Abstract
Mammalian hibernators provide an extreme example of naturally occurring challenges to muscle homeostasis. The annual hibernation cycle is characterized by shifts between summer euthermy with tissue anabolism and accumulation of body fat reserves, and winter heterothermy with fasting and tissue catabolism. The circannual patterns of skeletal muscle remodelling must accommodate extended inactivity during winter torpor, the motor requirements of transient winter active periods, and sustained activity following spring emergence. Muscle volume in thirteen-lined ground squirrels (Ictidomys tridecemlineatus) calculated from MRI upper hindlimb images (n=6 squirrels, n=10 serial scans) declined from hibernation onset, reaching a nadir in early February. Paradoxically, mean muscle volume rose sharply after February despite ongoing hibernation, and continued total body mass decline until April. Correspondingly, the ratio of muscle volume to body mass was steady during winter atrophy (October–February) but increased (+70%) from February to May, which significantly outpaced changes in liver or kidney examined by the same method. Generally stable myocyte cross-sectional area and density indicated that muscle remodelling is well regulated in this hibernator, despite vastly altered seasonal fuel and activity levels. Body composition analysis by echo MRI showed lean tissue preservation throughout hibernation amid declining fat mass by the end of winter. Muscle protein synthesis was 66% depressed in early but not late winter compared with a summer fasted baseline, while no significant changes were observed in the heart, liver or intestine, providing evidence that could support a transition in skeletal muscle regulation between early and late winter, prior to spring emergence and re-feeding.
KEY WORDS: Spermophilus, Disuse atrophy, Muscle regeneration, SUnSET, Echo magnetic resonance imaging
INTRODUCTION
Mammalian hibernators display annual cycles of extreme changes in physiology and morphology (for a review, see Carey et al., 2003). In ground squirrels, this pattern segregates an active period of reproduction, growth and fattening in spring and summer from an inactive period of fasting and hibernation in autumn and winter. The energy stores accumulated during the active season provide the metabolic fuel for hibernation, made possible by the energy savings derived from depressed metabolic rate and low body temperature during winter torpor bouts (for a review, see Lyman et al., 1982). However, for hibernation to be of net benefit, animal cells and tissues must cope with the pitfalls of extended inactivity. Such coping strategies have promising potential for translation to medicine in areas such as: disuse atrophy in bone, muscle and gut; body weight regulation; and ischemia–reperfusion injury (Carey et al., 2003; Andres-Mateos et al., 2013).
Recurring periods of torpor commonly last 1–2 weeks in thirteen-lined ground squirrels, punctuated by brief rewarming arousals to euthermia (~12 h) (Carey et al., 2003). The animals remain in their burrows during arousal, typically inactive and sleeping (Lyman et al., 1982). In other inactivity scenarios for similarly sized mammals (e.g. rats), skeletal muscle atrophy is detectable within a time frame consistent with the duration of a single ground squirrel torpor bout (Musacchia et al., 1990; Bodine, 2013). Yet skeletal muscle function and locomotor ability is sufficiently preserved across hibernation to support not only shivering thermogenesis during repeated rewarming, but also the possibility of activity during interbout arousal periods. Most importantly, ground squirrels must exit the hibernation season and emerge from their burrows capable of foraging, predator avoidance and reproduction. Multiple studies document muscle preservation or limited atrophy during hibernation in rodents (e.g. Wickler et al., 1987; Steffen et al., 1991; Cotton and Harlow, 2010; Nowell et al., 2011; Gao et al., 2012; Andres-Mateos et al., 2013), suggesting that some degree of protection against inactivity may exist and that its regulation is muscle-type specific (e.g. Reid et al., 1995; Nowell et al., 2011). Several hypotheses have been advanced to explain retention of muscle mass in small mammals during winter, including: the preservation benefits of intermittent shivering activity (e.g. Nowell et al., 2011); a reduction in muscle degradation capacity at low temperature (Nagano et al., 2003; Velickovska et al., 2005); continued transcriptional activity in satellite cell nuclei throughout winter (Malatesta et al., 2009); and up-regulation of candidate muscle-preserving proteins (Lee et al., 2010; Nowell et al., 2011; Kornfeld et al., 2012; Andres-Mateos et al., 2013; Rouble et al., 2013; Xu et al., 2013).
To preserve muscle mass, protein degradation must be balanced by protein synthesis. Several lines of evidence converge on the importance of maintaining protein synthesis during hibernation by activating the mechanistic/mammalian target of rapamycin (mTOR) signalling cascade (e.g. Lee et al., 2010; Nowell et al., 2011; Andres-Mateos et al., 2013; Fedorov et al., 2014). mTOR is a kinase that regulates many cellular processes involved in proliferation and growth, including protein synthesis, in part,
List of symbols and abbreviations
- CSA
cross-sectional area
- L0
muscle resting length
- Mb
body mass
- MRI
magnetic resonance imaging
- mTOR
mechanistic/mammalian target of rapamycin
- SUnSET
surface sensing of translations
through the phosphorylation and activation of eIF4G, 4E-BP1 and p70S6K (Ma and Blenis, 2009; Schiaffino et al., 2013). Golden-mantled ground squirrels (Callospermophilus lateralis) depress myostatin (a protein kinase B and thus mTOR inhibitor) in two muscles that are spared of winter atrophy (diaphragm and soleus), possibly facilitating mTOR signalling by reducing this inhibition (Nowell et al., 2011). Periodic arousals from winter torpor in greater tube-nosed bats (Murina leucogaster) as well as thirteen-lined ground squirrels (Ictidomys tridecemlineatus) appear to be associated with oscillations in the activation of mTOR (Lee et al., 2010; Wu and Storey, 2012). Increases in the phosphorylation status of both 4E-BP1 and p70S6K have been documented in hibernating thirteen-lined ground squirrels, and this evidence of activation in the mTOR protein synthesis cascade was shown to be controlled by SGK1, a previously unknown regulator of muscle mass in both hibernators and non-hibernators (Andres-Mateos et al., 2013).
Reliance on elevated protein synthesis, rather than impaired protein degradation, seems at odds with the energy conserving hibernation strategy. Moreover, animals enter and exit hibernation with dramatic differences in whole-body metrics such as mass, energy stores and exercise patterns (e.g. Hindle and Martin, 2014), and probably many yet-to-be documented cell-level distinctions, raising the question of potentially differing muscle maintenance strategies in early versus late winter, and immediately in advance of spring emergence. Are protein synthesis rates a function of time within the winter fast?
Detecting the incorporation of radioactivity-labelled tracers into proteins is the traditional method to assess protein synthesis rates (Wolfe, 2005). Recently, however, a non-radioactive technique, SUnSET (Surface Sensing of Translations), has been developed to measure protein synthesis (Schmidt et al., 2009). In the SUnSET method, an animal is treated with the antibiotic puromycin, which is incorporated into elongating peptide chains. Following an in vivo incubation, the degree of puromycin incorporation into peptides can be evaluated in tissues of interest using a puromycin antibody. SUnSET has been validated in vitro and in vivo for mice against standard radioactive-based methods (Schmidt et al., 2009; Goodman et al., 2011). This novel technique is promising for settings where use of radioactivity is undesirable, as well as to measure protein synthesis spatially within tissues via immunohistochemistry (Goodman and Hornberger, 2013).
Here, we incorporate the recently developed SUnSET method to measure rates of protein synthesis with a characterization of the prevalence and patterns of skeletal muscle atrophy in the thirteen-lined ground squirrel (Ictidomys tridecemlineatus Mitchill 1821) across the hibernation season. To a large extent we exploited sampling opportunities available as part of other studies. We used several different tissue collections to specifically explore the relationships between skeletal muscle morphology, protein synthesis rates and body composition in hibernating thirteen-lined ground squirrels, focusing on differences between early and late winter and the transition towards spring emergence.
RESULTS
Annual cycle in tissue size
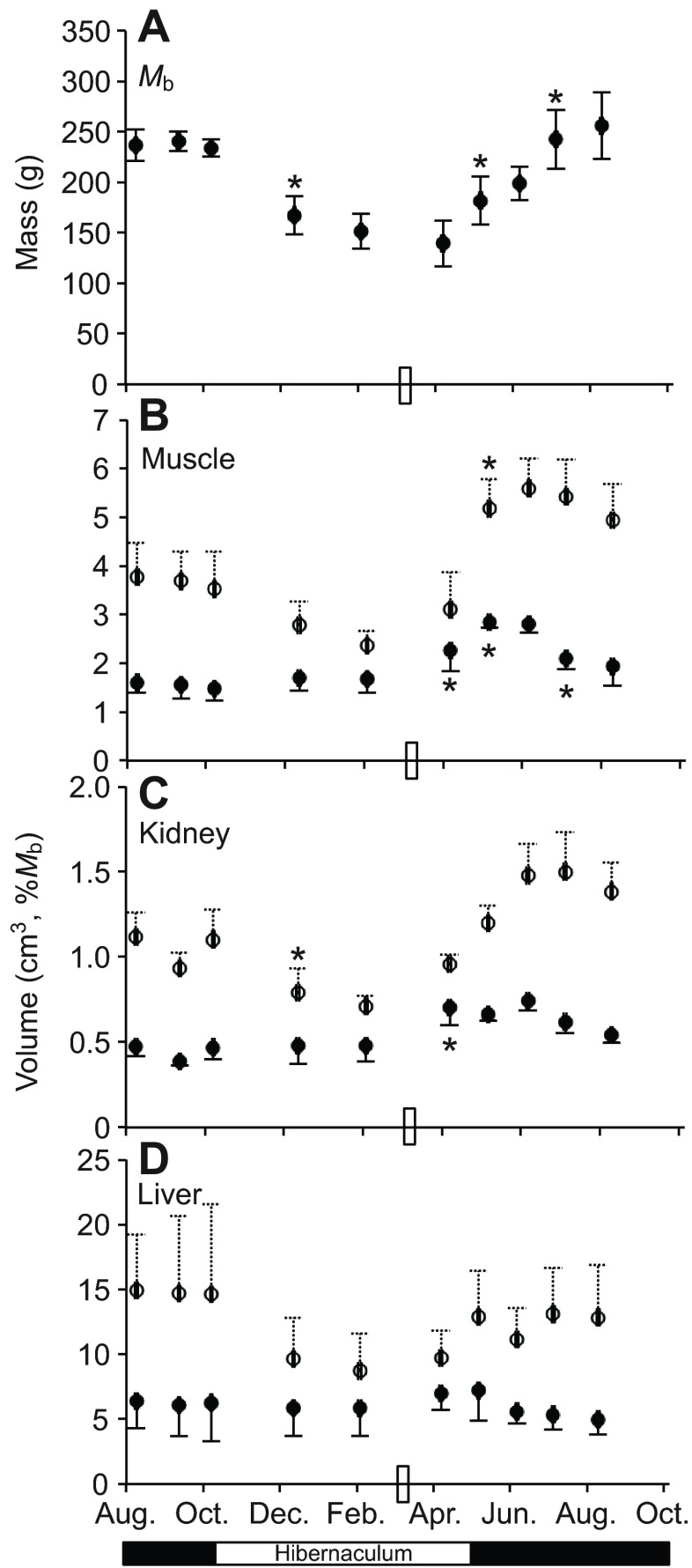
Thirteen-lined ground squirrels were serially sampled 10 times across a single year by magnetic resonance imaging (MRI). These animals had a body mass (Mb) nadir on 9 April (Fig. 1A, data from N=5–6 individuals per time point), reflecting a 42% average drop in Mb from the previous 12 September and a 48% reduction compared with the following August (Fig. 1A). Hindlimb muscle, kidney and liver volumes calculated from MRI images of these ground squirrels displayed a pattern similar to Mb, except the nadir occurred in the preceding sampling point, on 4 February (Fig. 1B–D, open circles). Specifically, hindlimb muscle volume declined 33% from the previous 12 September to 4 February (P<0.05). When sequential time points were compared statistically, Mb (P<0.05) and kidney volume (P<0.05) declined significantly between 8 October and 13 December. The significant Mb increase (P<0.05) from 9 April to 9 May was matched only by an increase in hindlimb muscle volume (P<0.05; Fig. 1A,B). We then normalized calculated tissue volumes to Mb at each time point to examine the departure of specific tissues from overall body responses across the year (Fig. 1B–D, filled circles). There were no changes in mass-normalized tissue volumes in early winter. Continued Mb decrease combined with volume increase in the tissues examined, especially hindlimb muscle, led to significantly increased individual mass-normalized volume in muscle (P<0.05) and kidney (P<0.05) between 4 February and 9 April – a period encompassing the late-winter phase of hibernation for these ground squirrels (Fig. 1B,C). Muscle exhibited the largest late-winter increase (70%) due to a volume increase that significantly outpaced Mb across two sampling points representing 3 months (P<0.05 from 4 February to 9 May, Fig. 1B). This annual pattern differed most from liver volume, which closely tracked changes in Mb (Fig. 1D).
Fig. 1.
Sequential animal mass and tissue volumes across a single year in thirteen-lined ground squirrels. (A) Body mass (Mb, mean ± s.d.) of the same six individuals collected at 10 time points across a single year, with N=1 individual dropped from the study after 9 April (time point no. 6). (B–D) Tissue volume (mean ± s.d.) monitored by MRI is presented in cm3 (open symbols) and normalized to individual Mb (filled symbols) of hindlimb muscle (B), kidney (C) and liver (D). Asterisks indicate a significant difference (Tukey's test, P<0.05) between the identified sample and the preceding time point. The rectangle on the x-axis represents the time point at which food and water were returned to the cages; hibernaculum versus standard rodent housing is indicated below the x-axis.
Skeletal muscle mass
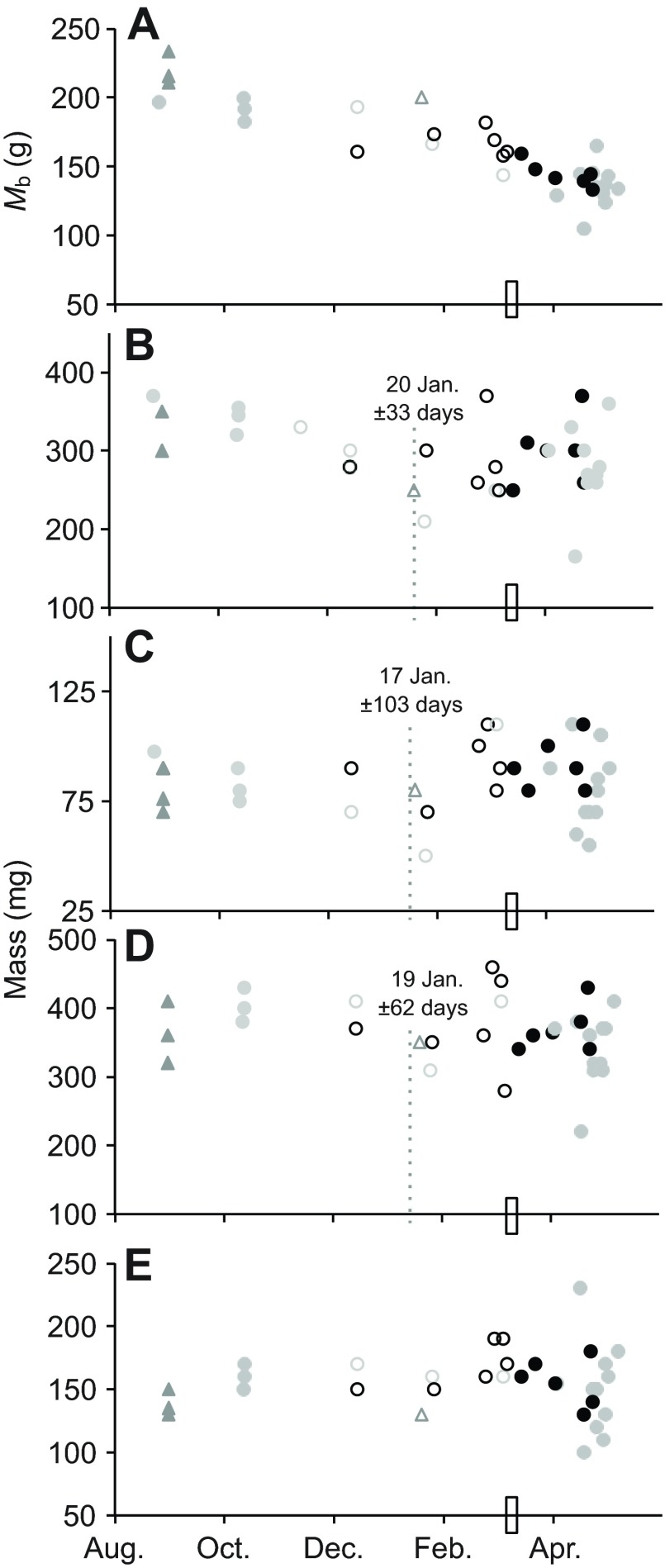
Skeletal muscle samples were collected across the hibernation season from non-weight-matched ground squirrels with specific focus on the late-winter hibernation period prior to spring emergence (Fig. 2). Muscle mass appeared generally stable across the hibernation season (soleus) or of similar pattern to the MRI volume calculations for upper hindlimb (rectus femoris, biceps brachii; Fig. 2), with a pattern of early winter decline then pre-emergence recovery. These patterns of muscle mass stability or late season recovery were most striking when compared against the declining Mb observed in the same animals over the course of hibernation (Fig. 2A). In all muscles but soleus, there was a mid-winter breakpoint in the time course pattern calculated from muscle mass normalized to the pre-hibernation Mb of each individual, indicating that mass did not follow the same relationship to time of year in early versus late winter (17–20 January; Fig. 2). These breakpoints in the time course relationship precede the nadir of Mb (Fig. 1).
Fig. 2.
Skeletal muscle mass in thirteen-lined ground squirrels. Mb at time of tissue collection in non-weight-matched animals (A) is plotted alongside wet mass of four muscles: rectus femoris (B); biceps brachii (C); gastrocnemius (D); and soleus (E). Animals were sampled pre-hibernation (N=6) and across the hibernation season (N=27). Open circles represent individuals that did not have access to food or water; closed symbols indicate individuals to whom food was available although consumption, if any, was not documented (the rectangle on the x-axis indicates the time point at which food and water were returned to the cages in the hibernaculum). Segmented regression analyses for all animals (day of year ± s.e.m., dotted lines) are included when they indicated that a single regression (slope and intercept) could not explain the time course data. Breakpoints were calculated after normalizing muscle mass to 1 October Mb, to control for the lack of weight-matched sampling groups. Animal gender is designated as female (grey symbols), male (black symbols) or unknown (triangles).
Stability in myocyte size and density across hibernation
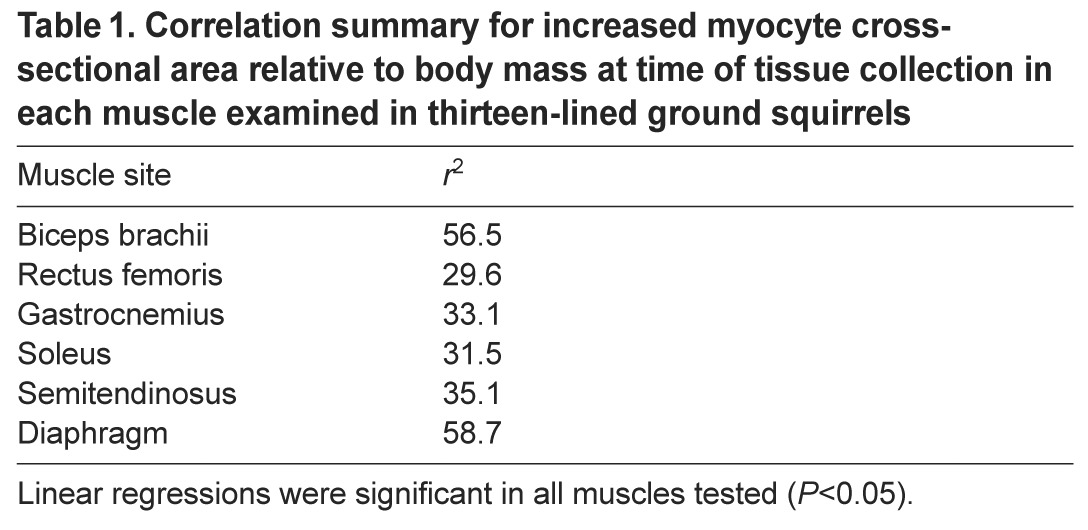
To examine cell-level morphology of specific muscles during hibernation, myocyte cross-sectional area (CSA) and densities were determined for each muscle collected (Fig. 3), with the addition of semitendinosus and diaphragm. The combination of these metrics can be informative in identifying the method of change in overall muscle appearance by suggesting atrophy/hypertrophy of individual myocytes or the loss/gain of cells (i.e. hypo- or hyperplasia). We found no evidence of over-winter atrophy of individual myocytes in any muscle examined. Positive relationships between time of year and CSA normalized to Mb at tissue collection (Table 1) indicate that CSA was preserved across hibernation in the face of Mb decline. Indeed, raw, non-normalized CSA values were either stable (semitendinosus, gastrocnemius and soleus) or increased within the sampling time frame (Fig. 3). In diaphragm, CSA was stable throughout winter, but increased between hibernation and pre-hibernation samples (Fig. 3A). There was a significant segmented breakpoint on 29 October and only the preceding data displayed a significant linear relationship with time of year (r2=0.61, P<0.05). CSA in biceps brachii was positively correlated with time of year (r2=0.28, P<0.05) until late winter (segmented breakpoint 17 April). Although rectus femoris data did produce a significant segmented breakpoint at 17 April (Fig. 3B), no linear relationship (P>0.05) between CSA and time of year was noted on either side of the breakpoint. Myocyte density was stable in semitendinosus and gastrocnemius muscles that also had stable fiber CSA. Co-ordinated changes in fiber density occurred with trends in CSA; density breakpoints matched corresponding CSA breakpoints in diaphragm, biceps brachii and rectus femoris (Fig. 3). In all cases, density changed in opposite pattern to CSA over time, decreasing in diaphragm and biceps brachii, while CSA increased. Despite no significant linearity in rectus femoris CSA (Fig. 3B), density increased prior to and decreased following the segmented breakpoint (17 April).
Fig. 3.
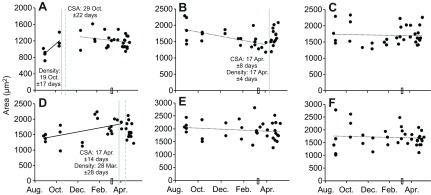
Myocyte cross-sectional area across hibernation in thirteen-lined ground squirrels. Rather than decline in winter atrophy, average myocyte cross-sectional area appears seasonally increased or stable. Cross-sectional area across the sampling period is presented for diaphragm (A), rectus femoris (B), soleus (C), biceps brachii (D), semitendinosus (E) and gastrocnemius (F). A segmented regression analysis indicated that a single regression (slope or intercept) could not explain the time course data for diaphragm, biceps brachii or rectus femoris (panels A–C), therefore the regression breakpoint estimates (±s.e.m.) derived from the analyses are indicated by grey dotted lines. Continuous vertical lines indicate the corresponding breakpoints in cell density for the same data set, indicating that myocyte size and density display co-ordinate patterns in hibernation. A rectangle on the x-axis represents the time point at which food and water were returned to the cages. Continuous lines denote significant linear relationships; broken lines reflect non-significance.
Table 1.
Correlation summary for increased myocyte cross-sectional area relative to body mass at time of tissue collection in each muscle examined in thirteen-lined ground squirrels
Myocyte density decreased across the entire sampling period only in soleus (r2=0.21, P<0.05).
Protein synthesis is differentially regulated across hibernation
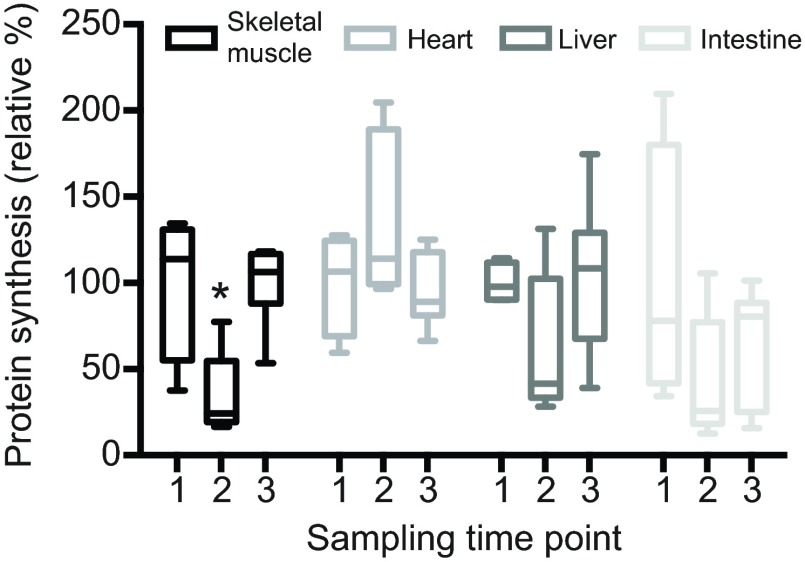
The late-winter maintenance and regrowth of skeletal muscles despite ongoing hibernation is supported by a return to summer baseline protein synthesis levels (Fig. 4). In this dataset, early hibernation season ground squirrels were sampled on 20 October, and showed a 66% decrease in protein synthesis from a baseline established by summer fasted ground squirrels (pairwise Tukey's post hoc test, P<0.05). However, protein synthesis significantly increased between early and late winter samples collected between 15 December and 13 January (P<0.05). This late-winter time point occurred prior to the regression breakpoints reported for the muscle mass data (Fig. 2; 17–20 January). By contrast, no significant changes in protein synthesis rates were documented for heart, liver or intestine (Fig. 4), highlighting the potentially unique regulation occurring in hibernating skeletal muscle.
Fig. 4.
Skeletal muscle protein synthesis increased from early to late winter. Protein synthesis in hindlimb muscle, heart, liver and intestine was detected by in vivo SUnSET in four summer-fasted ground squirrels (label 1), and compared with five early (label 2) and six late winter aroused hibernators after at least five consecutive days of torpor (label 3). Early winter animals were sampled 33–35 days after the onset of hibernation; late winter animals were sampled after 78–106 days. Protein synthesis is expressed relative to summer-fasted levels and does not change in the heart, liver or intestine across the time points analyzed (ANOVA, P>0.05). Skeletal muscle protein synthesis decreased in early winter, relative to both the summer baseline and the late winter hibernators (asterisk, ANOVA, P<0.05).
Body composition is stable until late winter
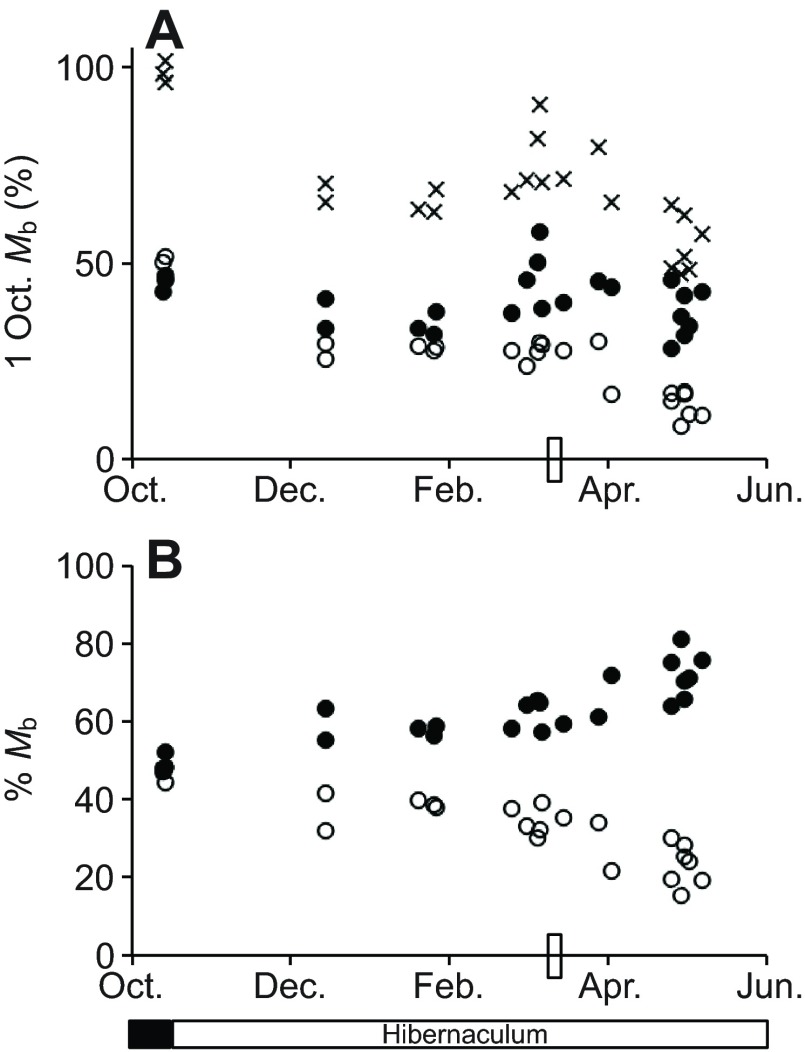
Body composition analyses with quantitative magnetic resonance (echo MRI) confirmed, as expected, that hibernating thirteen-lined ground squirrels held lean mass more constant than fat mass across this dataset (Fig. 5A). Although the variability in both lean (30%) and fat (22%) mass are similar in hibernating ground squirrels when normalized to the 1 October Mb of each individual, fat mass variability across the entire dataset was considerable. When three pre-hibernation animals were included, the maximum discrepancy in body fat between pre-hibernation and late hibernators was 43%, reflecting large fat stores acquired in anticipation of hibernation (Dark, 2005). Animals sampled throughout winter and in the transition period before spring emergence clearly demonstrated an increased contribution of lean tissue to overall body composition in late winter, while fat content declined (Fig. 5B).
Fig. 5.
Body composition of thirteen-lined ground squirrels through the hibernation season determined by echo MRI. (A) Individual Mb (crosses), lean content (filled circles) and fat content (open circles) of N=3 ground squirrels pre-hibernation and N=20 ground squirrels exhibiting torpor–arousal cycles during winter and up to spring emergence were normalized to the 1 October Mb of each individual to control for the mass range of the animals. (B) The contribution of lean (closed circles) and fat mass (open circles) to overall body composition, from independently calculated lean and fat mass normalized to Mb at sacrifice. The rectangle on the x-axis represents the time point at which food and water were returned to the cages; hibernaculum versus standard rodent housing is indicated below the x-axis.
DISCUSSION
A changing pattern of morphology prepares muscle for spring emergence
Winter hibernation in thirteen-lined ground squirrels is associated with a considerable loss of Mb, with corresponding declines in the volume of internal organs and skeletal muscle (Fig. 1). Endogenous fuel reserves and body composition of ground squirrels differ substantially between entrance to and exit from hibernation, as ground squirrels rely entirely on endogenous reserves during this period. The majority of observed Mb decline across hibernation was due to diminishing fat reserves and lipid content of tissues rather than the consumption of lean mass (Fig. 5). A shift away from carbohydrates and proteins and towards lipid fuels is a hallmark of hibernation physiology, and this shift has been well documented in ground squirrels (for a review, see Carey et al., 2003). A protein-sparing, fasting-tolerant physiology in hibernating ground squirrels has also been proposed through urea recycling, which would contribute to protein conservation during hibernation (Riedesel and Steffen, 1980) and limit the need to catabolize inactive muscles for amino acid substrates. The combination of these metabolic adjustments should lead to exploitation of endogenous lipid reserves over carbohydrates and proteins; therefore, we examined time course data across hibernation to detect changing patterns of tissue morphology and protein synthesis associated with diminishing body fat reserves.
As skeletal muscle comprises the largest pool of tissue protein in the body, examination of skeletal muscle dynamics across the hibernation season can provide insight into its role in fasting physiology and hibernation. Muscle volume reaches its winter minimum fully 2 months before a minimum occurs in ground squirrel Mb (Fig. 1), suggesting a change in fasting strategy mid–late winter designed to preserve remaining lean tissue. Indeed, this uncoupling of muscle volume from Mb decline corresponds to altered body composition (Fig. 5), driven by an increased rate of fat mass loss but no change in lean mass. Explaining the difference between the minimum observed hindlimb muscle volume (4 February) and Mb (9 April) is complicated by the sampling design. This was one of the longest gaps between sampled time points in the dataset presented in Fig. 1, and the ground squirrels were emerging from hibernation over this time and therefore were provided with food during this interval. Despite the difficulty in pinpointing differences in the time courses of muscle volume versus Mb, it is noteworthy that within this interval, hindlimb muscle had the highest rate of increase, particularly compared with liver in the same individuals. This evidence supports the interpretation of muscle rebuilding in late hibernation, prioritized relative to some other tissues.
The early winter decline followed by pre-spring increase in muscle volume was matched by late winter stability or even increase in muscle mass in biceps brachii, rectus femoris, gastrocnemius and soleus (Fig. 2). The altered dynamics of muscle mass after late January occurs prior to refeeding, which began on 10 March. The combination of muscle mass observations and a statistical analyses of the data's time course indicating that muscle recovery begins in advance of refeeding suggests that locomotory capability may be a prioritized element of spring arousal.
Previous investigations of muscle atrophy in hibernating rodents have produced varied results. A similar investigation of thirteen-lined ground squirrels (Andres-Mateos et al., 2013) found no evidence of altered muscle morphology across the hibernation season. As noted in our study, although a striking disuse atrophy does not manifest in this species, it still exhibits a muscle mass decline that is on pace with declining Mb in early winter. As similar muscle groups were examined, it is difficult to explain these between-study differences. It is not clear where precisely the morphology measurements collected at 2–3 months post-torpor initiation (Andres-Mateos et al., 2013) would fall along our early–late winter time scale. Certainly the molecular measurements collected at 4–5 months post-torpor initiation represent our late winter time points and are discussed in more detail below. Consistent with other hibernation studies, we found that degree of muscle loss is generally muscle specific (e.g. Wickler et al., 1991; Tinker et al., 1998; Nowell et al., 2011). In contrast to other mammalian disuse models, hibernating ground squirrels preserve slower oxidative fibers (types I and IIx) relative to fast glycolytic type IIb (Egginton et al., 2001; Rourke et al., 2004; Rourke et al., 2006; Hershey et al., 2008; Nowell et al., 2011; Gao et al., 2012; Bodine, 2013; Xu et al., 2013). This response may be a mechanism to preserve oxidative ability in skeletal muscles and to support a shift to a lipid-based metabolism. Soleus is composed predominantly of type I fibers (e.g. Delp and Duan, 1996) (for rats) and is therefore the slowest-twitch muscle examined here; selective preservation of slow-twitch fibers may contribute to the limited atrophy observed in soleus in this and other studies (Nowell et al., 2011). Consistency between muscle types and the fiber compositions of specific muscles may be an important normalizer in comparing muscle responses to hibernation across studies and species.
Consistent with previous observations in various muscle groups (e.g. Tinker et al., 1998; Hershey et al., 2008; Gao et al., 2012; Andres-Mateos et al., 2013), we detected no change in myocyte CSA or density across hibernation in semitendinosus or gastrocnemius (Fig. 3). Myocyte size (CSA) was generally stable, with only limited increases noted for diaphragm and biceps brachii, resulting in an increased Mb-specific myocyte CSA across the hibernation season in all six muscles examined (Table 1). This suggests that the observed decline in volume of the lower hindlimb muscles (12 September to 4 February, P<0.05; Fig. 1), consisting of rectus femoris and semitendinosus, may have been due to the loss of fibers (hypoplasia). This process maintains the size and spacing of existing myocytes, and is consistent with a selective reduction of type IIb fibers in winter muscles. Conversely, this stability in CSA implies that late winter regrowth occurs via hyperplasia. While Andres-Mateos et al. (Andres-Mateos et al., 2013) found no differences in the expression of myogenic factors in torpid, hibernating thirteen-lined ground squirrels, Tessier and Storey (Tessier and Storey, 2010) documented transcript oscillations of MyoD, MEF2a and MEF2c in the hindlimb muscles across torpor arousal cycles of the same species. If these changes do reflect activation of satellite cells they could support a mechanism for new fiber production (i.e. hyperplasia) in mid–late winter (samples were collected December–February). Contrary to the other muscles examined, the CSA of diaphragm increases in early winter and was stable throughout hibernation. A similar finding has been reported in golden-mantled ground squirrels, and this hypertrophy is explained by the need to overcome elevated resistive forces in respiration due to a stiffer chest wall in the cold (Reid et al., 1995). Again, we observed a unique response in soleus compared with the other locomotory muscles examined – soleus exhibited a relatively stable myofiber CSA across hibernation, while cell density decreased across the data set. While these patterns do not point to changes in cell number, they do imply that the architecture of this muscle is changing in late winter, in advance of refeeding and emergence from hibernation.
Despite the very disparate results reported for muscle atrophy in hibernators, even just for ground squirrels, some commonalities emerge. In cases where atrophy patterns are documented across the time course of winter, ground squirrels appear to lose muscle mass early before atrophy stabilizes through extended hibernation. This is also the pattern of immobilization atrophy in humans (for a review, see Bodine, 2013). We also found muscle-specific loss in early hibernation, but demonstrate that this period of loss is followed by stabilization and possible regrowth as animals progress into late hibernation/pre-spring emergence. Here we provide a unique addition to the hibernation literature by demonstrating skeletal muscle regrowth in some muscles, beginning in late hibernation (despite the continuation of regular torpor bouts) and apparently prior to return of food and water to Colorado animals. Another study that overlays the annual cycle of hibernation onto observations of muscle atrophy in golden-mantled ground squirrels (Nowell et al., 2011) shows a similarly increased muscle mass in end-of-winter (March vs December) samples. This timing is concordant with reported increases in circulating steroid hormones in sciurid hibernators (e.g. Barnes, 1986), whose mechanistic role in muscle function remains a topic of investigation in humans (Phillips et al., 2012). Increased circulating levels of steroid hormones could stimulate an increase in lean tissue production via increased protein synthesis or depressed degradation (Urban, 2011).
Late winter protein synthesis as a mechanism for muscle maintenance and regrowth
Differential regulation of protein synthesis underlies the observed differences in patterns of muscle morphology across hibernation (Fig. 4). By comparing early and later winter hibernators to fasted summer ground squirrels, we demonstrated that skeletal muscle protein synthesis is 66% depressed in early hibernation (20 October), but returns to baseline summer values by late winter. A depression and restoration of protein synthesis levels is more pronounced in skeletal muscle compared with liver and intestine, as well as less variable. This uniquely significant protein synthesis pattern is especially distinct compared with a constitutively active contractile tissue – the heart. Our data support elevated protein synthesis in quadriceps of thirteen-lined ground squirrels sampled between 15 December and 13 January, preceding significant changes in the patterns (slopes) of muscle mass (~20 January) and volumes (after 4 February) in this species. Protein synthesis data most appropriately support the observed mass changes in rectus femoris, which belongs to the quadriceps muscle group. This striking difference in protein synthesis occurred in thirteen-lined ground squirrels that were never offered food or water in the hibernaculum, suggesting that fasting physiology is regulated during hibernation to support locomotory muscle function in advance of spring emergence.
Although some of our data are confounded by the availability of food for still-hibernating Colorado ground squirrels, it is important to note that food availability does not necessarily indicate consumption, and that the return of food and water to cages did not disrupt the ongoing cycles of torpor-arousal to the same minimum body temperature in Colorado ground squirrels as monitored by telemetry. The data are presented in the context of food availability, and we consistently observe the adoption of a late winter, muscle-maintaining or even muscle-building phenotype prior to refeeding. This is particularly clear in the protein synthesis experiments conducted on Wisconsin ground squirrels, in which hibernating animals were not refed. It is interesting to speculate about the source of amino acid resources necessary for protein synthesis under fasting conditions. MRI data show disparate patterns between liver and muscle volumes in late winter (Fig. 1), highlighting liver as a candidate organ potentially tapped for substrates to rebuild muscle. Indeed, there was no significant change in liver protein synthesis by SUnSET between early and late winter hibernators. Furthermore, the possibility of liver autophagy in hibernating thirteen-lined ground squirrels was recently reported (Hindle et al., 2014), which could provide a mechanism and source of substrates for muscle growth. Protein synthesis in the intestine appeared winter-depressed compared with fasted summer ground squirrels, although high variability did not produce a significant difference (Fig. 4). Although atrophy of small intestinal tissue is well documented in hibernating rodents (Carey, 1990; Carey, 1992; Carey and Sills, 1992; Hume et al., 2002), there is little evidence linking intestinal tissue atrophy with provision of free amino acids for new protein synthesis during hibernation or other fasting states.
While there is conflicting evidence that supports both enhanced protein synthesis and dampened protein degradation as factors that limit muscle loss in mammalian hibernators (e.g. Velickovska et al., 2005; Velickovska and van Breukelen, 2007; Cotton and Harlow, 2010; Lee et al., 2010; Nowell et al., 2011; Kornfeld et al., 2012; Andres-Mateos et al., 2013; Fedorov et al., 2014), several studies have proposed that hibernation-specific adjustments to the mTOR pathway are key to enhancing muscle protein synthesis and limiting proteolysis, despite inactivity and fasting (e.g. Nowell et al., 2011; Wu and Storey, 2012; Andres-Mateos et al., 2013). Regulation of protein synthesis to preserve and augment muscle mass in late winter, as observed here, could be driven by activation of mTOR signalling. It is relevant that we examined protein synthesis in aroused hibernators, where activated mTOR should be high. Based on our entire data set, we propose that protein synthesis, and possibly degradation, are differentially regulated across the time course of hibernation – and that timing of sampling within the hibernation season is a potential confounding element in comparisons among published results.
Summary
Here we show several lines of evidence that support a basic shift in locomotory muscle physiology across hibernation. Our data demonstrate reduced protein synthesis and limited muscle loss in early winter, which does not outpace the observed decline in Mb of thirteen-lined ground squirrels. The results indicated a late-winter stabilization and potential recovery from the muscle loss of early hibernation, supported by a return of protein synthesis levels to summer baseline by late winter. Notably, this transition commences in ground squirrels hibernating in cold, dark conditions without access to food or water. Given the immediate need for locomotory output from muscles at spring emergence, these data may reflect prioritization of muscle tissue for maintenance and potential regrowth during hibernation, a process that appears to begin even while regular bouts of deep torpor continue.
MATERIALS AND METHODS
Thirteen-lined ground squirrel laboratory hibernation
This study presents data collected from thirteen-lined ground squirrels housed at either the University of Colorado School of Medicine (Colorado ground squirrels) or the University of Wisconsin Madison (Wisconsin ground squirrels). Animals were maintained in standard laboratory housing during the summer active period (temperature (T) 18–21°C, 12 h:12 h light:dark, May–September). Animals were provided with rodent chow (Colorado: 2920X Harlan-Teklad; Wisconsin: Purina #7001) and water ad libitum, and their diets were supplemented with sunflower seeds and for Colorado ground squirrels, dry cat food (2060 Harlan-Teklad). In early October, ground squirrels were transferred to a hibernaculum (T=4°C, 0 h:24 h light:dark) to facilitate entry into torpor. Food and water were removed once regular torpor bouts were established, and, for Colorado ground squirrels only, were returned prior to spring emergence though torpor bouts persisted (return of food and water is denoted in all figures on the x-axis, time of year). Specific relevant housing and experiment details are provided for each group of study animals below. All animal care and procedures were approved by the University of Colorado School of Medicine (protocol no. 44309) or the University of Wisconsin Madison (protocol no. V01134) Institutional Animal Care and Use Committees.
Serial magnetic resonance imaging of tissue volume
Six Colorado thirteen-lined ground squirrels (three males, three females) were serially imaged at 10 time points across the year (9 August, 12 September, 8 October, 13 December, 4 February, 9 April, 9 May, 9 June, 8 July and 13 August) by MRI. These animals were acquired from the captive breeding program at the University of Wisconsin Oshkosh. Four of six individuals were juveniles at the start of the imaging series and thus were hibernation naive. One individual was removed from the study after the 9 April sampling point when it died under anaesthesia. No body temperature records were collected from these ground squirrels, as implanted devices were incompatible with imaging technology. Animals were housed in the hibernaculum between the 8 October and 9 May sampling points (see Fig. 1). For volumetric MRI assessment, animals were weighed and anaesthetized by isoflurane (4% for induction, followed by 2–2.5% isoflurane during the scans on active ground squirrels; ~1% for torpid animals). Scans were performed at room temperature (~20°C) with indirect dim lighting. The squirrel was positioned on a Bruker rat animal bed and inserted into a 4.7 Tesla Bruker PharmaScan MRI scanner (Bruker, Billerica, MA, USA). A Bruker body volume coil (67 mm diameter) tuned to the 1H frequency of 200 MHz, was used as a radiofrequency (RF) transmitter and receiver. A fast spin echo RARE (Rapid Acquisition with Relaxation Enhancement) tri-pilot scan was performed for anatomical localization for all three dimensions (axial, coronal and sagittal) for the first bed position (upper abdominal MRI). Then, a gradient-based FIST (Fast Imaging with Steady State Precession) sequence was used for body MRI: field of view=6.4 cm; slice thickness 1.5 mm; inter-slice distance 1.5 mm (no gap allowed); echo time/repetition time=1.9/3.8 ms; slice orientation axial; number of slices 24–36 (depending on the size of the animal); number of averages 4; matrix size 256×256; flip angle 60 deg; total acquisition time 2 min 25 s. The bed was then moved to the lower body region and the scans were repeated using the same protocol.
Abdominal scans contained the complete image of liver and both kidneys, while the lower body scan contained skeletal muscle of the upper hindlimb (knee to hip). Large vessels and bone (femur in the case of skeletal muscle upper hindlimb) were measured and excluded. Complete tissue volume was calculated by summing the cross-sectional area of each image slice (determined with Adobe Photoshop CS3 or ImageJ, National Institutes of Health, version 1.46E) and multiplying each area by the image spacing (i.e. thickness). Data presented represent the calculated tissue volume for liver, left kidney only and the average of skeletal muscle in the left and right upper hindlimb.
Muscle collection for wet mass and histology
Muscle samples were collected from an additional N=35 Colorado thirteen-lined ground squirrels, acquired from both the captive breeding program and a commercial live-trapping source (TLS Research, Bloomingdale, IL, USA) as part of other studies. Although muscles were sampled opportunistically from these animals across the winter and spring according to the sampling designs of the other studies, the body temperature history of each individual was known due to a temperature logger (iButton, Embedded Data Systems, Dallas, TX, USA) and a temperature transmitter (Minimitter, Bend, OR, USA) implanted abdominally in early September. For tissue collections, ground squirrels were deeply anaesthetized with isoflurane, exsanguinated by cardiac puncture and perfused with ice-cold saline at various time points across the annual cycle. Forelimb (biceps brachii) and hindlimb (rectus femoris, semitendinosus, gastrocnemius and soleus) as well as diaphragm were immediately dissected free and weighed to determine wet mass. Semitendinosus and diaphragm were only incompletely dissected so mass data were not collected. In preparation for histological analyses, muscles were mounted at L0 in OCT (optimum cutting temperature compound; Sakura Finnetek, Torrance, CA, USA), frozen in cold isopentane and stored at −80°C.
Histochemical analyses
Skeletal muscle transverse cross-sections (10 μm) were cut on a cryostat at −20°C. Slides were air-dried, rinsed in phosphate-buffered saline (PBS), then stained with hematoxylin (1 min) for myocyte cross-sectional area and density evaluations. Images were captured with an Olympus BX61 microscope and analyzed in ImageJ. Mean CSA was determined from a minimum of 100 individual myocytes observed in three separate locations within the transverse muscle image.
Echo MRI assessment of whole-body composition
A subset of animals (N=23 of 35) for which muscle masses are reported were only minimally dissected after sacrifice for other studies. It was therefore possible to determine body composition for these individuals by quantitative magnetic resonance (EchoMRI-9000 Body Composition Analyzer; Echo Medical Systems, Houston, TX, USA) (Tinsley et al., 2004). Body composition (fat and lean mass) was determined within 1 h of sacrifice and minimal dissection. For each dissection we maintained consistent specimen hydration and dissection protocol, and considered the isolated skeletal muscles, liver and kidneys as lean Mb.
Determination of skeletal muscle protein synthesis
Protein synthesis was measured in Wisconsin ground squirrels with the in vivo SUnSET technique as described previously (Goodman et al., 2011). For this experiment, adult ground squirrels of both sexes were obtained by live trapping near Madison, WI, USA from July to September. Food was removed once Wisconsin ground squirrels exhibited regular torpor bouts in the hibernaculum (October) and was not available to animals at the time of the experiments. To minimize variation associated with recent food ingestion, baseline protein synthesis was measured in summer animals after 48 h of fasting (N=4). Hibernating ground squirrels were sampled in both early (N=5; 33–35 days after hibernation start) or late winter (N=6; 78–106 days). These animals were not telemetered, but were monitored daily under dim light (~5 min) to ascertain hibernation state. Prior to the experiment, hibernating ground squirrels were artificially aroused after at least 5 days of consecutive torpor by moving them to a lighted room at 22°C for 3 h. Animals were then anaesthetized with isoflurane (3%) and treated with puromycin (IP, 0.04 μmol g−1 in PBS, Calbiochem 540222). Ground squirrels recovered from anaesthesia and remained active in their cages for 25 min. At exactly 25 min post-puromycin injection animals were anaesthetized a second time. Exactly 30 min post-puromycin injection anaesthetized ground squirrels were euthanized by decapitation and the heart, liver, intestine, and a mixed quadriceps sample were collected and frozen immediately in liquid nitrogen. iButton body temperature logs were recovered from four late winter squirrels and showed that body temperature remained ≥34°C during the entire 30 min following puromycin injection. We therefore assumed that the early winter and additional late winter squirrels also had body temperatures ≥34°C during the 30 min puromycin treatment as they were handled identically. We also verified a rectal temperature of ≥35°C prior to puromycin injection and at euthanasia for all animals. Tissues were stored at −80°C.
Elongating peptides that had incorporated puromycin were detected by western immunoblotting, as previously described (Goodman et al., 2011). Specifically, 100 mg of tissue was homogenized in 1 ml of ice-cold buffer (40 mmol l−1 Tris, pH=7.5, 1 mmol l−1 EDTA, 5 mmol l−1 EGTA, 0.5% Triton X-100, 25 mmol l−1 β-glycerophosphate, 25 mmol l−1 NaF, 1 mmol l−1 Na3VO4, 10 μg ml−1 leupeptin and 1 mmol l−1 PMSF). Protein concentration of the homogenate was measured by bicinchoninic acid (BCA) assay (Pierce). Samples (35 μg) were combined with Laemmli buffer and boiled at 100°C for 5 min. Next, the samples were fractionated on 10% SDS-PAGE acrylamide gels and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% milk-TBST (Tris-buffered saline with Tween 20) for 1 h at room temperature, then incubated with mouse IgG2a monoclonal anti-puromycin antibody (clone 12D10; 1:5000;) overnight at 4°C. Membranes were washed 30 min in TBST and incubated for 45 min at room temperature with horseradish peroxidase conjugated anti-mouse IgG Fc 2a antibody (1:50 000; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA). After another 30 min wash at room temperature in TBST, membranes were developed with enhanced chemiluminescence (ECL) reagent (Pierce) and imaged using film. Densitometric measurements of entire lanes were made with ImageJ. Following image capture, membranes were stained with Coomassie Blue to verify equal protein loading.
Statistical analyses
All analyses were conducted in R (version 3.0.2, R Core Team, 2013). Changes across the annual cycle in Mb as well as tissue volumes (liver, kidney, skeletal muscle hindlimb) were evaluated with repeated measures ANOVA and Tukey's post hoc pairwise comparisons. The time course of muscle mass collected from independent ground squirrels was examined for a significant breakpoint in the slope of the regression versus time using the ‘segmented’ regression analysis package (Muggeo, 2003; Muggeo, 2008). Changes in cross-sectional area and density of myocytes were likewise examined by both linear and segmented regression analyses. Differences in protein synthesis between early and late winter animals were examined by one-way ANOVA and Tukey's post hoc comparisons. Significance was set at P<0.05. All means are presented ± standard deviation (s.d.) unless otherwise noted.
ACKNOWLEDGEMENTS
We thank Rae Russell, Katie Grabek and Jessica Martin for their assistance in obtaining muscle samples from ground squirrels. We are grateful to Ginger Johnson for performing the echo MRIs, and to Kendra Hasebroock and Natalie Serkova for collecting the serial MRIs. We appreciate the assistance of Julia Weiss in muscle cryo-sectioning. Kim Bjugstad kindly provided microscope and imaging capabilities, and Michael Grahn provided technical assistance with western blots.
FOOTNOTES
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health (R01HL-89049 to S.L.M., R01 AR057347 to T.A.H.) and the University of Wisconsin-Madison School of Veterinary Medicine (to H.V.C.). MRI images were collected in the Animal Imaging and Metabolomics Shared Resources Core facility, supported in part by NIH grants to the University of Colorado Cancer Center (P30 CA046934) and the Colorado Clinical and Translational Sciences Institute (UL1 TR001082). Quantitative magnetic resonance imaging was supported by the Colorado Nutrition and Obesity Research (NORC) NIH P30 DK048520. Deposited in PMC for release after 12 months.
References
- Andres-Mateos E., Brinkmeier H., Burks T. N., Mejias R., Files D. C., Steinberger M., Soleimani A., Marx R., Simmers J. L., Lin B., et al. (2013). Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Mol. Med. 5, 80-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes B. M. (1986). Annual cycles of gonadotropins and androgens in the hibernating golden-mantled ground squirrel. Gen. Comp. Endocrinol. 62, 13-22. [DOI] [PubMed] [Google Scholar]
- Bodine S. C. (2013). Hibernation: the search for treatments to prevent disuse-induced skeletal muscle atrophy. Exp. Neurol. 248, 129-135. [DOI] [PubMed] [Google Scholar]
- Carey H. V. (1990). Seasonal changes in mucosal structure and function in ground squirrel intestine. Am. J. Physiol. 259, R385-R392. [DOI] [PubMed] [Google Scholar]
- Carey H. V. (1992). Effects of fasting and hibernation on ion secretion in ground squirrel intestine. Am. J. Physiol. 263, R1203-R1208. [DOI] [PubMed] [Google Scholar]
- Carey H. V., Sills N. S. (1992). Maintenance of intestinal nutrient transport during hibernation. Am. J. Physiol. 263, R517-R523. [DOI] [PubMed] [Google Scholar]
- Carey H. V., Andrews M. T., Martin S. L. (2003). Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153-1181. [DOI] [PubMed] [Google Scholar]
- Cotton C. J., Harlow H. J. (2010). Avoidance of skeletal muscle atrophy in spontaneous and facultative hibernators. Physiol. Biochem. Zool. 83, 551-560. [DOI] [PubMed] [Google Scholar]
- Dark J. (2005). Annual lipid cycles in hibernators: integration of physiology and behavior. Annu. Rev. Nutr. 25, 469-497. [DOI] [PubMed] [Google Scholar]
- Delp M. D., Duan C. (1996). Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J. Appl. Physiol. 80, 261-270. [DOI] [PubMed] [Google Scholar]
- Egginton S., Fairney J., Bratcher J. (2001). Differential effects of cold exposure on muscle fibre composition and capillary supply in hibernator and non-hibernator rodents. Exp. Physiol. 86, 629-639. [DOI] [PubMed] [Google Scholar]
- Fedorov V. B., Goropashnaya A. V., Stewart N. C., Tøien Ø., Chang C., Wang H., Yan J., Showe L. C., Showe M. K., Barnes B. M. (2014). Comparative functional genomics of adaptation to muscular disuse in hibernating mammals. Mol. Ecol. 23, 5524-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. F., Wang J., Wang H. P., Feng B., Dang K., Wang Q., Hinghofer-Szalkay H. G. (2012). Skeletal muscle is protected from disuse in hibernating dauria ground squirrels. Comp. Biochem. Physiol. 161A, 296-300. [DOI] [PubMed] [Google Scholar]
- Goodman C. A., Hornberger T. A. (2013). Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc. Sport Sci. Rev. 41, 107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman C. A., Mabrey D. M., Frey J. W., Miu M. H., Schmidt E. K., Pierre P., Hornberger T. A. (2011). Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new non-radioactive in vivo technique. FASEB J. 25, 1028-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J. D., Robbins C. T., Nelson O. L., Lin D. C. (2008). Minimal seasonal alterations in the skeletal muscle of captive brown bears. Physiol. Biochem. Zool. 81, 138-147. [DOI] [PubMed] [Google Scholar]
- Hindle A. G., Martin S. L. (2014). Intrinsic circannual regulation of brown adipose tissue form and function in tune with hibernation. Am. J. Physiol. 306, E284-E299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle A. G., Grabek K. R., Epperson L. E., Karimpour-Fard A., Martin S. L. (2014). Metabolic changes associated with the long winter fast dominate the liver proteome in 13-lined ground squirrels. Physiol. Genomics 46, 348-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D., Beiglböck C., Ruf T., Frey-Roos F., Bruns U., Arnold W. (2002). Seasonal changes in morphology and function of the gastrointestinal tract of free-living alpine marmots (Marmota marmota). J. Comp. Physiol. B 172, 197-207. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. F., Biggar K. K., Storey K. B. (2012). Differential expression of mature microRNAs involved in muscle maintenance of hibernating little brown bats, Myotis lucifugus: a model of muscle atrophy resistance. Genomics Proteomics Bioinformatics 10, 295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., So H., Gwag T., Ju H., Lee J.-W., Yamashita M., Choi I. (2010). Molecular mechanism underlying muscle mass retention in hibernating bats: role of periodic arousal. J. Cell. Physiol. 222, 313-319. [DOI] [PubMed] [Google Scholar]
- Lyman C. P., Willis J. S., Malan A., Wang L. C. H. (1982). Hibernation and Torpor in Mammals and Birds. New York, NY: Academic Press. [Google Scholar]
- Ma X. M., Blenis J. (2009). Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10, 307-318. [DOI] [PubMed] [Google Scholar]
- Malatesta M., Perdoni F., Battistelli S., Muller S., Zancanaro C. (2009). The cell nuclei of skeletal muscle cells are transcriptionally active in hibernating edible dormice. BMC Cell Biol. 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggeo V. M. (2003). Estimating regression models with unknown break-points. Stat. Med. 22, 3055-3071. [DOI] [PubMed] [Google Scholar]
- Muggeo V. M. R. (2008). Segmented: an R package to fit regression models with broken-line relationships. R News 8, 20-25. [Google Scholar]
- Musacchia X. J., Steffen J. M., Fell R. D., Dombrowski M. J. (1990). Skeletal muscle response to spaceflight, whole body suspension, and recovery in rats. J. Appl. Physiol. 69, 2248-2253. [DOI] [PubMed] [Google Scholar]
- Nagano K., Kajihara H., Suzaki E., Suzuto M., Kataoka K., Yoshii M., Ozawa K. (2003). Disuse atrophy alterations in normal and low temperature environments during hindlimb unloading in Syrian hamsters. Cryo Letters 24, 245-252. [PubMed] [Google Scholar]
- Nowell M. M., Choi H., Rourke B. C. (2011). Muscle plasticity in hibernating ground squirrels (Spermophilus lateralis) is induced by seasonal, but not low-temperature, mechanisms. J. Comp. Physiol. B 181, 147-164. [DOI] [PubMed] [Google Scholar]
- Phillips B. E., Hill D. S., Atherton P. J. (2012). Regulation of muscle protein synthesis in humans. Curr. Opin. Clin. Nutr. Metab. Care 15, 58-63. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reid W. D., Ng A., Wilton R., Milsom W. K. (1995). Characteristics of diaphragm muscle fibre types in hibernating squirrels. Respir. Physiol. 101, 301-309. [DOI] [PubMed] [Google Scholar]
- Riedesel M. L., Steffen J. M. (1980). Protein metabolism and urea recycling in rodent hibernators. Fed. Proc. 39, 2959-2963. [PubMed] [Google Scholar]
- Rouble A. N., Hefler J., Mamady H., Storey K. B., Tessier S. N. (2013). Anti-apoptotic signaling as a cytoprotective mechanism in mammalian hibernation. PeerJ 1, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke B. C., Yokoyama Y., Milsom W. K., Caiozzo V. J. (2004). Myosin isoform expression and MAFbx mRNA levels in hibernating golden-mantled ground squirrels (Spermophilus lateralis). Physiol. Biochem. Zool. 77, 582-593. [DOI] [PubMed] [Google Scholar]
- Rourke B. C., Cotton C. J., Harlow H. J., Caiozzo V. J. (2006). Maintenance of slow type I myosin protein and mRNA expression in overwintering prairie dogs (Cynomys leucurus and ludovicianus) and black bears (Ursus americanus). J. Comp. Physiol. B 176, 709-720. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Dyar K. A., Ciciliot S., Blaauw B., Sandri M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294-4314. [DOI] [PubMed] [Google Scholar]
- Schmidt E. K., Clavarino G., Ceppi M., Pierre P. (2009). SUnSET, a non-radioactive method to monitor protein synthesis. Nat. Methods 6, 275-277. [DOI] [PubMed] [Google Scholar]
- Steffen J. M., Koebel D. A., Musacchia X. J., Milsom W. K. (1991). Morphometric and metabolic indices of disuse in muscles of hibernating ground squirrels. Comp. Biochem. Physiol. 99B, 815-819. [DOI] [PubMed] [Google Scholar]
- Tessier S. N., Storey K. B. (2010). Expression of myocyte enhancer factor-2 and downstream genes in ground squirrel skeletal muscle during hibernation. Mol. Cell. Biochem. 344, 151-162. [DOI] [PubMed] [Google Scholar]
- Tinker D. B., Harlow H. J., Beck T. D. (1998). Protein use and muscle-fiber changes in free-ranging, hibernating black bears. Physiol. Zool. 71, 414-424. [DOI] [PubMed] [Google Scholar]
- Tinsley F. C., Taicher G. Z., Heiman M. L. (2004). Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes. Res. 12, 150-160. [DOI] [PubMed] [Google Scholar]
- Urban R. J. (2011). Growth hormone and testosterone: anabolic effects on muscle. Horm. Res. Paediatr. 76, suppl. 1, 81-83. [DOI] [PubMed] [Google Scholar]
- Velickovska V., van Breukelen F. (2007). Ubiquitylation of proteins in livers of hibernating golden-mantled ground squirrels, Spermophilus lateralis. Cryobiology 55, 230-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velickovska V., Lloyd B. P., Qureshi S., van Breukelen F. (2005). Proteolysis is depressed during torpor in hibernators at the level of the 20S core protease. J. Comp. Physiol. B 175, 329-335. [DOI] [PubMed] [Google Scholar]
- Wickler S. J., Horwitz B. A., Kott K. S. (1987). Muscle function in hibernating hamsters: a natural analog to bed rest? J. Therm. Biol. 12, 163-166. [Google Scholar]
- Wickler S. J., Hoyt D. F., van Breukelen F. (1991). Disuse atrophy in the hibernating golden-mantled ground squirrel, Spermophilus lateralis. Am. J. Physiol. 261, R1214-R1217. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R. (2005). Regulation of skeletal muscle protein metabolism in catabolic states. Curr. Opin. Clin. Nutr. Metab. Care 8, 61-65. [DOI] [PubMed] [Google Scholar]
- Wu C. W., Storey K. B. (2012). Regulation of the mTOR signaling network in hibernating thirteen-lined ground squirrels. J. Exp. Biol. 215, 1720-1727. [DOI] [PubMed] [Google Scholar]
- Xu R., Andres-Mateos E., Mejias R., MacDonald E. M., Leinwand L. A., Merriman D. K., Fink R. H. A., Cohn R. D. (2013). Hibernating squirrel muscle activates the endurance exercise pathway despite prolonged immobilization. Exp. Neurol. 247, 392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]