Abstract
Introduction
HIV infections and the use of amphetamine-type stimulants (ATS) among men who have sex with men (MSM) have been increasing internationally, but the role of ATS use as a co-factor for HIV infection remains unclear. We aimed to summarize the association between ATS use and HIV infection among MSM.
Methods
We conducted a systematic search of MEDLINE, EMBASE, GLOBAL HEALTH and PsycINFO for relevant English, peer-reviewed articles of quantitative studies published between 1980 and 25 April 2013. Pooled estimates of the association – prevalence rate ratios (PRR, cross-sectional studies), odds ratio (OR, case-control studies) and hazard ratio (HR, longitudinal studies), with 95% Confidence Intervals (CI) – were calculated using random-effects models stratified by study design and ATS group (meth/amphetamines vs. ecstasy). We assessed the existence of publication bias in funnel plots and checked for sources of heterogeneity using meta-regression and subgroup analysis.
Results
We identified 6710 article titles, screened 1716 abstracts and reviewed 267 full text articles. A total of 35 publications were eligible for data abstraction and meta-analysis, resulting in 56 records of ATS use. Most studies (31/35) were conducted in high-income countries. Published studies used different research designs, samples and measures of ATS use. The pooled association between meth/amphetamine use and HIV infection was statistically significant in all three designs (PRR=1.86; 95% CI: 1.57–2.17; OR=2.73; 95% CI: 2.16–3.46 and HR=3.43; 95% CI: 2.98–3.95, respectively, for cross-sectional, case-control and longitudinal studies). Ecstasy use was not associated with HIV infection in cross-sectional studies (PRR=1.15; 95% CI: 0.88–1.49; OR=3.04; 95% CI: 1.29–7.18 and HR=2.48; 95% CI: 1.42–4.35, respectively, for cross-sectional, case-control and longitudinal studies). Results in cross-sectional studies were highly heterogeneous due to issues with ATS measurement and different sampling frames.
Conclusions
While meth/amphetamine use was significantly associated with HIV infection among MSM in high-income countries in all study designs, evidence of the role of ecstasy in HIV infection was lacking in cross-sectional studies. Cross-sectional study design, measurement approaches and source populations may also be important modifiers of the strength and the direction of associations. Event-specific measure of individual drug is required to establish temporal relationship between ATS use and HIV infection. HIV prevention programmes targeting MSM should consider including interventions designed to address meth/amphetamine use.
Keywords: HIV, amphetamine-type stimulants, MSM, systematic review, meta-analysis, risk behaviour, meth/amphetamine, ecstasy
Introduction
Amphetamine-type stimulants (ATS) are the second most popular group of illegal drugs globally and are increasingly used in different populations and in different parts of the world [1,2]. ATS can be classified into two main subgroups: meth/amphetamines, which include amphetamine sulphate, amphetamine hydrochloride, methamphetamine and methcathinone, and ecstasy subgroup, which comprises MDMA (3,4-methylenedioxy-N-methylamphetamine) and its analogue (called meth/amphetamines and ecstasy hereafter) [1,3]. Both groups are synthetic neurotropic stimulants that can be ingested orally, injected, inhaled, smoked or “shafted” (inserted in the anus) and have immediate accelerated physical and psychological effects which last up to 10–12 hours (meth/amphetamines) or 3–6 hours (ecstasy) [4,5]. Ecstasy is the most common street name for MDMA [6]. As to methamphetamine, its street slang names vary geographically, and some of them “crystal,” “speed,” “ice,” “crank,” “batu,” “glass,” “chalk” and “go-fast” [7,8].
In relation to sex, meth/amphetamine and ecstasy have been documented to have different effects. Meth/amphetamines are often used to increase sexual desire, make intercourse more pleasurable, facilitate sexual experimentation and decrease sexual inhibition [9,10]. Meth/amphetamines may increase sexual pleasure, help prolong sexual performance, facilitate sexual marathons, make anal intercourse easier and less painful, particularly during more forceful and traumatic sexual penetration [11]. Such attributes have been valued in more sexually adventurous gay community subcultures [12]. Meth/amphetamine use clearly affects both physiological and psychological aspects of sexual behaviour and may facilitate risky sexual practices, including unprotected sex, thereby increasing the risk of HIV transmission.
While some studies suggest that ecstasy use may also increase sexual satisfaction, prolong and enhance sexual arousal [13–17], other studies found no effect on sexual desire in penetrative sexual intercourse [18,19]. Ecstasy has also been reported to increase feelings of sensuality and emotional closeness [20,21]. Therefore, it may be used in the context of less risky sex and its impact on HIV transmission is less well defined.
In the past decade, ATS use has become increasingly popular among men who have sex with men (MSM) in North America, Asia, Western and South Western Europe [22–33]. In high-income countries such as the United Kingdom and the United States, the prevalence of recent (past 12 months) amphetamine use among MSM was reported to be between 7.2 and 18.8% [22,23], recent meth/amphetamine use – between 2.8 and 18.0% [23–25] and recent ecstasy use – between 18.5 and 36.7% [23,34,35]. The prevalence of lifetime use of these substances among MSM in seven US cities was found to be much higher [26,32,33]. An online study of drug use among MSM in 12 countries in Asia in 2010 reported an overall prevalence of recreational drug use over a six-month period of 16.7%, with ecstasy the most commonly used drug (8.1%) [30]. Data from studies assessing drug use during specific gay community events and venues in Western countries, (e.g. circuit parties, dance clubs, bars and bathhouses) have found the prevalence of both meth/amphetamine and ecstasy use to be even higher [34,36].
A growing body of literature documents significant associations between meth/amphetamine and ecstasy use and unprotected anal intercourse (UAI), including receptive UAI – a practice which carries the highest risk of HIV infection [11,22,28,32,37–43]. ATS use and UAI are co-occurring risk behaviours with the potential to facilitate HIV transmission among MSM. Since ATS can also be administered parenterally, exposure to HIV can also occur via unsafe injecting practices [44,45].
A number of studies have directly focused on the association between ATS use and HIV infection or included measures of ATS use in their analyses of associates/risk factors of HIV infection among MSM [8,25,42,46–77]. However, the results of these studies have been inconsistent as to the significance of this association. Furthermore, the interpretation of their findings may be complicated given the variety of study designs, sampling frames and measures of ATS use. The main objective of this systematic review and meta-analysis was to evaluate and summarize the association between ATS use and HIV infection among MSM in different study designs and by ATS subgroup (meth/amphetamines and ecstasy).
Methods
This paper followed the guidelines for reporting a meta-analysis of observational studies (MOOSE) proposed by Stroup et al. [78].
Search strategy
We conducted a systematic search in MEDLINE, EMBASE, GLOBAL HEALTH and PsysINFO for relevant publications from 1980 until 25 April 2013. The search used a combination of free terms and the Medline subject headings, including (1) MSM OR homosexual men OR bisexual men OR gay men OR male homosexual OR bisexual male OR homosexuality OR bisexuality AND (2) risk factors OR determinants OR associations OR correlates OR correlations OR predictors OR high-risk behaviours OR predictor variables AND (3) HIV prevalence OR HIV incidence OR HIV seroconversion OR HIV status OR human immunodeficiency virus OR human immunodeficiency virus prevalence/infection. Some articles reported only a combined drug use measure, did not specify the drug(s) used, did not provide a quantitative effect measure with an associated 95% confidence interval (95% CI) and did not include the original data. In these instances, we contacted the corresponding authors by email to obtain the effect measure or a descriptive tabulation of ATS use and HIV infection. If no reply was received within four weeks, the corresponding articles were excluded from further review. The search was carried out by Nga Thi Thu Vu and Julia Kennedy.
Inclusion and exclusion criteria
Articles were eligible for inclusion in the review if they satisfied all of the following criteria: 1) cross-sectional, case-control or longitudinal study design; 2) quantitative data collection; 3) MSM as a target population; 4) the article reported a crude quantitative measure of association between ATS use and HIV infection or provided data to calculate it; 4) HIV status of participants was confirmed by a standardized laboratory method, and 5) the article was published in a peer-reviewed English language journal. Studies were excluded if: 1) they applied only qualitative methods or mathematical modelling; 2) specifically targeted only HIV positive MSM or only ATS users; 3) quantitative data could not be extracted and/or were not provided by the authors; 4) HIV status of participants was self-reported; 5) the publication included only conference proceedings; and 6) was published in a language other than English. These inclusion and exclusion criteria aimed to minimize any classification bias as to HIV status and to exclude articles which did not provide a quantitative measure of the association between ATS use and HIV infection.
Quality assessment
The article quality was assessed using quality assessment criteria adapted for cross-sectional studies from Boyle [79] and for case-control and longitudinal studies from Wells et al. [80] (the checklist is provided in Supplementary 1). According to these quality criteria, a score of 1 was assigned for each of the items included and articles were assigned a summative score on a scale of 0 to 9 for cross-sectional studies, 0–10 for case-control studies and 0–11 for longitudinal studies. All scores were categorized into high- and low-quality groups based on the cut off of 50%.
Data extraction
Extracted information included the primary author, year of publication, country of research, sampling method(s), sample size, type of drug(s) examined and recall periods, basic participant characteristics (e.g. age, sexual identification) and either a crude measure of association with 95% CI or data to calculate it. If articles reported more than one drug or used more than one recall period, each measure of drug use at each recall period was extracted as a separate record. Measures of association reported without 95% CIs were not extracted. Extracted data from cross-sectional and case-control studies were used to calculate prevalence rate ratios (PRR) [81] and odds ratios (OR), respectively. For longitudinal studies, we directly extracted hazard ratios (HR) or relative risk (RR) with 95% CI as a measure of association between ATS use and HIV seroconversion. Data extraction was carried out by Nga Thi Thu Vu and Julia Kennedy.
Statistical analysis
Meta-analysis was performed using STATA 13.0 (StataCorp, College Station, TX, USA) and was stratified by study design and ATS subgroup (meth/amphetamine vs. ecstasy). We did not combine effect measures (i.e. PRR, OR and HR) of all selected studies because of differences in the nature and calculation methods for each of these measures. In the group of longitudinal studies, all articles reported HR as a measure of association, and only Burcham et al. [74] used RR. We treated this RR as equivalent to HR. The pooled estimates of the association and their 95% CI were estimated using random-effects models, as suggested by DerSimonian and Laird. Heterogeneity was defined by Q statistic when p>0.1 as Hardy et al. [82] had previously reported this method to have low power. Based on the I2 classification suggested by Higgins and Thompson [83], we used the cut-offs of 25, 50 and 75% to define low, medium and high levels of heterogeneity, respectively [84]. Sources of heterogeneity were checked using subgroup analysis and meta-regression [85]. The variables for meta-regression included the study quality score (high vs. low), ATS group (meth/amphetamines vs. ecstasy) and study location (high vs. low- and middle-income countries (LMIC), as according to World Bank income classification, sampling location (clinic based vs. other), drug use recall period (recent use vs. lifetime use), injecting drug use reporting (Yes vs. No) and other specific drug use measurements, that is, nitrite inhalants, heroin, cocaine and EDM use (Yes vs. No). Injecting drug use, specifically needle and syringe sharing and these specific drug use behaviours were assessed because they were found to be associated with HIV infection and/ or unprotected risky sexual behaviours [45,69,72,86,87], therefore, may be confounders of the association between ATS use and sexually transmitted HIV infection. Sensitivity analysis was performed by the Comprehensive Meta-Analysis software V2.0 (Biostat, Englewood, New Jersey) to explore any possible influence of abnormal or outlier data on pooled estimates. Publication bias and the effects of small sample sizes were evaluated in a funnel plot [88]. Asymmetry of the funnel plot was tested as recommended by Egger et al. [89].
Results
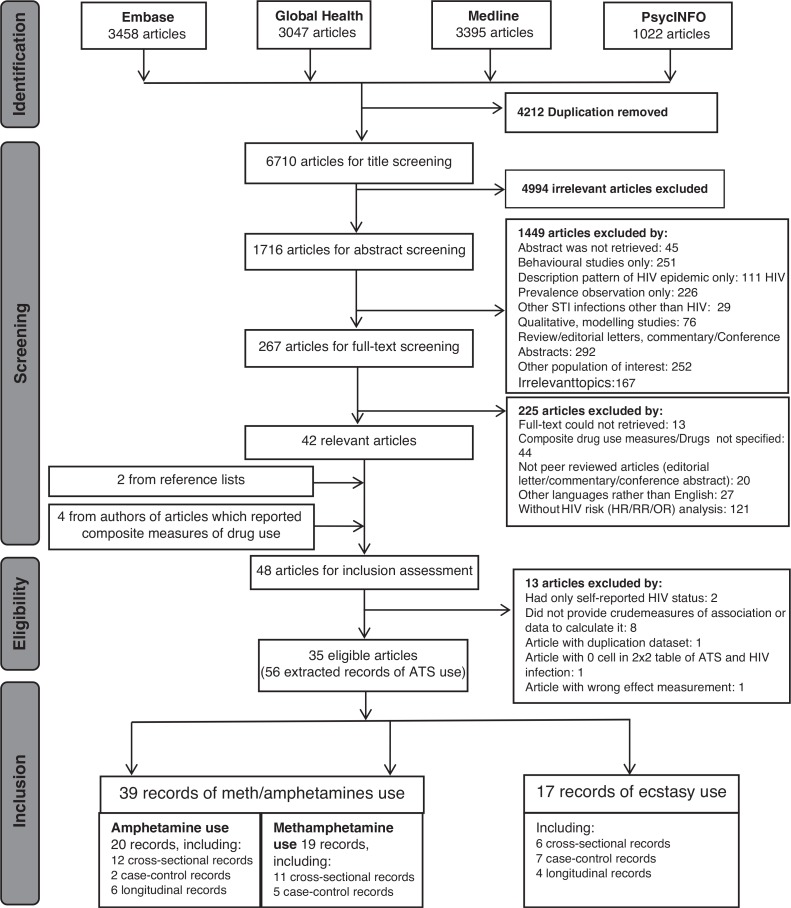
The flow of the review process is shown in Figure 1. We identified 6710 unique article titles, 262 of which progressed to full text screening, resulting in 42 articles relevant for this review (list of excluded articles is provided upon request). The review yielded six additional articles: two from reference lists [69,77] and four from corresponding authors of articles reporting only composite measures of drug use [46,47,53,59]. We contacted by email 30 authors of manuscripts which reported composite drug use measures and received seven responses: four [46,47,53,59] responded with tabulations of ATS use and HIV infection and three clarified that ATS had not been measured in their study or was not analyzed [90–92]. Seven articles provided a descriptive tabulation of ATS and HIV without analysis of the association with HIV infection [25,42,49,54,64,65,72]. Because some articles reported more than one drug used and/or more than one recall period, 58 records were extracted from 36 articles [8,25,42,46–77]. Two records were excluded from analysis because of a 0 cell for a 2x2 table [49,93]. Finally, 56 records from 35 studies were retained for meta-analysis. Records from Van Griensven et al. [46], Menza et al. [54] and Chesney et al. [69] were taken from the baseline data of their longitudinal studies; therefore, these records were treated as a cross-sectional design such that PRR was calculated for these records. The HR reported in Chesney [69] were not comparable with that measurement in other studies; therefore, these HR were not included in the analysis.
Figure 1.
Flow chart for selection of studies with number of articles.
Description of the selected studies and their participants
Among 35 selected articles, only five were from low- and middle-income countries (LMIC), while 30 were from the United States and other high-income countries, specifically The Netherlands, Australia and the United Kingdom. The majority of studies (30/35) used convenience, non-random sampling, and recruited participants using such approaches as advertising, community outreach, referrals from gay community and networks, clients of MSM-specific clinics or HIV testing centres. Nine studies used purely clinic-based recruitment, sixteen used community-based recruitment and ten used both. Most of the articles (26/35) reported a global measure of drug use with different recall periods, including 1, 3, 6, or 12 months and lifetime use; five articles [8,51,55,58,63] reported a contextual measure of ATS use in relation to sex, and the remaining articles did not specify the recall period. Almost all of the study had a quality score larger than 50%, only seven studies, among which one from the LMIC countries, had a quality score lower than 50%.
Table 1 presents the characteristics of studies selected for meta-analysis and their participants. Regarding ATS use, the majority of articles reported the use of methamphetamine (n=19), amphetamine (n=14), ecstasy (n=14) and speed (n=3). Almost all articles (n=34) also reported the use of other drugs of which the most popular reported drugs including cocaine (n=24), nitrites/poppers (n=23), marijuana (n=18) and alcohol (n=17) and heroin (n=13). Among 29 cross-sectional studies, 25 reported high HIV prevalence (9–34%) and only five reported HIV prevalence of less than 9%. All longitudinal studies found an HIV incidence between 1.90 and 2.55 per 100 person years.
Table 1.
Articles in the analysis (n = 35): description of studies and their participants
| Author, yeara | Countryb | World Bank rankingb | Data collection period | Study typec | Quality score | Sampling (method, sample size) | Age mean (SD)/median (range) | Sexual orientation (%) | Reporting IDU (%) | Drug use measure | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Recall period | ATS use (%) | Other drug use | ||||||||||
| Van Griensven et al. 2013 [46] | Thailand | 1 | 2006 | 1 | 77.8 | CS: 1744 | Baseline: Median: 26 (18–56) | NR | NR | Sexual: P4M drug: lifetime; P4M | Lifetime: Ecs: 7.4 Meth: 11.2 P4M: Ecs: 3.3 Meth: 6.0 | Alcohol; nitrite; EDM5 |
| Pham et al. 2012 [47] | Vietnam | 1 | 8–12/2009 | 1 | 55.6 | CS: 381 | Median: 20.4 (18–25.1) | Gay: 39.6; trans: 20.0; Hetero: 40.4 | Yes (16.5%) | Sexual: P1M drug use: lifetime | Meth: 16.7 | IDU alcohol |
| Ackers et al. 2012 [48] | USA | 2 | 6/1998–10/1999 | 3 | 72.7 | CS: 4684 | Baseline: median: 35 (18–62) 18–30: 25.0% | NR | Yes (baseline: 0.23%) | P6M | Baseline: Amp: 9.0 | IDU; crack; cocaine; poppers; tranquilizers; EDM5; hallucinogens; alcohol |
| Oster et al. 2011 [49] | USA | 2 | 2–4/2008 | 2 | 50.0 | CS: 110 | Mean: 21 | Case/control: gay: 76.0/61.0; bisexual: 12.0/27.0; hetero & other: 12.0/12.0 | Yes (0.0%) | P12M | Case/control: Ecs: 4.0/79.0 Meth: 0.0/7.0 | IDU; other non-injection drugs |
| Chariyalertsak et al. 2011 [50] | Thailand | 1 | 2008–2009 | 1 | 44.4 | CS: 551 | <30: 88.7% | Gay: 56.1 bisexual: 18.5 trans: 25.4 | NR | Lifetime | Meth: 12.7 | Marijuana; heroin |
| Morineau et al. 2011 [51] | Indonesia | 1 | 8–11/2007 | 1 | 55.6 | TLS, RDS: 749 | NR | NR | NR | 1–3 months | Meth: 14.6 | NR |
| Truong et al. 2011 [52] | USA | 2 | 1/2004–12/2006 | 1 | 77.8 | CS: 6859 | NR | NR | Yes (NR) | P12M | Amp (NRa) | IDU |
| Forrest et al. 2010 [25] | USA | 2 | 2004–2005 | 1 | TLS: 946 | NR | NR | Yes (3.0%) | P12M | Meth: 18.0 Ecs: 17.8 | Viagra; IDU | |
| Feng et al. 2010 [53] | China | 1 | 3–7/2007 | 1 | 66.7 | CS: 513 | Median: 24 (16.8–44.5) | Gay: 72.9; bisexual: 25.34; hetero: 7.02 | NR | Sexual: P6M drug: NR | Amp: 13.3 | IDU; ketamine; alcohol; heroin |
| Menza et al. 2009 [54] | USA | 2 | 10/2001–5/2008 | 1 | 77.8 | CS: 1903 | <40: 79.72% | NR | NR | P6M | Meth: 6.73 | Nitrite; crack/cocaine |
| Carey et al. 2009 [55] | USA | 2 | 2003–2005 | 2 | 60.0 | CS: 444 | ≤30: 47.5 | NR | No | Sexual:P6M drug use: sex-related drug use/P6M | Case/control: Meth: 28.8/11.4 Ecs: 6.3/4.5 | Alcohol; ketamine; GHB; viagra; poppers; marijuana; cocaine; LSD; heroin |
| Drumright et al. 2009 [56] | USA | 2 | 5/2002–2/2006 | 2 | 50.0 | CS: 145 | Median: 32 | NR | No | Sexual: P12M drug: P12M; sex-related drug use; P12M | Case/control: P6M: Meth: 28.8/11.4 Ecs: 6.3/4.5 Sex-related/3 partners: Meth: 44.2/28.2 Ecs: 14.0/8.5 | Nitrite; marijuana; GHB; Cocaine; EDM5 |
| Rudy et al. 2009 [57] | USA | 2 | 2006–2007 | 1 | 44.4 | CS: 6435 | 18–24: 15.0% 25–34: 37.0% ≥35: 48.0% | NR | NR | Sexual: P3M drug: P12M | Meth: 13.0 | EDM; nitrite; Ecs; ketamine |
| Thiede et al. 2009 [58] | USA | 2 | 7/2002–5/2005 | 2 | 60.0 | CS: 142 | <30: case: 31.3% control: 40.0% | Gay: case: 96.6 control: 76.4 | Yes (10.6%) | P6M | Case/control: Meth: 43.4/12.7 Ecs: 18.8 /0.9 | IDU; popper; viagra; ketamine; GHB; cocaine; alcohol |
| Prestage et al. 2009 [59] | Australia | 2 | 6/2001–12/2004 | 3 | CS: 1427 | Baseline: 37 (18–75) | Homosexual: 95% | No | P6M | Meth: 38.4; Ecs & other ATS: 58.9 | Cocaine; cannabis; heroin; EDM; barbiturates; amyl nitrite; psychedelics | |
| Raymond et al. 2008 [60] | USA | 2 | 10/2003–12/2004 | 1 | 88.9 | TLS: 794 | 18–30: 41% | Gay: 83.0; Bisexual: 15.0; Hetero: 1.0; Other: 1.0 | NR | Sexual: P6M Drug: P12M | Ecs: 6.9 Speed: 14.1 | Cocaine; Marijuana; Crack; Poppers |
| Macdonald et al. 2008 [61] | UK | 2 | 9/2002–10/2004 | 2 | 70.0 | CS: 232 | Mean: Case: 35.2 (20–58) Control: 35.1 (20–66) | Gay: 77.0 | Yes (Case: 8.0%, Control: 3.0%) | P2Y | Case/Control: Meth: 16.0/13.0, Ecs: 67.0/44.0, speed: 25.0/18.0 | Alcohol; Nitrite; Cocaine; Cannabis; Ketamine; Viagra; GHB; LSD; Valium |
| Schwarcz et al. 2007 [42] | USA | 2 | 6/2002–1/2003 | 1 | RS: 1976 | Median: 42 (18–92) | NR | NR | NR | Meth: 16.8 | Viagra; Nitrite; Nocaine; other club drugs (Ketamine, Ecstasy, GHB) | |
| Plankey et al. 2007 [62] | USA | 2 | 4/1984–9/1991 & 10/1996–9/2004 | 3 | 63.6 | CS: 4003 | Baseline: Mean: 34.4 (SD: 8.6) | NR | Yes (baseline: 17.0%) | P6M | Baseline: Meth: 23.0 Ecs: 12.0 | Poppers; Cocaine |
| Koblin et al. 2006 [63] | USA | 2 | 1/1999–2/2001 | 3 | 63.6 | CS: 4295 | Baseline: Mean: 34 ≤25: 19.0% | NR | Yes (baseline: 10.0%) | P6M | Baseline: Amp: 12.3 | Alcohol; IDU; non-injection drugs |
| Fuller et al. 2005 [64] | USA | 2 | 8/2000–2/2004 | 1 | 55.6 | CS: 95 | Median: 28 (18–40) | Gay/bisexual: 72.0; Hetero: 28.0 | Yes (25.0%) | Sexual: P2M Drug: life-time | Meth: 9.0 Ecs: 20.0 | IDU; heroin; cocaine; crack |
| Kral et al. 2005 [65] | USA | 2 | 1998–2002 | 1 | 77.8 | TS: 357 | <30: 22.0% | Gay: 34.0; bisexual: 44.0; hetero: 22.0 | Yes (sharing needle: 84.0%) | P6M | Amp: 79.0 | IDU; heroin; cocaine; crack |
| Buchbinder et al. 2005 [66] | USA | 2 | 4/1995–5/1997 | 3 | 63.6 | CS: 3257 | Enrolment: ≤35: 34.6% | NR | Yes (baseline: 1.5%) | P6M | % visit: Amp: 8.8 | Nitrite; cocaine; hallucinogens; IDU |
| Robertson et al. 2004 [67] | USA | 2 | 4/1996–12/1997 | 1 | 66.7 | RS: 475 | <30: 65.0% | Gay/bisexual: 75.5; Hetero: 24.5 | Yes (58.3%) | Life-time | Meth: 46.4 | IDU, heroine, cocaine |
| Weber et al. 2003 [68] | Canada | 2 | 1995–12/2000 | 3 | 45.5 | CS: 673 | Baseline: median: 25 (22–28) | NR | Yes (NR) | P11M | Meth (NR); Ecs (NR) | Crack; cocaine; poppers; marijuana; alcohol |
| Chesney et al. 1998 [69] | USA | 2 | 1985 | 1 | 70.0 | CS: 337 | Mean 34.8–36 | NR | NR | P6M | Amp: 19.3 | Alcohol; marijuana; nitrite; cocaine; barbiturate; hallucinogens; heroin |
| Molitor et al. 1998 [68] | USA | 2 | 7/1994–12/1995 | 1 | 66.7 | CS: 32,321 | Mean 28 | Gay: 49.6; bisexual: 50.4 | NR | Sex-related drug use | Meth: 3.5 | NR |
| Ruiz et al. 1998 [70] | USA | 2 | 2–11/1994 | 1 | 66.7 | CS: 824 | 17–22: 50.6% 22–25: 49.4% | NR | Yes (sharing needle: 6.4%) | P6M | Ecs: 22.6 Amp: 44.1 | Poppers; crack; cocaine; heroin; IDU |
| Page-Shafer et al. 1997 [71] | USA, Australia, Canada, Holland | 2 | 1982–1985 | 2 | 60.0 | CS: 690 | Mean: 35.3 (7.7) | NR | No | P6M | Case/control: Amp: 26.9/13.3 | Cannabis; nitrite; alcohol |
| Buchbinder et al. 1996 [72] | USA | 2 | 1/1993–7/1994 | 3 | 77.8 | CS: 1975 | Baseline: median: 31 | NR | Yes (NR) | P6M | Baseline: Amp/P12M: 15.7 | IDU; cocaine; popper; marijuana; barbiturate |
| Seage et al. 1992 [73] | USA | 2 | 5/1985–12/1988 | 1 | 66.7 | CS: 481 | <30: 34.1 | NR | NR | P5Y | Amp: 28.5 | Marijuana; nitrite; cocaine; heroin; LSD; PCP; barbiturate; methaquolone; nitrous oxide |
| Burcham et al. 1989 [74] | Australia | 2 | 1/1984–7/1987 | 3 | 45.5 | CS: 643 | Enrolment: HIV seroconverts: Mean 33 (17–65) HIV negative: 34 (15–64) | NR | No | P6M | Amp (NR) Ecs (NR) | Cocaine; nitrite; marijuana |
| Rietmeijer et al. 1989 [75] | USA | 2 | 11/1982–12/1985 | 1 | 55.6 | CS: 216 | <30: 40% | NR | Yes (17.8%) | Not specified | Amp: 66.4 | IDU; alcohol; marijuana; nitrites; cocaine; LSD; heroin; barbiturate; alcohol |
| Van Griensven et al. 1987 [76] | Holland | 2 | 10/1984–05/1985 | 1 | 33.3 | CS: 741 | Mean: 35 | Bisexual: 34.0; gay 34 | NR | Not specified | Amp: 3.0 | Marijuana; nitrite; cocaine; LSD |
| Jeffries et al. 1985 [77] | Canada | 2 | 11/1982–2/1984 | 2 | 50.0 | CS: 448 | Mean: 32 | NR | No | P8M | Case/control: Ecs: 65.0/44.0 | LSD; cocaine; marijuana; nitrite |
Number in the reference list.
World Bank's country name (USA: United States; UK: United Kingdom); World Bank ranking, 1: low- and middle-income country, 2: high-income country.
1: cross-sectional study; 2: case-control study; 3: longitudinal study.
NR: not reported; CS: convenience sampling; TS: targeted sampling; RS: random sampling; TLS: time location sampling; RDS: respondent driven sampling; IDU: injecting drug users; Trans: transgender; hetero: heterosexual; P1M: past one month; P2M: past two months; P3M: past three months; P4M: past four months; P6M: past six months; P8M: past eight months; P11M: past 11 months; P12M: past 12 months; P2Y: past two years; P5Y: past five years; Meth: methamphetamine; Amp: amphetamine; Ecs: ecstasy; ATS: amphetamine-type stimulants; EDM: erectile dysfunction medications; GHB: gamma hydroxybutyrate; LSD: lysergic acid diethylamide; PCP: phencyclidine.
Seventeen of 35 articles reported injecting drug use (eight of 21 cross-sectional, three of seven case-control and six of seven longitudinal studies) and just three measured needle and syringe sharing. Prevalence of injecting drug use varied markedly between 0 and 58%. Out of eight articles which investigated the relationship between injecting drug use and HIV infection, seven found a significant univariate association. Only three articles confirmed a significant association between ATS use and HIV infection when injecting drug use was included in the model.
Association between ATS use and HIV
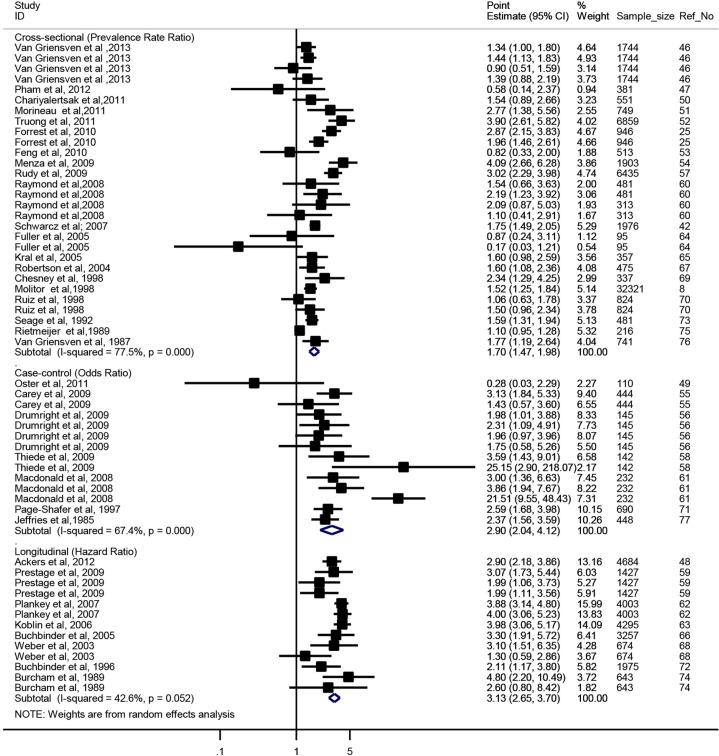
Association between ATS and HIV infection was significant in all study designs (Figure 2). In cross-sectional studies, MSM who reported ever using ATS were 1.70 times more likely to be infected with HIV than non-users (PRR=1.70; 95% CI: 1.47–1.98). Results in cross-sectional studies were highly heterogeneous (Q28=124.68, p=0.000 and I2=77.5%). In case-control studies, the pooled OR was 2.90 (95% CI: 2.04–4.12), with high heterogeneity (Q13=39.89, p=0.000 and I2=67.4%). In longitudinal studies, the pooled HR was 3.13 (95% CI: 2.65–3.70) with medium heterogeneity (Q12=20.92, p=0.052 and I2=42.6%).
Figure 2.
Summarized effect measure of the association between ATS use and HIV infection, by study design.
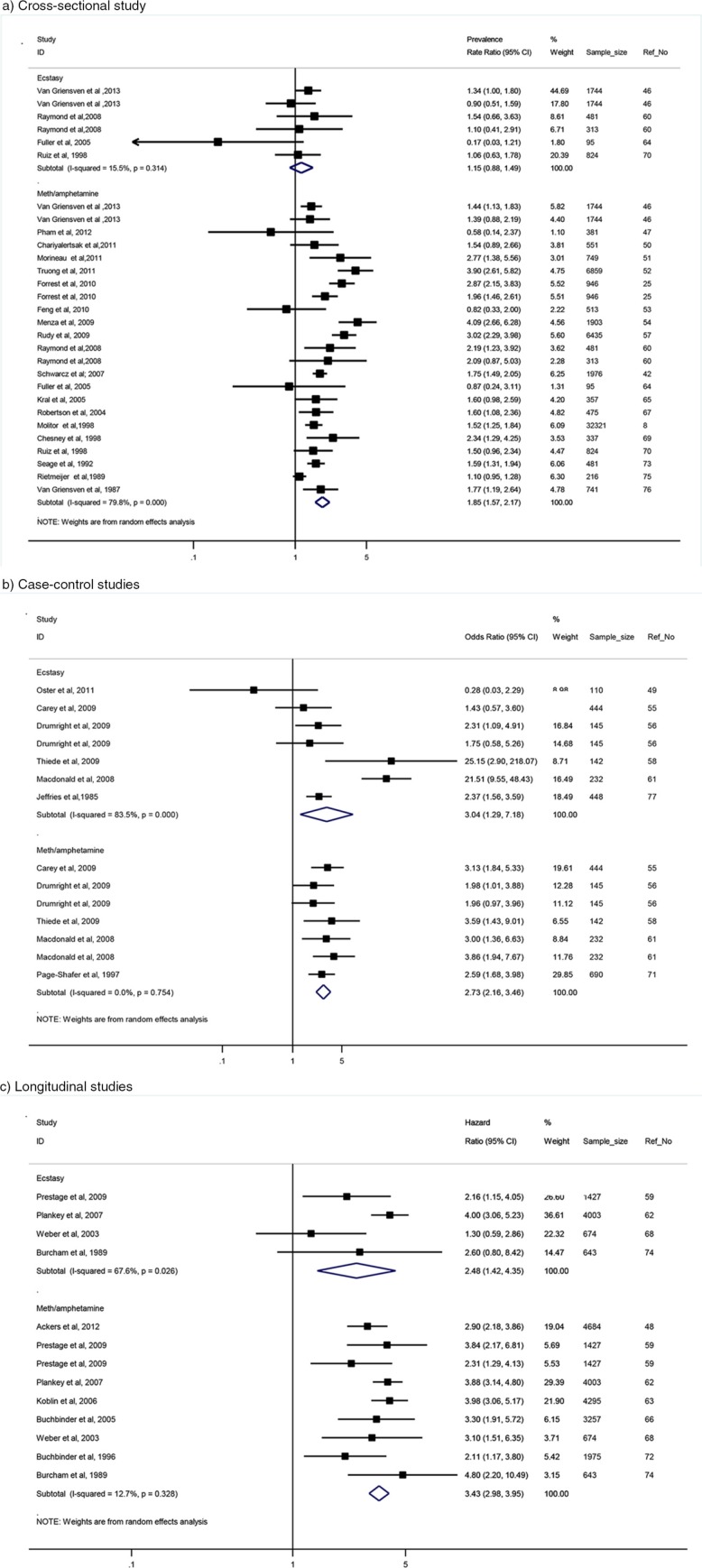
In the meth/amphetamine subgroup (Figure 3), the pooled estimate was statistically significant in all study designs (PRR for cross-sectional studies was 1.85; 95% CI: 1.57–2.17; OR for case-control studies was 2.73; 95% CI: 2.16–3.46 and HR for longitudinal studies was 3.43; 95% CI: 2.98–3.95). Heterogeneity in longitudinal and case-control studies was low (Q7=9.17, p=0.328, I2=12.7% and Q5=3.42, p=0.754, I2=0.0%, respectively) while the results of cross-sectional studies were highly heterogeneous (Q22=109.11, p<0.001 and I2=79.8%). However, in the ecstasy subgroup (Figure 3), in cross-sectional studies, the pooled PR estimate was not statistically significant (PR=1.15; 95% CI: 0.88–1.49), with low heterogeneity (Q5=5.92, p=0.314 and I2=15.5%). In case-control studies, the pooled OR estimate was significant (OR=3.04 (95% CI: 1.29–7.18), with high heterogeneity (Q5=36.33, p=0.000 and I2=83.5%). Similarly, the pooled HR estimate was statistically significant (HR=2.48; 95% CI: 1.42–4.35), with high heterogeneity (Q3=9.26, p=0.026 and I2=67.6%). Sources of heterogeneity among cross-sectional studies were presented in Table 2. Due to the limited number of selected case-control and longitudinal articles and because of low power of Q statistic [82], the test of heterogeneity in these study designs was not conducted.
Figure 3.
Summarized effect measure of the association between ATS use and HIV infection, by study design and drug type. (a) Cross-sectional study; (b) case-control studies; (c) longitudinal studies.
Table 2.
Stratification analysis for cross-sectional studies
| Study characteristic | No. of records | Meta-regression (β, p-value)a | Pooled PR (95% CI)b |
|---|---|---|---|
| Study location | |||
| LMIC countries | 8 | β=0.72, p=0.086 | 1.36 (1.12–1.65) |
| High-income countries | 21 | 1.85 (1.54–2.21) | |
| Study quality | |||
| Low | 3 | β=1.34, p=0.289 | 2.10 (1.36–3.27) |
| High | 26 | 1.66 (1.42–1.94) | |
| Sampling locations | |||
| Clinic-based sample | 6 | β=1.66, p=0.005 | 2.53 (1.70–3.77) |
| Other venues | 23 | 1.52 (1.32–1.76) | |
| Drug use recall period | |||
| Recent use | 19 | β=1.42, p=0.047 | 1.93 (1.57–2.37) |
| Lifetime use | 9 | 1.38 (1.19–1.61) | |
| Reported injecting drugs | |||
| No | 20 | β=1.02, p=0.914 | 1.70 (1.46–1.97) |
| Yes | 9 | 1.62 (1.09–2.42) | |
| Type of ATS | |||
| Amphetamines | 23 | β=0.59, p=0.02 | 1.85 (1.57–2.17) |
| Ecstasy | 6 | 1.15 (0.88–1.49) | |
| Reported alcohol use | |||
| No | 21 | β=0.69, p=0.039 | 1.90 (1.61–2.45) |
| Yes | 8 | 1.30 (1.10–1.54) | |
| Cocaine use | |||
| No | 13 | β=0.74, p=0.106 | 1.78 (1.40–2.25) |
| Yes | 16 | 1.63 (1.34–1.98) | |
| Heroin use | |||
| No | 17 | β=1.04, p=0.84 | 1.96 (1.63–2.36) |
| Yes | 12 | 1.35 (1.12–1.64) | |
| EDM use | |||
| No | 21 | β=0.60, p=0.003 | 1.67 (1.37–2.03) |
| Yes | 8 | 1.77 (1.40–2.24) |
Significant p-value indicates significant source of heterogeneity. Results from meta-regression analysis.
Results from subgroup analysis.
LMIC: low- and middle-income countries.
Sources of heterogeneity in cross-sectional studies
The results of subgroup analysis are presented in Table 2. Sampling locations, ATS subgroup, recall period for drug use, reporting EDM use and alcohol consumption were responsible for a high heterogeneity of the results in cross-sectional studies. The pooled estimates of the association between ATS use and HIV were significantly higher in studies which recruited participants in clinics rather than in other locations; used measures of recent versus lifetime drug use; reported EDM use (yes vs. no) or alcohol consumption (yes vs. no). Finally, the pooled PRR is higher in studies that reported meth/amphetamine use versus ecstasy use.
Sensitivity analysis
None of the individual study results noticeably affected the pooled estimate for longitudinal and cross-sectional studies. In relation to case-control studies, the pooled OR decreased by 13.6% (from OR=2.9; 95% CI: 2.04–4.11 to OR=2.51; 95% CI: 2.02–3.12) when one high OR of a record of ecstasy use reported by Macdonald et al. [61] was excluded from the analysis. This record explained 50.4% of the heterogeneity of the results.
When restricted to the ecstasy subgroup among case-control studies, the pooled estimate of the association with HIV infection was also noticeably affected by the same record, which was responsible for 34.9% of the heterogeneity. After excluding this record, the pooled OR decreased by 32.2% (from OR=3.04; 95% CI: 1.29–7.18 to OR=2.06; 95% CI: 1.19–3.58).
Publication bias
The funnel plot of all selected studies (Supplementary 2) indicates potential publication bias. However, the result of the test for symmetry of the funnel plot was not statistically significant, suggesting no small sample size effect.
Discussion
Our review and meta-analysis of the published evidence found a statistically significant relationship between ATS use and HIV infection. The use of meth/amphetamines was significantly associated with HIV infection in all study designs, while ecstasy use was not associated with HIV in cross-sectional studies. The pooled estimate from case-control studies had low heterogeneity and the significant pooled HR from longitudinal studies was affected by studies with large samples and highly significant results [62]. The pooled estimates of case-control studies were affected by a record from study of Macdonald et al. [61]; however, while the exclusion of this record in the analysis resulted in decreasing the effect size; it did not change the significance of the overall effect size. The pooled estimates of cross-sectional studies were heterogeneous as a result of sampling location approach, different drug use recall periods and the diversity of different drug use measurement. Our findings of the relationship between ATS and HIV infection are consistent with results from a previous review by Drumright et al. [45]; that review covered fewer studies. It found that meth/amphetamine use was associated with HIV infection and reported insufficient evidence of an association between ecstasy and HIV infection. More recently, a meta-analysis of the relationship between ecstasy use and risky sexual behaviour by Hittner et al. found ecstasy use to be significantly associated with behaviours associated with HIV infection [20], but that review combined different sexual outcomes and did not specifically focus on MSM. Our finding of consistently significant pooled estimates of the association between meth/amphetamine use and HIV infection in all study designs proves the robustness of this association and echoes the finding of Vosburgh et al. [87] that methamphetamine was associated with event-level measurement of sexual risk behaviour among MSM.
Differences in the relationship between meth/amphetamine and ecstasy with HIV infection can potentially be explained by their different sexual behavioural effects. Previous research has found that meth/amphetamines facilitate sexual disinhibition and experimentation [9], increase sexual desire and facilitate sexual marathons [11] in which men practice prolonged sexual encounters with different sexual partners for hours and days [94]. Prestage et al. found that meth/amphetamines have often been combined with orally administered erectile dysfunction medications to further enhance sexual performance [59]. Unprotected sex is common in these contexts, as are lesions due to forceful sexual penetration and increased likelihood of condom failure, all of which can increase the risk of sexual transmission of HIV [94]. Furthermore, high dose of methamphetamine was found to increase anal sensation for receptive partners, thus promoting receptive positioning in anal sex which is the practice of highest risk in sexual transmission of HIV among MSM [44]. In relation to ecstasy, where reported effects include improved sexual performance and satisfaction [13,14], participants also reported enhanced sensuality rather than sexuality [17] and increased feelings of intimacy and emotional closeness [20,21]. Such effects may compensate for the negative effects associated with condom use such as decreased sensuality and sexual satisfaction. These effects may account for the lack of consistency of findings in relation to ecstasy observed across different studies included in our review. However, it is important to acknowledge that the pooled estimate of association between ecstasy and HIV infection was significant in case-control and longitudinal studies which provided stronger evidence than cross-sectional studies. This finding may suggest that a more robust approach to study the relationship between ecstasy and HIV infection should be explored in future studies.
Our review highlights the methodological limitations of current research. First, many studies used composite measures of drug use (e.g. any drug use) which ignore the different effects of specific drugs on sexual behaviour and ultimately on HIV transmission. Second, most studies used global measures of ATS use (that is measures unrelated to sexual encounters) with various recall periods from one month to lifetime use. Only five articles [8,51,55,58,63] reported situational or contextual drug use in which ATS were taken before or during sexual intercourse, but not during a specific event. As early as 1993, Leigh and Stall [95] recommended the use of event-specific measures of ATS use in relation to sexual encounters to enable assessment of the causal relationship between ATS use and HIV infection. Our review, conducted in 2013, was unable to find any studies which used the recommended measures. Third, a number of studies, including reviews, explored the relationship between ATS use and HIV infection [5,45,96] but not its nature or pathway; therefore, the question about causality of this relationship remains largely unanswered. Future research should take into account the methodological limitations of current studies on ATS use. Studies should adopt study designs, sampling methods and ATS use measures which would allow investigating and better understanding the temporal relationship between ATS use and HIV infection among MSM. Our analysis found that most studies were also based on opportunistic samples recruited from different source populations. Our finding of a higher pooled prevalence ratio in cross-sectional studies using samples purely recruited from clinical settings, compared to studies which relied on community-based and/or other recruitment approaches may be explained by the higher prevalence of ATS use and HIV infection among clinic patients.
Our review also identified an important gap in current research. While ATS use and HIV infections among MSM are increasing in many settings, there is little published research from LMIC. We excluded 27 articles published in languages other than English. Since 25 of them were from studies conducted in LMIC countries, it is possible that research from these countries is underrepresented in this analysis. We were not able to assess whether these studies investigated the association between ATS use and HIV infection. We found only five studies published in English language conducted in LMIC compared to 30 in high-income countries (all five studies were cross-sectional in design). As such, generalization of the relationship between ATS use and HIV infection to LMIC may not be appropriate. Further investigation is warranted in regions where ATS use is highly prevalent, such as South East Asia, and may be an important co-factor in increasing HIV transmission among MSM [97].
Our study has limitations that should be born in mind in interpreting the results. As with all meta-analyses, we were restricted to data from reports written in English [88]. Our meta-analysis cannot improve the quality of the results reported by the original studies and depends on their validity. The study diversity with respect to designs, sampling frames, populations, ATS use measures and other drug use measurement, and the heterogeneity of their results, particularly in cross-sectional and longitudinal studies, may have implications for our pooled estimates of the association between ATS use and HIV infection. We assessed heterogeneity of cross-sectional studies but unfortunately we were not able to do the same analysis for other study designs due to the small number of published articles from the longitudinal and case-control studies. They leave a potential for biased results and limit their generalizability. An inherent limitation of meta-analysis is that we could only analyze the role of ATS use in explaining the variance in HIV infections, and could not account for the possibility of various confounding factors which could also explain the association between ATS use and HIV infections (e.g. the concurrent injecting of drugs, specific sexual practices and characteristics of MSM and their networks which are the known risk factors for HIV infection). We should also acknowledge that the cross-sectional or case-control studies pooled together do not provide information about the temporal sequence between ATS use and HIV infection and, therefore, cannot attest to the causality of this relationship.
Conclusions
The findings from our meta-analysis confirmed the significant association between meth/amphetamine use and HIV infection in all study designs, but there is lack of evidence (particularly in cross-sectional studies) regarding the role of ecstasy in HIV infection. Our review and meta-analysis also revealed important methodological limitations as to the currently used measures of drug use and their ability to establish the causal relationship between ATS use and HIV infection. Finally, our results have implications for policy and practice. Because ATS are often used in the context of high-risk unprotected sex, particularly among more adventurous MSM [11], and a significant number of HIV infections happen in these contexts [98], HIV prevention programmes targeting MSM should take into account the role of ATS use, particularly meth/amphetamines, in HIV transmission. They should also consider including interventions designed to address meth/amphetamine use in this population and adopt novel HIV prevention approaches for MSM at high risk for HIV.
Competing interests
No declared competing interests.
Authors' contributions
Nga Thi Thu Vu contributed significant efforts in the development and conduct of the review, performance of the statistical analysis and drafting of the manuscript. Iryna Zablotska and Lisa Maher provided oversight in the design, implementation and interpretation of findings and provided significant input into the preparation of this manuscript. All authors have seen and approved the final version of this paper.
Supplementary Material
Acknowledgements
We are grateful to Julia Kennedy for assistance with reviewing the abstracts, Eric Chow for assistance with data extraction and Lei Zhang for advice on the methods of meta-analysis. We thank the researchers who provided information and further data on our request, including Frits van Griensven, Sarika Pattanasin and Timothy H. Holtz (Thailand MOPH – US CDC Collaboration, TUC); Ying Li and Michael W. Plankey (Department of Medicine, Division of Infectious Diseases, Georgetown University); Quang Duy Pham (Pasteur Institute, Ho Chi Minh city, Vietnam and Kirby Institute, University of New South Wales, Australia); Garrett Prestage and Jeff Jin (Kirby Institute, University of New South Wales); Yuji Feng (Department of Epidemiology, School of Public Health, University of California, Los Angeles, CA, USA); Alexa Oster (Incidence and Viral Resistance Team, Division of HIV/AIDS Prevention Centers for Disease Control and Prevention, USA); Wolfgang Hladik (CGH/DGHA, Centers for Disease Control and Prevention, USA) and Hillard Weinstock (Surveillance and Special Studies Team, Epidemiology and Surveillance Branch, Division of STD Prevention, Centers for Disease Control and Prevention, USA).
Funding
This study was carried out without funding.
References
- 1.United Nations Office on Drugs and Crime. New York: United Nations; 2010. World drug report 2010. [Google Scholar]
- 2.United Nations Office on Drugs and Crime. New York: United Nations; 2013. World drug report 2013. [Google Scholar]
- 3.Ministerial Council on Drug Strategy. Australia: Ministerial Council on Drug Strategy; 2008. National amphetamine-type stimulants strategy, 2008–2011. [Google Scholar]
- 4.Colfax G, Guzman R. Club drugs and HIV infection: a review. Clin Infect Dis. 2006;42(10):1463–9. doi: 10.1086/503259. [DOI] [PubMed] [Google Scholar]
- 5.Colfax G, Santos GM, Chu P, Vittinghoff E, Pluddemann A, Kumar S, et al. Amphetamine-group substances and HIV. Lancet. 2010;376(9739):458–74. doi: 10.1016/S0140-6736(10)60753-2. [DOI] [PubMed] [Google Scholar]
- 6.Davison D, Parrott A. Ecstasy (MDMA) in recreational users: self-reported psychological and physiological effects. Hum Psychopharmacol Clin Exp. 1997;12(3):221–6. [Google Scholar]
- 7.Hall JN, Broderick PM. Community networks for response to abuse outbreaks of methamphetamine and its analogs. NIDA Res Monogr. 1991;115:109–20. [PubMed] [Google Scholar]
- 8.Molitor F, Truax SR, Ruiz JD, Sun RK. Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. West J Med. 1998;168(2):93–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22(3):149–56. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz SP. Post-circuit blues: motivations and consequences of crystal meth use among gay men in Miami. AIDS Behav. 2005;9(1):63–72. doi: 10.1007/s10461-005-1682-3. [DOI] [PubMed] [Google Scholar]
- 11.Prestage G, Grierson J, Bradley J, Hurley M, Hudson J. The role of drugs during group sex among gay men in Australia. Sex Health. 2009;6(4):310–7. doi: 10.1071/SH09014. [DOI] [PubMed] [Google Scholar]
- 12.Green AI, Halkitis PN. Crystal methamphetamine and sexual sociality in an urban gay subculture: an elective affinity. Cult Health Sex. 2006;8(4):317–33. doi: 10.1080/13691050600783320. [DOI] [PubMed] [Google Scholar]
- 13.Zemishlany Z, Aizenberg D, Weizman A. Subjective effects of MDMA (‘Ecstasy’) on human sexual function. Eur Psychiatry. 2001;16(2):127–30. doi: 10.1016/s0924-9338(01)00550-8. [DOI] [PubMed] [Google Scholar]
- 14.Baylen CA, Rosenberg H. A review of the acute subjective effects of MDMA/ecstasy. Addiction. 2006;101(7):933–47. doi: 10.1111/j.1360-0443.2006.01423.x. [DOI] [PubMed] [Google Scholar]
- 15.Solowij N, Hall W, Lee N. Recreational MDMA use in Sydney: a profile of ‘Ecstacy’ users and their experiences with the drug. Br J Addict. 1992;87(8):1161–72. doi: 10.1111/j.1360-0443.1992.tb02003.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen RS. Subjective reports on the effects of the MDMA (‘ecstasy’) experience in humans. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(7):1137–45. doi: 10.1016/0278-5846(95)00231-6. [DOI] [PubMed] [Google Scholar]
- 17.Parrott AC. Human psychopharmacology of ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol. 2001;16(8):557–77. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- 18.McElrath K. MDMA and sexual behavior: ecstasy users’ perceptions about sexuality and sexual risk. Subst Use Misuse. 2005;40(9–10):1461–77. doi: 10.1081/JA-200066814. [DOI] [PubMed] [Google Scholar]
- 19.Schilder AJ, Lampinen TM, Miller ML, Hogg RS. Crystal methamphetamine and ecstasy differ in relation to unsafe sex among young gay men. Can J Public Health. 2005;96(5):340–3. doi: 10.1007/BF03404028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hittner JB, Schachne ER. Meta-analysis of the association between ecstasy use and risky sexual behavior. Addict Behav. 2012;37(7):790–6. doi: 10.1016/j.addbeh.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39(4):1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koblin BA, Murrill C, Camacho M, Xu G, Liu KL, Raj-Singh S, et al. Amphetamine use and sexual risk among men who have sex with men: results from the National HIV Behavioral Surveillance study–New York City. Subst Use Misuse. 2007;42(10):1613–28. doi: 10.1080/10826080701212519. [DOI] [PubMed] [Google Scholar]
- 23.Hichson F, Weatherburn P, Reid D, Jessup K, Hammond G. Consuming passions: findings from the United Kingdom Gay Men's Sex Survey 2005. London: Sigma Research; 2007. [Google Scholar]
- 24.Spindler HH, Scheer S, Chen SY, Klausner JD, Katz MH, Valleroy LA, et al. Viagra, methamphetamine, and HIV risk: results from a probability sample of MSM, San Francisco. Sex Transm Dis. 2007;34(8):586–91. doi: 10.1097/01.olq.0000258339.17325.93. [DOI] [PubMed] [Google Scholar]
- 25.Forrest DW, Metsch LR, LaLota M, Cardenas G, Beck DW, Jeanty Y. Crystal methamphetamine use and sexual risk behaviors among HIV-positive and HIV-negative men who have sex with men in South Florida. J Urban Health. 2010;87(3):480–5. doi: 10.1007/s11524-009-9422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiede H, Valleroy LA, MacKellar DA, Celentano DD, Ford WL, Hagan H, et al. Regional patterns and correlates of substance use among young men who have sex with men in 7 US urban areas. Am J Public Health. 2003;93(11):1915–21. doi: 10.2105/ajph.93.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirshfield S, Remien RH, Humberstone M, Walavalkar I, Chiasson MA. Substance use and high-risk sex among men who have sex with men: a national online study in the USA. AIDS Care. 2004;16(8):1036–47. doi: 10.1080/09540120412331292525. [DOI] [PubMed] [Google Scholar]
- 28.Klitzman RL, Greenberg JD, Pollack LM, Dolezal C. MDMA (‘ecstasy’) use, and its association with high risk behaviors, mental health, and other factors among gay/bisexual men in New York City. Drug Alcohol Depend. 2002;66(2):115–25. doi: 10.1016/s0376-8716(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 29.Stall R, Paul JP, Greenwood G, Pollack LM, Bein E, Crosby GM, et al. Alcohol use, drug use and alcohol-related problems among men who have sex with men: the Urban Men's Health Study. Addiction. 2001;96(11):1589–601. doi: 10.1046/j.1360-0443.2001.961115896.x. [DOI] [PubMed] [Google Scholar]
- 30.Wei C, Guadamuz TE, Lim SH, Huang Y, Koe S. Patterns and levels of illicit drug use among men who have sex with men in Asia. Drug Alcohol Depend. 2012;120(1–3):246–9. doi: 10.1016/j.drugalcdep.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei C, Guadamuz TE, Lim SH, Koe S. Sexual transmission behaviors and serodiscordant partnerships among HIV-positive men who have sex with men in Asia. Sex Transm Dis. 2012;39(4):312–5. doi: 10.1097/OLQ.0b013e31824018e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colfax G, Coates TJ, Husnik MJ, Huang Y, Buchbinder S, Koblin B, et al. Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of San Francisco men who have sex with men. J Urban Health. 2005;82(Suppl 1):62–70. doi: 10.1093/jurban/jti025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawstorne P, Digiusto E, Worth H, Zablotska I. Associations between crystal methamphetamine use and potentially unsafe sexual activity among gay men in Australia. Arch Sex Behav. 2007;36(5):646–54. doi: 10.1007/s10508-007-9206-z. [DOI] [PubMed] [Google Scholar]
- 34.Mansergh G, Colfax GN, Marks G, Rader M, Guzman R, Buchbinder S. The Circuit Party Men's Health Survey: findings and implications for gay and bisexual men. Am J Public Health. 2001;91(6):953–8. doi: 10.2105/ajph.91.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenwood GL, White EW, Page-Shafer K, Bein E, Osmond DH, Paul J, et al. Correlates of heavy substance use among young gay and bisexual men: The San Francisco Young Men's Health Study. Drug Alcohol Depend. 2001;61(2):105–12. doi: 10.1016/s0376-8716(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 36.Colfax GN, Mansergh G, Guzman R, Vittinghoff E, Marks G, Rader M, et al. Drug use and sexual risk behavior among gay and bisexual men who attend circuit parties: a venue-based comparison. J Acquir Immune Defic Syndr. 2001;28(4):373–9. doi: 10.1097/00126334-200112010-00011. [DOI] [PubMed] [Google Scholar]
- 37.Darrow WW, Biersteker S, Geiss T, Chevalier K, Clark J, Marrero Y, et al. Risky sexual behaviors associated with recreational drug use among men who have sex with men in an international resort area: challenges and opportunities. J Urban Health. 2005;82(4):601–9. doi: 10.1093/jurban/jti122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansergh G, Shouse RL, Marks G, Guzman R, Rader M, Buchbinder S, et al. Methamphetamine and sildenafil (Viagra) use are linked to unprotected receptive and insertive anal sex, respectively, in a sample of men who have sex with men. Sex Transm Infect. 2006;82(2):131–4. doi: 10.1136/sti.2005.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rusch M, Lampinen TM, Schilder A, Hogg RS. Unprotected anal intercourse associated with recreational drug use among young men who have sex with men depends on partner type and intercourse role. Sex Transm Dis. 2004;31(8):492–8. doi: 10.1097/01.olq.0000135991.21755.18. [DOI] [PubMed] [Google Scholar]
- 40.Woody GE, Donnell D, Seage GR, Metzger D, Marmor M, Koblin BA, et al. Non-injection substance use correlates with risky sex among men having sex with men: data from HIVNET. Drug Alcohol Depend. 1999;53(3):197–205. doi: 10.1016/s0376-8716(98)00134-3. [DOI] [PubMed] [Google Scholar]
- 41.Colfax G, Vittinghoff E, Husnik MJ, McKirnan D, Buchbinder S, Koblin B, et al. Substance use and sexual risk: a participant- and episode-level analysis among a cohort of men who have sex with men. Am J Epidemiol. 2004;159(10):1002–12. doi: 10.1093/aje/kwh135. [DOI] [PubMed] [Google Scholar]
- 42.Schwarcz S, Scheer S, McFarland W, Katz M, Valleroy L, Chen S, et al. Prevalence of HIV infection and predictors of high-transmission sexual risk behaviors among men who have sex with men. Am J Public Health. 2007;97(6):1067–75. doi: 10.2105/AJPH.2005.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clatts MC, Goldsamt LA, Yi H. Drug and sexual risk in four men who have sex with men populations: evidence for a sustained HIV epidemic in New York City. J Urban Health. 2005;82(Suppl 1):i9–17. doi: 10.1093/jurban/jti019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex. 2001;41(2):17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- 45.Drumright LN, Patterson TL, Strathdee SA. Club drugs as causal risk factors for HIV acquisition among men who have sex with men: a review. Subst Use Misuse. 2006;41(10–12):1551–601. doi: 10.1080/10826080600847894. [DOI] [PubMed] [Google Scholar]
- 46.Van Griensven F, Thienkrua W, McNicholl J, Wimonsate W, Chaikummao S, Chonwattana W, et al. Evidence of an explosive epidemic of HIV infection in a cohort of men who have sex with men in Thailand. AIDS. 2013;27(5):825–32. doi: 10.1097/QAD.0b013e32835c546e. [DOI] [PubMed] [Google Scholar]
- 47.Pham QD, Nguyen TV, Hoang CQ, Cao V, Khuu NV, Phan HT, et al. Prevalence of HIV/STIs and associated factors among men who have sex with men in An Giang, Vietnam. Sex Transm Dis. 2012;39(10):799–806. doi: 10.1097/OLQ.0b013e318265b180. [DOI] [PubMed] [Google Scholar]
- 48.Ackers ML, Greenberg AE, Lin CY, Bartholow BN, Goodman AH, Longhi M, et al. High and persistent HIV seroincidence in men who have sex with men across 47 U.S. cities. PLoS One. 2012;7(4):34972. doi: 10.1371/journal.pone.0034972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oster AM, Dorell CG, Mena LA, Thomas PE, Toledo CA, Heffelfinger JD. HIV risk among young African American men who have sex with men: a case-control study in Mississippi. Am J Public Health. 2011;101(1):137–43. doi: 10.2105/AJPH.2009.185850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chariyalertsak S, Kosachunhanan N, Saokhieo P, Songsupa R, Wongthanee A, Chariyalertsak C, et al. HIV incidence, risk factors, and motivation for biomedical intervention among gay, bisexual men, and transgender persons in Northern Thailand. PLoS One. 2011;6(9):24295. doi: 10.1371/journal.pone.0024295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morineau G, Nugrahini N, Riono P, Nurhayati , Girault P, Mustikawati DE, et al. Sexual risk taking, STI and HIV prevalence among men who have sex with men in six Indonesian cities. AIDS Behav. 2011;15(5):1033–44. doi: 10.1007/s10461-009-9590-6. [DOI] [PubMed] [Google Scholar]
- 52.Truong HM, Kellogg TA, McFarland W, Louie B, Klausner JD, Philip SS, et al. Sentinel surveillance of HIV-1 transmitted drug resistance, acute infection and recent infection. PLoS One. 2011;6(10):25281. doi: 10.1371/journal.pone.0025281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng Y, Wu Z, Detels R, Qin G, Liu L, Wang X, et al. HIV/STD prevalence among men who have sex with men in Chengdu, China and associated risk factors for HIV infection. J Acquir Immune Defic Syndr. 53 Suppl. 2010;1:S74–80. doi: 10.1097/QAI.0b013e3181c7dd16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menza TW, Hughes JP, Celum CL, Golden MR. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36(9):547–55. doi: 10.1097/OLQ.0b013e3181a9cc41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carey JW, Mejia R, Bingham T, Ciesielski C, Gelaude D, Herbst JH, et al. Drug use, high-risk sex behaviors, and increased risk for recent HIV infection among men who have sex with men in Chicago and Los Angeles. AIDS Behav. 2009;13(6):1084–96. doi: 10.1007/s10461-008-9403-3. [DOI] [PubMed] [Google Scholar]
- 56.Drumright LN, Gorbach PM, Little SJ, Strathdee SA. Associations between substance use, erectile dysfunction medication and recent HIV infection among men who have sex with men. AIDS Behav. 2009;13(2):328–36. doi: 10.1007/s10461-007-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rudy ET, Shoptaw S, Lazzar M, Bolan RK, Tilekar SD, Kerndt PR. Methamphetamine use and other club drug use differ in relation to HIV status and risk behavior among gay and bisexual men. Sex Transm Dis. 2009;36(11):693–5. doi: 10.1097/OLQ.0b013e3181ad54a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thiede H, Jenkins RA, Carey JW, Hutcheson R, Thomas KK, Stall RD, et al. Determinants of recent HIV infection among Seattle-area men who have sex with men. Am J Public Health. 2009;99(Suppl 1):S157–64. doi: 10.2105/AJPH.2006.098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prestage G, Jin F, Kippax S, Zablotska I, Imrie J, Grulich A. Use of illicit drugs and erectile dysfunction medications and subsequent HIV infection among gay men in Sydney, Australia. J Sex Med. 2009;6(8):2311–20. doi: 10.1111/j.1743-6109.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 60.Raymond HF, Bingham T, McFarland W. Locating unrecognized HIV infections among men who have sex with men: San Francisco and Los Angeles. AIDS Educ Prev. 2008;20(5):408–19. doi: 10.1521/aeap.2008.20.5.408. [DOI] [PubMed] [Google Scholar]
- 61.Macdonald N, Elam G, Hickson F, Imrie J, McGarrigle CA, Fenton KA, et al. Factors associated with HIV seroconversion in gay men in England at the start of the 21st century. Sex Transm Infect. 2008;84(1):8–13. doi: 10.1136/sti.2007.027946. [DOI] [PubMed] [Google Scholar]
- 62.Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45(1):85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20(5):731–9. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 64.Fuller CM, Absalon J, Ompad DC, Nash D, Koblin B, Blaney S, et al. A comparison of HIV seropositive and seronegative young adult heroin- and cocaine-using men who have sex with men in New York City, 2000–2003. J Urban Health. 2005;82(Suppl 1):i51–61. doi: 10.1093/jurban/jti024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kral AH, Lorvick J, Ciccarone D, Wenger L, Gee L, Martinez A, et al. HIV prevalence and risk behaviors among men who have sex with men and inject drugs in San Francisco. J Urban Health. 2005;82(Suppl 1):i43–50. doi: 10.1093/jurban/jti023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buchbinder SP, Vittinghoff E, Heagerty PJ, Celum CL, Seage GR, 3rd, Judson FN, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2005;39(1):82–9. doi: 10.1097/01.qai.0000134740.41585.f4. [DOI] [PubMed] [Google Scholar]
- 67.Robertson MJ, Clark RA, Charlebois ED, Tulsky J, Long HL, Bangsberg DR, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health. 2004;94(7):1207–17. doi: 10.2105/ajph.94.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weber AE, Craib KJ, Chan K, Martindale S, Miller ML, Cook DA, et al. Determinants of HIV serconversion in an era of increasing HIV infection among young gay and bisexual men. AIDS. 2003;17(5):774–7. doi: 10.1097/00002030-200303280-00024. [DOI] [PubMed] [Google Scholar]
- 69.Chesney MA, Barrett DC, Stall R. Histories of substance use and risk behavior: precursors to HIV seroconversion in homosexual men. Am J Public Health. 1998;88(1):113–6. doi: 10.2105/ajph.88.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruiz J, Facer M, Sun RK. Risk factors for human immunodeficiency virus infection and unprotected anal intercourse among young men who have sex with men. Sex Transm Dis. 1998;25(2):100–7. doi: 10.1097/00007435-199802000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Page-Shafer K, Veugelers PJ, Moss AR, Strathdee S, Kaldor JM, van Griensven GJ. Sexual risk behavior and risk factors for HIV-1 seroconversion in homosexual men participating in the Tricontinental Seroconverter Study, 1982–1994. Am J Epidemiol. 1997;146(7):531–42. doi: 10.1093/oxfordjournals.aje.a009311. [DOI] [PubMed] [Google Scholar]
- 72.Buchbinder SP, Douglas JM, Jr, McKirnan DJ, Judson FN, Katz MH, MacQueen KM. Feasibility of human immunodeficiency virus vaccine trials in homosexual men in the United States: risk behavior, seroincidence, and willingness to participate. J Infect Dis. 1996;174(5):954–61. doi: 10.1093/infdis/174.5.954. [DOI] [PubMed] [Google Scholar]
- 73.Seage GR, 3rd, Mayer KH, Horsburgh CR, Jr, Holmberg SD, Moon MW, Lamb GA. The relation between nitrite inhalants, unprotected receptive anal intercourse, and the risk of human immunodeficiency virus infection. Am J Epidemiol. 1992;135(1):1–11. doi: 10.1093/oxfordjournals.aje.a116186. [DOI] [PubMed] [Google Scholar]
- 74.Burcham JL, Tindall B, Marmor M, Cooper DA, Berry G, Penny R. Incidence and risk factors for human immunodeficiency virus seroconversion in a cohort of Sydney homosexual men. Med J Aust. 1989;150(11):634–9. doi: 10.5694/j.1326-5377.1989.tb136727.x. [DOI] [PubMed] [Google Scholar]
- 75.Rietmeijer CA, Penley KA, Cohn DL, Davidson AJ, Horsburgh CRJr, Judson FN. Factors influencing the risk of infection with human immunodeficiency virus in homosexual men, Denver 1982–1985. Sex Transm Dis. 1989;16(2):95–102. doi: 10.1097/00007435-198904000-00011. [DOI] [PubMed] [Google Scholar]
- 76.Van Griensven GJ, Tielman RA, Goudsmit J, van der Noordaa J, de Wolf F, de Vroome EM, et al. Risk factors and prevalence of HIV antibodies in homosexual men in the Netherlands. Am J Epidemiol. 1987;125(6):1048–57. doi: 10.1093/oxfordjournals.aje.a114620. [DOI] [PubMed] [Google Scholar]
- 77.Jeffries E, Willoughby B, Boyko WJ, Schechter MT, Wiggs B, Fay S, et al. The Vancouver Lymphadenopathy-AIDS Study: 2. Seroepidemiology of HTLV-III antibody. Can Med Assoc J. 1985;132(12):1373–7. [PMC free article] [PubMed] [Google Scholar]
- 78.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 79.Boyle MH. Guidelines for evaluating prevalence studies. Evid Base Ment Health. 1998;1(2):37–9. [Google Scholar]
- 80.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. [cited 2015 Jan 15]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 81.Skov T, Deddens J, Petersen MR, Endahl L. Prevalence proportion ratios: estimation and hypothesis testing. Int J Epidemiol. 1998;27(1):91–5. doi: 10.1093/ije/27.1.91. [DOI] [PubMed] [Google Scholar]
- 82.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med. 1998;17(8):841–56. doi: 10.1002/(sici)1097-0258(19980430)17:8<841::aid-sim781>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 83.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 84.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. 2nd ed. London: BMJ Publishing Group; 2001. [Google Scholar]
- 86.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007;4(12):339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vosburgh HW, Mansergh G, Sullivan PS, Purcell DW. A review of the literature on event-level substance use and sexual risk behavior among men who have sex with men. AIDS Behav. 2012;16(6):1394–410. doi: 10.1007/s10461-011-0131-8. [DOI] [PubMed] [Google Scholar]
- 88.Rothstein HR, Sutton AJ, Borenstein M, editors. Publication bias in meta-analysis: prevention, assessment and adjustments. Chichester: John Wiley & Sons, Ltd; 2005. [Google Scholar]
- 89.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hladik W, Barker J, Ssenkusu JM, Opio A, Tappero JW, Hakim A, et al. HIV infection among men who have sex with men in Kampala, Uganda – a respondent driven sampling survey. PLoS One. 2012;7(5):38143. doi: 10.1371/journal.pone.0038143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oster AM, Wiegand RE, Sionean C, Miles IJ, Thomas PE, Melendez-Morales L, et al. Understanding disparities in HIV infection between black and white MSM in the United States. AIDS. 2011;25(8):1103–12. doi: 10.1097/QAD.0b013e3283471efa. [DOI] [PubMed] [Google Scholar]
- 92.Weinstock H, Sweeney S, Satten GA, Gwinn M. HIV seroincidence and risk factors among patients repeatedly tested for HIV attending sexually transmitted disease clinics in the United States, 1991 to 1996. STD Clinic HIV Seroincidence Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(5):506–12. doi: 10.1097/00042560-199812150-00010. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Baker JJ, Korostyshevskiy VR, Slack RS, Plankey MW. The association of intimate partner violence, recreational drug use with HIV seroprevalence among MSM. AIDS Behav. 2012;16(3):491–8. doi: 10.1007/s10461-012-0157-6. [DOI] [PubMed] [Google Scholar]
- 94.Semple S, Zians J, Strathdee S, Patterson T. Sexual marathons and methamphetamine use among HIV-positive men who have sex with men. Arch Sex Behav. 2009;38(4):583–90. doi: 10.1007/s10508-007-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leigh BC, Stall R. Substance use and risky sexual behavior for exposure to HIV. Issues in methodology, interpretation, and prevention. Am Psychol. 1993;48(10):1035–45. doi: 10.1037//0003-066x.48.10.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Degenhardt L, Mathers B, Guarinieri M, Panda S, Phillips B, Strathdee SA, et al. Meth/amphetamine use and associated HIV: implications for global policy and public health. Int J Drug Policy. 2010;21(5):347–58. doi: 10.1016/j.drugpo.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 97.Fischer A, Curruthers S, Power R, Allsop S, Degenhardt L. The link between amphetamine-type stimulant use and the transmission of HIV and other blood-borne viruses in the Southeast Asia region. Canberra: Australian National Council on Drugs; 2012. [Google Scholar]
- 98.Poynten IM, Jin F, Prestage GP, Kaldor JM, Kippax S, Grulich AE. Defining high HIV incidence subgroups of Australian homosexual men: implications for conducting HIV prevention trials in low HIV prevalence settings. HIV Med. 2010;11(10):635–41. doi: 10.1111/j.1468-1293.2010.00833.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.