closely when they used sideways evasion compared with classical evasion plus jinking. These results reinforce the argument that visual perception plays a significant role in determining the success of prey-evasion maneuvers (Eilam, 2005; Shifferman and Eilam, 2004).
The goshawk was found to assume impact posture for lure captures and landings at times and distances that lay within a narrow range consistent with values reported by Goslow (Goslow, 1971). To study its approach to collision, we used the tau function 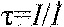 provide a quantitative measure of prey looming dynamics. Previous studies have shown that hummingbirds and pigeons maintain constant tau-dot,
provide a quantitative measure of prey looming dynamics. Previous studies have shown that hummingbirds and pigeons maintain constant tau-dot, 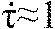 , during aerial docking and landing (Lee et al., 1993; Lee et al., 1991). Plunge-diving gannets use tau-based optical flow cues for streamlining prior to water impact (Lee and Reddish, 1981), hawks use tau-related cues to initiate landing (Davies and Green, 1990) and bats use acoustic tau to intercept prey (Lee et al., 1992). The linearity of our Z versus time data for 59% of flights toward stationary targets and 91% of pursuits of live prey is equivalent to the goshawk maintaining constant
, during aerial docking and landing (Lee et al., 1993; Lee et al., 1991). Plunge-diving gannets use tau-based optical flow cues for streamlining prior to water impact (Lee and Reddish, 1981), hawks use tau-related cues to initiate landing (Davies and Green, 1990) and bats use acoustic tau to intercept prey (Lee et al., 1992). The linearity of our Z versus time data for 59% of flights toward stationary targets and 91% of pursuits of live prey is equivalent to the goshawk maintaining constant 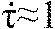 before prey interception (Lee et al., 1993). This shows that the goshawk also used a tau-based interception strategy: it cannot achieve
before prey interception (Lee et al., 1993). This shows that the goshawk also used a tau-based interception strategy: it cannot achieve 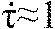 simply by flying at constant velocity when the prey constantly maneuvers, so the goshawk must regulate its motion using looming cues as a control signal.
simply by flying at constant velocity when the prey constantly maneuvers, so the goshawk must regulate its motion using looming cues as a control signal.
Looming objects elicit a reflexive escape response in many taxa, including birds (Schiff, 1965), fish (Dill, 1974), crabs (Oliva et al., 2007), frogs (Yamamoto et al., 2003) and humans (Regan and Vincent, 1995). This reaction is due to neurons sensitive to looming and time to collision, and is independent of binocular depth cues (Sun and Frost, 1998; Wang and Frost, 1992). Some animals have evolved behavior to exploit this widespread response to looming. For example, the painted redstart has been shown to mimic looming by spreading and pivoting its conspicuously patterned wings and tail to flush out insect prey (Arbanas, 2006; Jabłoński and Strausfeld, 2000) and the vampire squid uses an antipredator display in which two circular photophores change diameter and its lighted arm tips move radially to simulate fleeing (National Geographic, 2007; Hunt, 1996). We hypothesize that sensory exploitation (Stevens, 2013) of the response to looming might explain the cognitive mechanism behind many other ‘startle effect’ displays in which prey appear abruptly larger in size just before attack by spreading conspicuous wings, tails or ruffs, inflating body parts, or erecting feathers or fur (Edmunds, 1974). Previously proposed functions include appearing larger to intimidate the predator (Cooper and Stankowich, 2010), confusing the predator by disrupting a search image (Bond, 2007), or deflecting attack to the displayed body part (Ruxton et al., 2004). However, many of these displays are very similar to those used by the painted redstart, and little empirical testing has been performed on these hypotheses (Ruxton et al., 2004).
Tests of this conjecture must distinguish among other mutually non-exclusive hypotheses (e.g. last-minute displays also deter early detection by the predator) (Ruxton et al., 2004). The appearance of these displays (Brooke, 1998; Sargent, 1990) on predator visual fields could be modeled to compare their tau-dot values with collision scenarios. Previous experiments have used presentations that were static or that simplified the actual dynamics while focusing on the effect of different patterns (Ingalls, 1993; Sargent, 1990; Stevens et al., 2008). Experiments could assess instead the predator's response to presentations corresponding to natural and variable effective tau-dot, with contrast manipulations probing the relationship between contrast and looming found in some taxa (Jabłoński, 1999; Landwehr et al., 2013).
In comparing the goshawk's observed hunting behavior with optimal foraging theory (Stephens and Krebs, 1986), one must consider that this raptor eats large, infrequent meals, placing a high premium on capture success rate as well as search strategy, pursuit duration and metabolic cost of different flight behaviors. This raptor must solve several distinct problems: find prey, reach its immediate vicinity and then kill it. For stationary prey, rapid interception via CP best preserves the element of surprise, facilitating capture. For moving prey, interception lets the goshawk reach its prey quickly, but allows only a brief time window for capture. Unlike stooping falcons, which often kill on impact, goshawks typically need time to subdue and kill prey with their talons (Sustaita and Hertel, 2010). Interception followed by CPE allows the goshawk to maneuver accurately as it attacks from behind, a strategy previously reported for bats (Kalko and Schnitzler, 1998) and owls (Hausmann et al., 2008). CATD followed by CPE also seems well-suited to the goshawk's habit of pursuing prey through dense forest and brush, where its central binocular field can process depth cues for obstacle avoidance. (In one pursuit, we observed the goshawk rapidly swerving to avoid a branch while pursuing a pheasant.) By contrast, the falcons in our previous study (Kane and Zamani, 2014) flew through the unobstructed sky, so viewing the prey at a lateral angle was unproblematic. As both species frequently fixed prey at non-zero visual angles, this supports the hypothesis that these birds may collide with man-made objects because they often focus attention away from their forward direction (Lima et al., 2014; Martin et al., 2012).
Other taxa have been found to use different pursuit–evasion strategies for different scenarios, including flies (Land, 1993), fiddler crabs (Land and Layne, 1995) and bats (Chiu et al., 2010; Ghose et al., 2006). Consequently, we caution that these findings for goshawks and falcons need not apply in every circumstance. Although hunting behavior is innate in raptors (Bustamante, 1999), juveniles develop skills through social play, prey transfer by parents, object play and practice. Northern goshawks begin with inefficient pursuits ending by colliding with prey, and only gradually develop targeting and capture techniques (Fox, 1995). Pursuit–evasion modeling studies have found that the optimal strategy depends on the assumed predator and prey behaviors (Nolfi, 2012; Pais and Leonard, 2010; Wei et al., 2009), highlighting the importance of integrated empirical studies of predator and prey behavior (Combes et al., 2012).
The pursuit strategy employed by birds may also relate to their hunger level and the pursuit's purpose (e.g. hunting for food, territorial defense, catching a lure, social play, etc.) (Treleaven, 1980). In future work, it will be important to address these issues by studying predation of stationary versus moving prey or lures, the effect of hunger (within humane limits), variation due to terrain or wind and differences between prey species (Fox, 1995), with birds pursuing insects a case of special interest (Schuler, 1990). As flocking models have had to rely on untested assumptions about visual guidance (Ballerini et al., 2008; Mischiati and Krishnaprasad, 2012), there is much to learn from the study of visual guidance in non-predation scenarios, such as flying or flocking with conspecifics (Shelton et al., 2014) and in birds with other retinal specializations, eye placement and eye motions (Martin, 2014). This presents a wide field for further investigation using headcams in combination with rapidly improving GPS (Pettit et al., 2013) and high-speed stereometric video (Theriault et al., 2014) tracking.
MATERIALS AND METHODS
Field recording with the headcam took place near Twenthe, The Netherlands, on 6 days (December 2012 to January 2013). The goshawk (female, 1.30 kg, 2.5 years old) was raised in captivity by a parent and flown by master falconer Robert Musters in her third hunting season. For ground video, all goshawks were raised by a parent and flown for falconry (Dale Mews, UK). All prey were wild animals encountered by the raptors during hunting in natural environments. All experiments were approved by the Haverford College Institutional Animal Care and Use Committee following the ARRIVE guidelines (NC3Rs 2010).
Headcam video was recorded using model 808 camcorders (Toplanter, Huizhou, China) (29.97 frames s−1; 1280×720 pixel resolution; shutter speed ~0.01 s; 2 h recording time; field of view: 52 deg horizontal, 31 deg vertical) mounted in a fiberglass hood (total headcam + hood mass=20 g, ≤1.5% body mass) made by Robert Musters. The camera axis was h=2.4±0.5 cm (95% CI) above the eyes. The headcam video was stable without deshake post-processing because the goshawk maintained head nystagmus (Jones et al., 2007), apart from some roll before impact or during short rapid turns, as confirmed by the level horizon and optical flow. We have described previously how ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to track target image coordinates in pixels and how these were converted into camera angles (θ, χ) in deg (±0.3 deg; 95% CI) (Kane and Zamani, 2014). Optical flow was measured manually by tracking background objects, because automated methods cannot accommodate large changes in perspective. Using optical flow during flight to determine the center of motion confirmed that θ≈0 deg agreed with the forward direction in most cases and that the goshawk did not use head saccades during pursuits. The prey's motion and pose onscreen was used to determine γ relative to optical flow. The target images were displaced downward before impact due to the mounting of the camera above the body axis and the goshawk's upward pitching before impact (Goslow, 1971). To offset this effect, the camera was mounted pitched downward such that χ≈15 deg corresponded to 0 deg in the goshawk's vertical visual field (Fig. 1B).
Target size, O, was measured directly for posts and lures and determined from average prey body dimensions corrected for perspective using estimated pose angles. Target distances, Z, were found from: Z=Of/I, where f is the camera focal length (pixels) and I is the image size (pixels) (≤2.7% accuracy for objects imaged ≤15 deg off-axis). When Z could be measured, linear fits to the Z versus time data were used to determine the projected collision time (time=0 s); otherwise, the origin for time was arbitrary. Relative goshawk–target speed, v, and acceleration, a, were found from polynomial least squares fits to Z versus time; error bars in v and a were calculated from uncertainty in measured I and f values (statistical), and uncertainty in prey species' size and pose (systematic). All fitting, statistical analysis and other data analysis were carried out in Origin 8.6 (OriginLab, Northampton, MA, USA). All error bars are s.d.
Ground videos were obtained from an online archive (A. Burn and D. Burn, Dale Mews videos; www.dalemews.com) filmed over a period of 3 years using various consumer camcorders (25 frames s−1; 720×1280 pixels) that showed several goshawks pursuing rabbits and pheasants flushed by dogs or ferrets. Goshawk and prey tracks on the video images were measured using ImageJ and, when necessary, corrected for small camera pan using the image tracks of stationary nearby background objects.
Computer-simulated headcam images were created using Python 2.7 code for stationary prey (CP) or moving and intermittently maneuvering prey (CATD). Goshawk and relative goshawk–prey speeds (vp=15 m s−1 and ve=10 m s−1) and initial Z values were taken from measured values. The simulated goshawk was allowed to maneuver without limits on acceleration and glided in a straight line. Changes in χ due to perspective changes as the goshawk assumed impact posture were modeled by linearly increasing h (camera–body axis distance) using measured time scales. Typical avian flicker fusion frequencies (Fox, 1995; Jones et al., 2007) set the simulation time step, Δt=11 ms, and the goshawk's reactions lagged prey motion by 66 ms (Potts, 1984).
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Robert Musters for flying Shinta the goshawk while she wore a headcam, and David and Adam Burn for permission to reproduce images from their videos. We benefitted greatly from advice on falconry from falconers Robert Musters, David Burn, Jack Hubley and Robert Giroux, and from email correspondence with Graham Taylor that resulted in several new ideas for headcam image analysis. Finally, we appreciate the many helpful suggestions made by two anonymous reviewers.
FOOTNOTES
Competing interests
The authors declare no competing financial interests.
Funding
This research was funded by a grant to Haverford College from the Howard Hughes Medical Institute and a Special Projects award from the Marion E. Koshland Integrated Natural Sciences Center of Haverford College.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.108597/-/DC1
References
- Alerstam T. (1987). Radar observations of the stoop of the peregrine falcon Falco peregrinus and the goshawk Accipiter gentilis. Ibis 129, 267-273. [Google Scholar]
- Angell T. (1969). A study of the ferruginous hawk: adult and brood behavior. Living Bird 8, 225-241. [Google Scholar]
- Arbanas L. (2006). Painted Redstart (Myioborus pictus), ML Video 464938. Macaulay Library. Ithaca NY: Cornell Lab of Ornithology; Available at: www.macaulaylibrary.org [Google Scholar]
- Ballerini M., Cabibbo N., Candelier R., Cavagna A., Cisbani E., Giardina I., Lecomte V., Orlandi A., Parisi G., Procaccini A., et al. (2008). Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl. Acad. Sci. USA 105, 1232-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BBC (2009). Flying with the Fastest Birds on the Planet: Peregrine Falcon and Goshawk (Animal Camera). London: BBC. [Google Scholar]
- Bond A. B. (2007). The evolution of color polymorphism: crypticity searching images, and apostatic selection. Annu. Rev. Ecol. Evol. Syst. 38, 489-514. [Google Scholar]
- Brooke M. De L. (1998). Ecological factors influencing the occurrence of ‘flash marks’ in wading birds. Funct. Ecol. 12, 339-346. [Google Scholar]
- Buck L. (2013a). Goshawk BM8E-Goshawk CU on Head Real Time (Birds in Slow Motion). Newtownmountkennedy, County Wicklow, Ireland. [Google Scholar]
- Buck L. (2013b). Goshawk Slow-Motion Pouncing on Prey Shot with Phantom HD Gold, (Birds in Slow Motion). Newtownmountkennedy, County Wicklow, Ireland. [Google Scholar]
- Bustamante J. (1999). Ecological factors affecting hunting behaviour during the post-fledging dependence period of raptors. In Proceedings of the 22nd International Ornithological Congress, Durban (ed. Adams N. J., Slotow R. H.), pp. 1381-1396 Johannesburg: BirdLife South Africa. [Google Scholar]
- Chiu C., Reddy P. V., Xian W., Krishnaprasad P. S., Moss C. F. (2010). Effects of competitive prey capture on flight behavior and sonar beam pattern in paired big brown bats, Eptesicus fuscus. J. Exp. Biol. 213, 3348-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett T. S., Land M. F. (1978). How hoverflies compute interception courses. J. Comp. Physiol. 125, 191-204. [Google Scholar]
- Combes S. A., Rundle D. E., Iwasaki J. M., Crall J. D. (2012). Linking biomechanics and ecology through predator-prey interactions: flight performance of dragonflies and their prey. J. Exp. Biol. 215, 903-913. [DOI] [PubMed] [Google Scholar]
- Cooper W. E., Jr, Stankowich T. (2010). Prey or predator? Body size of an approaching animal affects decisions to attack or escape. Behav. Ecol. 21, 1278-1284. [Google Scholar]
- Curio E. (1976). The Ethology of Predation. Berlin: Springer. [Google Scholar]
- Davies M. N. O., Green P. R. (1990). Optic flow-field variables trigger landing in hawk but not in pigeons. Naturwissenschaften 77, 142-144. [DOI] [PubMed] [Google Scholar]
- Devereux C. L., Fernández-Juricic E., Krebs J. R., Whittingham M. J. (2008). Habitat affects escape behaviour and alarm calling in common starlings Sturnus vulgaris. Ibis 150, 191-198. [Google Scholar]
- Dill L. M. (1974). Escape response of zebra Danio (Brachydanio rerio) 1. Stimulus for escape. Anim. Behav. 22, 711-722. [Google Scholar]
- Driver P. M., Humphries D. A. (1988). Protean Behaviour. The Biology of Unpredictability. Oxford: Clarendon Press. [Google Scholar]
- EarthRangers (2011). Kingfisher Diving-Feeding, Vol. 2014 Woodbridge, ON: Earth Rangers Foundation. [Google Scholar]
- Edmunds M. (1974). Defence in Animals: a Survey of Anti-predator Defences. Harlow, UK: Longman. [Google Scholar]
- Edut S., Eilam D. (2004). Protean behavior under barn-owl attack: voles alternate between freezing and fleeing and spiny mice flee in alternating patterns. Behav. Brain Res. 155, 207-216. [DOI] [PubMed] [Google Scholar]
- Eilam D. (2005). Die hard: a blend of freezing and fleeing as a dynamic defense – implications for the control of defensive behavior. Neurosci. Biobehav. Rev. 29, 1181-1191. [DOI] [PubMed] [Google Scholar]
- Fajen B. R., Warren W. H. (2004). Visual guidance of intercepting a moving target on foot. Perception 33, 689-715. [DOI] [PubMed] [Google Scholar]
- Fernández-Juricic E. (2012). Sensory basis of vigilance behavior in birds: synthesis and future prospects. Behav. Processes 89, 143-152. [DOI] [PubMed] [Google Scholar]
- Fernández-Juricic E., Venier M. P., Renison D., Blumstein D. T. (2005). Sensitivity of wildlife to spatial patterns of recreationist behavior: a critical assessment of minimum approaching distances and buffer areas for grassland birds. Biol. Conserv. 125, 225-235. [Google Scholar]
- Fite K. V., Rosenfield-Wessels S. (1975). A comparative study of deep avian foveas. Brain Behav. Evol. 12, 97-115. [DOI] [PubMed] [Google Scholar]
- Flack A., Zsuzsa A., Nagy M., Vicsek T., Biro D. (2013). Robustness of flight leadership relations in pigeons. Anim. Behav. 86, 723-732. [Google Scholar]
- Fox N. (1981). The hunting behaviour of trained northern goshawks. In Understanding the Goshawk (ed. Kenward R. E., Lindsay I. M.), pp. 121-133 Oxford, UK: International Association for Falconry and the Conservation of Birds of Prey. [Google Scholar]
- Fox N. (1995). Understanding the Bird of Prey. Blaine, WA: Hancock House Publishers. [Google Scholar]
- Fux M., Eilam D. (2009). How barn owls (Tyto alba) visually follow moving voles (Microtus socialis) before attacking them. Physiol. Behav. 98, 359-366. [DOI] [PubMed] [Google Scholar]
- Garland T. (1983). The relation between maximal running speed and body-mass in terrestrial mammals. J. Zool. 199, 157-170. [Google Scholar]
- Geographic N. (2007). Vampyroteuthis infernalis, Vol. 2014 Washington, DC: National Geographic. [Google Scholar]
- Ghose K., Horiuchi T. K., Krishnaprasad P. S., Moss C. F. (2006). Echolocating bats use a nearly time-optimal strategy to intercept prey. PLoS Biol. 4, e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose K., Triblehorn J. D., Bohn K., Yager D. D., Moss C. F. (2009). Behavioral responses of big brown bats to dives by praying mantises. J. Exp. Biol. 212, 693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. (1997). Visual control of cursorial prey pursuit by tiger beetles (Cicindelidae). J. Comp. Physiol. A 181, 217-230. [Google Scholar]
- Giudice J. H., Ratti J. T. (2001). Ring-necked pheasant (Phasianus colchicus). In The Birds of North America Online, Vol. 2014 (ed. Poole A.). Ithaca, NY: Cornell Lab. of Ornithology. [Google Scholar]
- Goslow G. E. (1971). The attack and strike of some North American raptors. Auk 88, 815-827. [Google Scholar]
- Green P. R., Davies M. N. O., Thorpe P. H. (1992). Head orientation in pigeons during landing flight. Vision Res. 32, 2229-2234. [DOI] [PubMed] [Google Scholar]
- Hausmann L., Plachta D. T. T., Singheiser M., Brill S., Wagner H. (2008). In-flight corrections in free-flying barn owls (Tyto alba) during sound localization tasks. J. Exp. Biol. 211, 2976-2988. [DOI] [PubMed] [Google Scholar]
- Hedenström A., Rosén M. (2001). Predator versus prey: on aerial hunting and escape strategies in birds. Behav. Ecol. 12, 150-156. [Google Scholar]
- Howland H. C. (1974). Optimal strategies for predator avoidance: the relative importance of speed and manoeuvrability. J. Theor. Biol. 47, 333-350. [DOI] [PubMed] [Google Scholar]
- Hunt J. C. (1996). The behavior and ecology of midwater cephalopods from Monterey Bay: submersible and laboratory observations. PhD dissertation, University of California, Los Angeles, CA, USA. [Google Scholar]
- Ilany A., Eilam D. (2008). Wait before running for your life: defensive tactics of spiny mice (Acomys cahirinus) in evading barn owl (Tyto alba) attack. Behav. Ecol. Sociobiol. 62, 923-933. [Google Scholar]
- Ingalls V. (1993). Startle and habituation responses of blue jays (Cyanocitta cristata) in a laboratory simulation of antipredator defenses of Catacala moths (Lepidoptera noctuidae). Behaviour 126, 77-95. [Google Scholar]
- Jabłoński P. G. (1999). A rare predator exploits prey escape behavior: the role of tail-fanning and plumage contrast in foraging of the painted redstart (Myioborus pictus). Behav. Ecol. 10, 7-14. [Google Scholar]
- Jabłoński P. G., Strausfeld N. J. (2000). Exploitation of an ancient escape circuit by an avian predator: prey sensitivity to model predator display in the field. Brain Behav. Evol. 56, 94-106. [DOI] [PubMed] [Google Scholar]
- Jones M. P., Pierce K. E., Jr, Ward D. (2007). Avian vision: a review of form and function with special consideration to birds of prey. Journal of Exotic Pet Medicine 16, 69-87. [Google Scholar]
- Justh E. W., Krishnaprasad P. S. (2006). Steering laws for motion camouflage. Proc. R. Soc. A 462, 3629-3643. [Google Scholar]
- Kalko E. K. V., Schnitzler H.-U. (1998). How echolocating bats approach and acquire food. In Bat Biology and Conservation (ed. Kunz T. H., Racey P. A.). Washington, DC: Smithsonian Institution Press. [Google Scholar]
- Kane S. A., Zamani M. (2014). Falcons pursue prey using visual motion cues: new perspectives from animal-borne cameras. J. Exp. Biol. 217, 225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzir G. (1994). Tuning of visuomotor coordination during prey capture in water birds. In Perception and Motor Control in Birds, pp. 315-338 Berlin: Springer. [Google Scholar]
- Kenward R. E. (1978). Hawks and doves – factors affecting success and selection in goshawk attacks on woodpigeons. J. Anim. Ecol. 47, 449-460. [Google Scholar]
- Kenward R. (2006). The Goshawk. Oxford, UK: T. & A. D. Poyser. [Google Scholar]
- Kullberg C., Jakobsson S., Fransson T. (2000). High migratory fuel loads impair predator evasion in sedge warblers. Auk 117, 1034-1038. [Google Scholar]
- Lanchester B. S., Mark R. F. (1975). Pursuit and prediction in the tracking of moving food by a teleost fish (Acanthaluteres spilomelanurus). J. Exp. Biol. 63, 627-645. [DOI] [PubMed] [Google Scholar]
- Land M. F. (1993). Chasing and pursuit in the dolichopodid fly Poecilobothrus nobilitatus. J. Comp. Physiol. A 173, 605-613. [Google Scholar]
- Land M., Layne J. (1995). The visual control of behavior in fiddler-crabs 2: tracking control-systems in courtship and defense. J. Comp. Physiol. A 177, 91-103. [Google Scholar]
- Landwehr K., Brendel E., Hecht H. (2013). Luminance and contrast in visual perception of time to collision. Vision Res. 89, 18-23. [DOI] [PubMed] [Google Scholar]
- Lee D. N., Kalmus H. (1980). The optic flow field: the foundation of vision (and Discussion). Philos. Trans. R. Soc. B 290, 169-179. [DOI] [PubMed] [Google Scholar]
- Lee D. N., Reddish P. E. (1981). Plummeting gannets – a paradigm of ecological optics. Nature 293, 293-294. [Google Scholar]
- Lee D. N., Reddish P. E., Rand D. T. (1991). Aerial docking by hummingbirds. Naturwissenschaften 78, 526-527. [Google Scholar]
- Lee D. N., Vanderweel F. R. R., Hitchcock T., Matejowsky E., Pettigrew J. D. (1992). Common principle of guidance by echolocation and vision. J. Comp. Physiol. A 171, 563-571. [DOI] [PubMed] [Google Scholar]
- Lee D. N., Davies M. N. O., Green P. R., Vanderweel F. R. R. (1993). Visual control of velocity of approach by pigeons when landing. J. Exp. Biol. 180, 85-104. [Google Scholar]
- Lima S. L. (1993). Ecological and evolutionary perspectives on escape from predatory attack – a survey of North American birds. Wilson Bull. 105, 1-47. [Google Scholar]
- Lima S. L., Bednekoff P. A. (2011). On the perception of targeting by predators during attacks on socially feeding birds. Anim. Behav. 82, 535-542. [Google Scholar]
- Lima S. L., Blackwell B. F., Devault T. L., Fernández-Juricic E. (2014). Animal reactions to oncoming vehicles: a conceptual review. Biol. Rev. Camb. Philos. Soc. [DOI] [PubMed] [Google Scholar]
- Lind J., Kaby U., Jakobsson S. (2002). Split-second escape decisions in blue tits (Parus caeruleus). Naturwissenschaften 89, 420-423. [DOI] [PubMed] [Google Scholar]
- Lord R. D., Jr (1956). A comparative study of the eyes of some falconiform and passeriform birds. Am. Midl. Nat. 56, 325-344. [Google Scholar]
- Machovsky-Capuska G. E., Howland H. C., Raubenheimer D., Vaughn-Hirshorn R., Würsig B., Hauber M. E., Katzir G. (2012). Visual accommodation and active pursuit of prey underwater in a plunge-diving bird: the Australasian gannet. Proc. Biol. Sci. 279, 4118-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. (2012). Through birds' eyes: insights into avian sensory ecology. J. Ornithol. 153 Suppl., 23-48. [Google Scholar]
- Martin G. R. (2014). The subtlety of simple eyes: the tuning of visual fields to perceptual challenges in birds. Philos. Trans. R. Soc. B 369, 20130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Portugal S. J., Murn C. P. (2012). Visual fields, foraging and collision vulnerability in Gyps vultures. Ibis 154, 626-631. [Google Scholar]
- McCormack J. E., Harvey M. G., Faircloth B. C., Crawford N. G., Glenn T. C., Brumfield R. T. (2013). A phylogeny of birds based on over 1,500 loci collected by target enrichment and high-throughput sequencing. PLoS ONE 8, e54848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischiati M., Krishnaprasad P. S. (2012). The dynamics of mutual motion camouflage. Syst. Control Lett. 61, 894-903. [Google Scholar]
- Moinard C., Rutherford K. M. D., Statham P., Green P. R. (2005). Visual fixation of a landing perch by chickens. Exp. Brain Res. 162, 165-171. [DOI] [PubMed] [Google Scholar]
- Nahin P. J. (2012). Chases and Escapes. Princeton, NJ: Princeton University Press. [Google Scholar]
- National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs) (2010). ARRIVE (Animal Research: Reporting in Vivo Experiments) Guidelines. London: NC3Rs. [Google Scholar]
- Nolfi S. (2012). Co-evolving predator and prey robots. Adapt. Behav. 20, 10-15. [Google Scholar]
- O'Rourke C. T., Hall M. I., Pitlik T., Fernández-Juricic E. (2010). Hawk eyes I: diurnal raptors differ in visual fields and degree of eye movement. PLoS ONE 5, e12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon S., Harmening W., Wagner H., Rivlin E. (2008). Through a barn owl's eyes: interactions between scene content and visual attention. Biol. Cybern. 98, 115-132. [DOI] [PubMed] [Google Scholar]
- Olberg R. M. (2012). Visual control of prey-capture flight in dragonflies. Curr. Opin. Neurobiol. 22, 267-271. [DOI] [PubMed] [Google Scholar]
- Oliva D., Medan V., Tomsic D. (2007). Escape behavior and neuronal responses to looming stimuli in the crab Chasmagnathus granulatus (Decapoda: Grapsidae). J. Exp. Biol. 210, 865-880. [DOI] [PubMed] [Google Scholar]
- Osorio D., Srinivasan M. V., Pinter R. B. (1990). What causes edge fixation in walking flies? J. Exp. Biol. 149, 281-292. [DOI] [PubMed] [Google Scholar]
- Pais D., Leonard N. E. (2010). Pursuit and evasion: evolutionary dynamics and collective motion. In AIAA Guidance, Navigation and Control Conference (ed. AIAA ). Toronto, ON: AIAA. [Google Scholar]
- Pennycuick C. J., Fuller M. R., Oar J. J., Kirkpatrick S. J. (1994). Falcon versus grouse: flight adaptations of a predator and its prey. J. Avian Biol. 25, 39-49. [Google Scholar]
- Pettit B., Perna A., Biro D., Sumpter D. J. T. (2013). Interaction rules underlying group decisions in homing pigeons. J. R. Soc. Interface 10, 20130529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponitz B., Schmitz A., Fischer D., Bleckmann H., Brücker C. (2014). Diving-flight aerodynamics of a peregrine falcon (Falco peregrinus). PLoS ONE 9, e86506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S. J., Hubel T. Y., Fritz J., Heese S., Trobe D., Voelkl B., Hailes S., Wilson A. M., Usherwood J. R. (2014). Upwash exploitation and downwash avoidance by flap phasing in ibis formation flight. Nature 505, 399-402. [DOI] [PubMed] [Google Scholar]
- Potts W. K. (1984). The chorus-line hypothesis of manoeuvre coordination in avian flocks. Nature 309, 344-345. [Google Scholar]
- Reddy P. V., Justh E. W., Krishnaprasad P. S. (2006). Motion camouflage in three dimensions. In Proceedings of the 45th IEEE Conference on Decision and Control (IEEE Cat. No. 06CH37770), San Diego, CA, pp. 3327-3332 New York, NY: IEEE. [Google Scholar]
- Reddy P. V., Justh E. W., Krishnaprasad P. S. (2007). Motion camouflage with sensorimotor delay. In Proceedings of the 46th IEEE Conference on Decision and Control, New Orleans, LA, pp. 1660-1665 New York, NY: IEEE. [Google Scholar]
- Regan D., Vincent A. (1995). Visual processing of looming and time to contact throughout the visual field. Vision Res. 35, 1845-1857. [DOI] [PubMed] [Google Scholar]
- Ros I. G., Bassman L. C., Badger M. A., Pierson A. N., Biewener A. A. (2011). Pigeons steer like helicopters and generate down- and upstroke lift during low speed turns. Proc. Natl. Acad. Sci. USA 108, 19990-19995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal Netherlands Meteorological Institute (2014). Daily weather data of the Netherlands. Available at: http://www.knmi.nl/climatology/daily_data/selection.cgi
- Rutz C. (2006). Home range size, habitat use, activity patterns and hunting behaviour of urban-breeding northern goshawks Accipiter gentilis. Ardea 94, 185-202. [Google Scholar]
- Rutz C., Troscianko J. (2013). Programmable, miniature video-loggers for deployment on wild birds and other wildlife. Methods Ecol. Evol. 4, 114-122. [Google Scholar]
- Rutz C., Bluff L. A., Weir A. A. S., Kacelnik A. (2007). Video cameras on wild birds. Science 318, 765-765. [DOI] [PubMed] [Google Scholar]
- Ruxton G. D., Sherratt T. N., Speed M. P. (2004). Avoiding Attack: the Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford, UK: Oxford University Press. [Google Scholar]
- Sargent T. D. (1990). Startle as an anti-predator mechanism with special reference to Catocala. In Insect Defenses: Adaptive Mechanisms and Strategies for Prey and Predators (ed. Evans D. L., Schmidt J. O.). Albany, NY: State University of New York Press. [Google Scholar]
- Schiff W. (1965). Perception of impending collision – a study of visually directed avoidant behavior. Psychol. Monogr. 79, 1-26. [DOI] [PubMed] [Google Scholar]
- Schuler W. (1990). Avian predatory behavior and prey distribution. In Insect Defenses Adaptive Mechanisms and Strategies of Prey and Predators (ed. Evans D. L., Schmidt J. O.), pp. 151-171 Albany, NY: State University of New York Press. [Google Scholar]
- Sebesta K., Baillieul J. (2012). Animal-inspired agile flight using optical flow sensing. In 51st IEEE Annual Conference on Decision and Control (CDC), pp. 3727-3734 Maui, HI: IEEE. [Google Scholar]
- Shaw R. L. (1985). Fighter Combat: Tactics and Maneuvering. Annapolis, MD: Naval Institute Press. [Google Scholar]
- Shelton R. M., Jackson B. E., Hedrick T. L. (2014). The mechanics and behavior of cliff swallows during tandem flights. J. Exp. Biol. 217, 2717-2725. [DOI] [PubMed] [Google Scholar]
- Shifferman E., Eilam D. (2004). Movement and direction of movement of a simulated prey affect the success rate in barn owl Tyto alba attack. J. Avian Biol. 35, 111-116. [Google Scholar]
- Shima T. (2007). Deviated velocity pursuit. In AIAA Guidance, Navigation and Control Conference and Exhibit, pp. 2007-6782 Hilton Head, SC: AAIA. [Google Scholar]
- Squires J. R., Reynolds R. T. (1997). Northern goshawk (Accipiter gentilis). In The Birds of North America Online, Vol. 2013 (ed. Poole A.). Ithaca, NY: Cornell Laboratory of Ornithology. [Google Scholar]
- Srinivasan M. V. (2011). Honeybees as a model for the study of visually guided flight, navigation, and biologically inspired robotics. Physiol. Rev. 91, 413-460. [DOI] [PubMed] [Google Scholar]
- Srinivasan M. V., Zhang S. (2004). Visual motor computations in insects. Annu. Rev. Neurosci. 27, 679-696. [DOI] [PubMed] [Google Scholar]
- Stankowich T., Blumstein D. T. (2005). Fear in animals: a meta-analysis and review of risk assessment. Proc. Biol. Sci. 272, 2627-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. W., Krebs J. R. (1986). Foraging Theory. Princeton, NJ: Princeton University Press. [Google Scholar]
- Stevens M. (2013). Sensory Ecology, Behavior and Evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- Stevens M., Hardman C. J., Stubbins C. L. (2008). Conspicuousness, not eye mimicry, makes ‘eyespots’ effective antipredator signals. Behav. Ecol. 19, 525-531. [Google Scholar]
- Sun H., Frost B. J. (1998). Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nat. Neurosci. 1, 296-303. [DOI] [PubMed] [Google Scholar]
- Sustaita D., Hertel F. (2010). In vivo bite and grip forces, morphology and prey-killing behavior of North American accipiters (Accipitridae) and falcons (Falconidae). J. Exp. Biol. 213, 2617-2628. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Sato K., Naito Y., Dunn M. J., Trathan P. N., Croxall J. P. (2004). Penguin-mounted cameras glimpse underwater group behaviour. Proc. Biol. Sci. 271 Suppl. 5, S281-S282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault D. H., Fuller N. W., Jackson B. E., Bluhm E., Evangelista D., Wu Z., Betke M., Hedrick T. L. (2014). A protocol and calibration method for accurate multi-camera field videography. J. Exp. Biol. 217, 1843-1848. [DOI] [PubMed] [Google Scholar]
- Tislerics A. (2000). Oryctolagus cuniculus: European rabbit. Animal Diversity Web. Ann Arbor, MI: University of Michigan Museum of Zoology. [Google Scholar]
- Tobalske B. W., Dial K. P. (2000). Effects of body size on take-off flight performance in the Phasianidae (Aves). J. Exp. Biol. 203, 3319-3332. [DOI] [PubMed] [Google Scholar]
- Treleaven R. B. (1980). High and low intensity hunting in raptors. Z. Tierpsychol. 54, 339-345. [Google Scholar]
- Trischler C., Kern R., Egelhaaf M. (2010). Chasing behavior and optomotor following in free-flying male blowflies: flight performance and interactions of the underlying control systems. Front. Behav. Neurosci. 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker V. A., Tucker A. E., Akers K., Enderson J. H. (2000). Curved flight paths and sideways vision in peregrine falcons (Falco peregrinus). J. Exp. Biol. 203, 3755-3763. [DOI] [PubMed] [Google Scholar]
- van den Hout P. J., Mathot K. J., Maas L. R. M., Piersma T. (2009). Predator escape tactics in birds: linking ecology and aerodynamics. Behav. Ecol. 21, 10. [Google Scholar]
- Wang Y., Frost B. J. (1992). Time to collision is signalled by neurons in the nucleus rotundus of pigeons. Nature 356, 236-238. [DOI] [PubMed] [Google Scholar]
- Warrick D. R., Bundle M. W., Dial K. P. (2002). Bird maneuvering flight: blurred bodies, clear heads. Integr. Comp. Biol. 42, 141-148. [DOI] [PubMed] [Google Scholar]
- Watanabe Y. Y., Takahashi A. (2013). Linking animal-borne video to accelerometers reveals prey capture variability. Proc. Natl. Acad. Sci. USA 110, 2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei E., Justh E. W., Krishnaprasad P. S. (2009). Pursuit and an evolutionary game. Proc. R. Soc. A 465, 1539-1559. [Google Scholar]
- Widen P. (1989). The hunting habitats of goshawks Accipeter gentilis in boreal forests of central Sweden. Ibis 131, 205-213. [Google Scholar]
- Yamamoto K., Nakata M., Nakagawa H. (2003). Input and output characteristics of collision avoidance behavior in the frog Rana catesbeiana. Brain Behav. Evol. 62, 201-211. [DOI] [PubMed] [Google Scholar]
- Yorzinski J. L., Platt M. L. (2014). Selective attention in peacocks during predator detection. Anim. Cogn. 17, 767-777. [DOI] [PubMed] [Google Scholar]
- Zhang S. W., Wang X. A., Liu Z. L., Srinivasan M. V. (1990). Visual tracking of moving targets by freely flying honeybees. Vis. Neurosci. 4, 379-386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.