Abstract
Background and Purpose
Despite the successful use of a ketogenic diet in pediatric epilepsy, its application in adults has been limited. The aim of this meta-analysis was to summarize the findings of relevant published studies in order to identify the efficacy of and compliance with a ketogenic diet and its main subtypes (i.e., classic ketogenic diet and modified Atkins diet) in adults with intractable epilepsy, and to provide useful information for clinical practice.
Methods
Electronic searches of PubMed, EMBASE, Google Scholar, and the ISI Web of Science were conducted to identify studies of the efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy; the included studies were reviewed. Meta-analyses were performed using STATA to determine combined efficacy rates and combined rates of compliance with the ketogenic diet and its main subtypes.
Results
In total, 12 studies qualified for inclusion, and data from 270 patients were evaluated.The results of the meta-analysis revealed combined efficacy rates of all types of ketogenic diet, a classical ketogenic diet, and a modified Atkins diet were 42%, 52%, and 34%, respectively; the corresponding combined compliance rates were 45%, 38%, and 56%.
Conclusions
The results indicate that a ketogenic diet is a promising complementary therapy in adult intractable epilepsy, and that while a classical ketogenic diet may be more effective, adult patients are likely to be less compliant with it than with a modified Atkins diet.
Keywords: ketogenic diet, adult, intractable epilepsy, efficacy, compliance
Introduction
A ketogenic diet (KD) was first reported to be an effective treatment for children with intractable epilepsy in the 1920s.1 The primary goal of a KD is to mimic the starvation state in the body's tissues and to produce urinary ketosis by restricting carbohydrate intake and shifting the predominant caloric source to fat.2 KDs include the classical KD (CKD), which is a high-fat, low-carbohydrate diet that usually has a ketogenic ratio (i.e., grams of fat: grams of carbohydrate+protein combined) of 3-4:1, and variants thereof, such as the modified Atkins diet (MAD), medium-chain triglyceride diet (MCT), and low glycemic index treatment, the aim of which is to improve the diet tolerability.2 The MAD, which was first introduced in 2006,3 is usually characterized by a ketogenic ratio of 1-2:1 and a net carbohydrate intake of 20 g/day. The most frequently used variants in adults are the CKD and MAD. The CKD is typically started in the hospital by a dietitian,4 while the MAD is usually initiated at outpatient departments without a fasting period. The latter has been used increasingly in the adult population due to its better operability and tolerability.5
Since its first use, the KD has been used mainly to treat pediatric epilepsy. There is increasing evidence for its high effectiveness, with reportedly 30-60% of children achieving a reduction in seizures of at least 50% after 6 months of treatment.6 Despite its successful use in children and given that approximately 30% of patients cannot be adequately controlled by drugs,7 the application of a KD in adult refractory epilepsy has been limited. The main reasons for this could be the ongoing development of new anticonvulsant drugs, the poor compliance of patients, and the lack of familiarity among physicians with dietary therapies. Therefore, studies on the efficacy of the KD in adults with refractory epilepsy are limited in both quantity and quality, which in turn constrains its clinical application.
We present herein a meta-analysis of the currently available evidence exploring the efficacy of and patient compliance with a KD, with a view to provide neurologists with more information for making clinical decisions. Such information is currently lacking in the literature.
Methods
Search strategy
Searches of the following electronic databases searched were conducted for studies reporting on the efficacy of and patient compliance with a KD in adults with intractable epilepsy: PubMed, EMBASE, Google Scholar, and the ISI Web of Science. The keywords "ketogenic diet", "modified Atkins diet", "medium chain triglyceride diet", and "low glycemic index treatment" were used, cross-referenced with "adults" and "epilepsy/seizure". The reference lists of suitable retrieved articles were manually searched for any missed references, as were the reference lists of existing relevant systematic reviews identified in the original search. The final search was carried out on June 10, 2014.
Inclusion and exclusion criteria
Published studies were included if they fulfilled the following criteria: 1) observed the efficacy of and patient compliance with a KD in adults with refractory epilepsy; 2) produced original information needed for the meta-analysis, such as the subtype of KD, number of enrolled patients, numbers of adherers (those who continued to follow the diet up to a given time point, usually the endpoint of each study) and dropouts (i.e., those who ceased following the diet prior to a given time point), number of patients achieving a seizure reduction of ≥50% (defined as therapeutic success for the purpose of meta- analysis); and 3) produced original information from which the aforementioned factors could be calculated. Non-English articles, small case series, and review articles were excluded.
Data extraction and statistical analysis
All of the included papers were carefully reviewed by two independent reviewers to extract relevant information. Discrepancies were resolved by consensus. The numbers of enrolled patients and patients achieving therapeutic success were used to calculate the efficacy rate of each included study; meta-analysis was then performed to calculate the combined efficacy rate of KD. A combined KD patient compliance rate was calculated using the numbers of enrolled patients and numbers of adherers in each study. The data extraction was based on the total diet duration except those lasting until all of the patients quitted the diet; and the medians of the follow-up durations were prespecified as cutoff points for the latter when calculating the combined efficacy and combined compliance rates. Subgroup analyses were performed according to the subtypes of KD (i.e., CKD, MAD, and MCT). The chi-square test was used to compare the combined results of two of the subgroups (CKD and MAD). The heterogeneity among studies was assessed using the I2 statistic and, where statistically significant heterogeneity was found (p<0.05), the random effects model was used to combine results. Publication bias was investigated using a funnel plot analysis. All analyses were performed using STATA (version 12.0, StataCorp, College Station, TX, USA).
Results
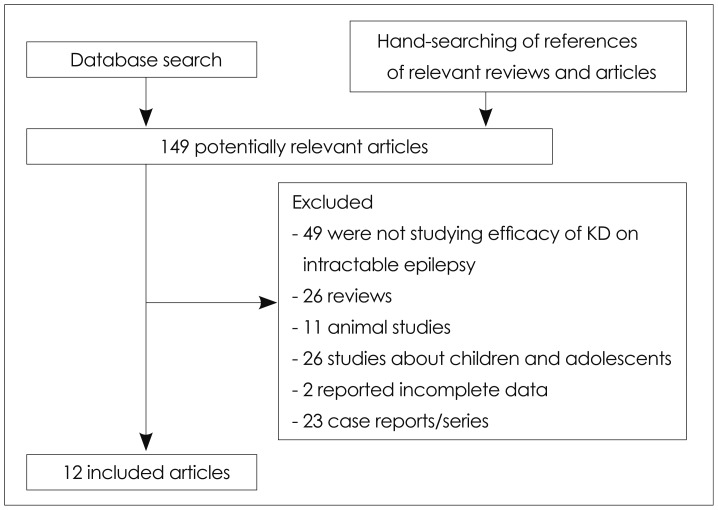
The literature search yielded 12 qualified studies,8,9,10,11,12,13,14,15,16,17,18,19 all in English and focusing on intractable epilepsy. Eleven of them were published as original research articles in professional journals, and the twelve was published as a conference paper.12 Fig. 1 shows the study inclusion/exclusion process. It had been planned that studies in which mainly pediatric patients were enrolled, but had at least five adults and complete data were present, would be included in order to boost the sample size, but in fact no such studies were found; three studies8,13,18 that included both adult and pediatric/adolescent patients were retained, but with only the data for the adults patients (i.e., aged ≥18 years) being entered into the meta-analysis. In addition, the data extraction was based on the total diet duration for all except two of the included studies.11,18
Fig. 1.
Flowchart of studies included and excluded. KD: ketogenic diet.
A final total of 270 patients was evaluated. In all of the studies, the KD was used as a complementary therapy to the previously prescribed anticonvulsant drugs. The CKD was used in six studies (n=168 patients) and the MAD was used in five (n=87 patients). The study of Lambrechts et al.19 (n=15 patients) used both MCT and CKD. Table 1 lists the baseline information and relevant data of all of the included studies.
Table 1.
Baseline information and relevant data of included studies
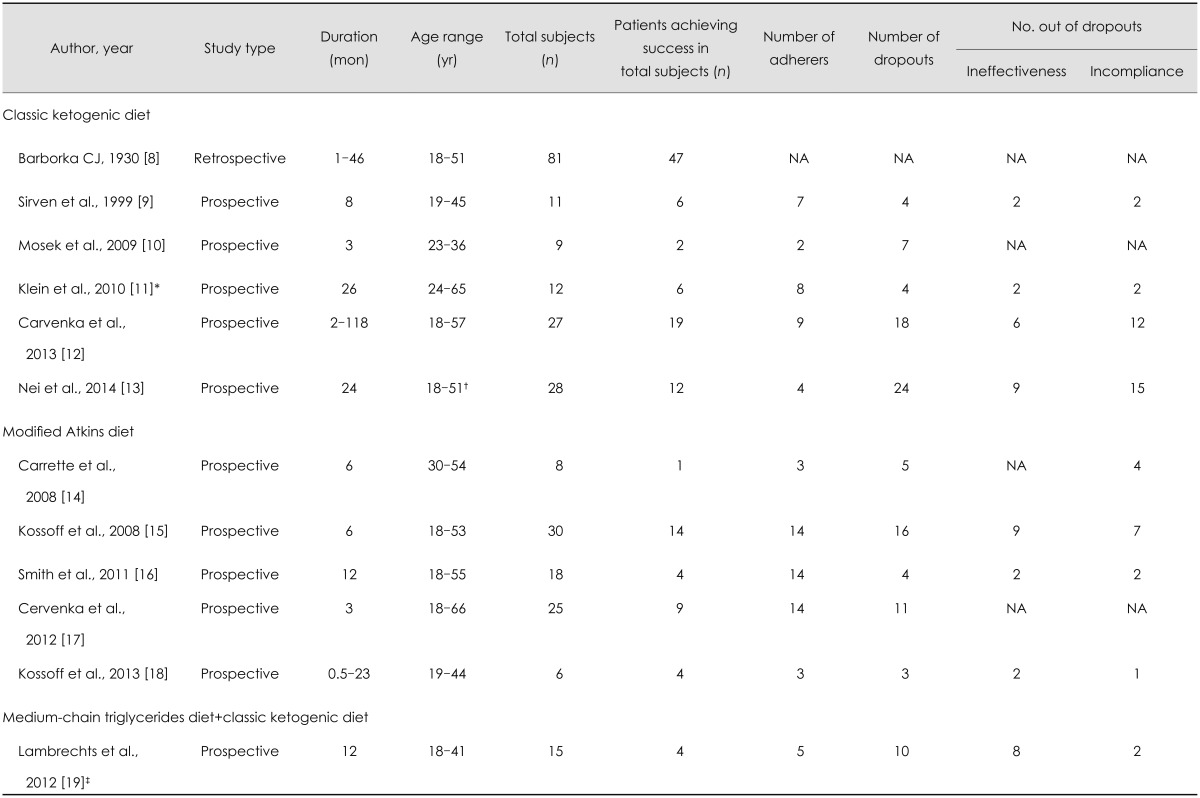
*KD with a 3:1 ketogenic ratio were used in Klein P's study, and the ketogenic ratio for the other five studies using CKD were 4:1, †All patients were >17 years at the time of diet initiation, except one (11 years) who was prespecified to have achieved success and adhered to diet for meta-analysis, ‡Medium-chain triglycerides diet and classic ketogenic diet were both used in this study.
CKD: classical ketogenic diet, NA: not available.
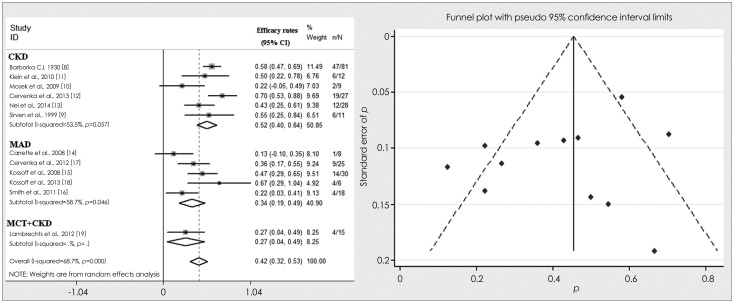
The efficacy rates of KD in adult intractable epilepsy ranged from 13%14 to 70%12 in the 12 included studies. The meta-analysis yielded a combined efficacy rate of the KD of 42% (random effects model). There was evidence for a significant heterogeneity among the studies (I2=68.7%, p<0.001). The combined efficacy rate of the CKD was 52%, while that of the MAD was 34%; the heterogeneity for both of these was less than for the KD. The chi-square test indicated that the odds ratio (OR) for the therapeutic success of the CKD relative to the MAD was 2.04 [95% confidence interval (CI)=1.19-3.49, p=0.009], indicating a significant difference between the efficacy rates of the two KD subtypes. The funnel plot revealed a fairly symmetrical distribution of the efficacy rates, with four studies falling outside the 95% CIs. The forest and funnel plots of the combined efficacy rates are shown in Fig. 2. Among the included studies, only that of Barborka8 was a retrospective one, and dated back 80 years. Therefore, that study was excluded from the meta-analysis of combined efficacy rates in order to evaluate the possible bias that it might cause. The combined efficacy rates of the KD, CKD, and MAD were 40%, 49%, and 34%, respectively. The OR of therapeutic success for the CKD relative to the MAD was 1.81 (95% CI=1.06-3.10, p=0.03), indicating that the efficacy rate differed significantly between the two KD subtypes. Therefore, it appears that the main findings of this study would not differ regardless of whether or not the study of Barborka8 was included.
Fig. 2.
The forest and funnel plots of the combined efficacy rates of ketogenic diet and its variants. CI: confidence interval, CKD: classical ketogenic diet, MAD: modified Atkins diet, MCT: medium-chain triglycerides diet, N: number of total subjects, n: number of patients achieving success in total subjects, % weight: percentage that each study counts in the results of the meta-analysis.
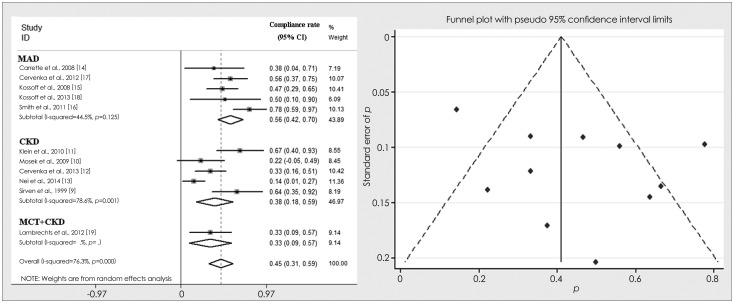
The number of adherers was reported for 11 studies. The meta-analysis revealed a combined compliance rate for the KD was 45% (random effects model). There was evidence for clear heterogeneity among the studies (I2=76.3%, p<0.001). The combined compliance rate of the CKD was 38%, with significant heterogeneity (I2=78.6%, p=0.001), while that of the MAD was 56%, with insignificant heterogeneity (I2=44.5%, p=0.125). The chi-square test revealed that the OR of the compliance rate for the CKD relative to the MAD was 0.48 (95% CI=0.28-0.81, p=0.006), indicating that the compliance rate differed significantly between the two KD subtypes. The funnel plot showed a relatively mild publication bias of the 11 studies, with 2 studies lying outside of the CIs. The forest and funnel plots of the combined compliance rates are shown in Fig. 3.
Fig. 3.
The forest and funnel plots of the combined compliance rates of ketogenic diet and its variants. CI: confidence interval, CKD: classical ketogenic diet, MAD: modified Atkins diet, MCT: medium-chain triglycerides diet, % weight: percentage that each study counts in the results of the meta-analysis.
Discussion
The aim of this study to summarize the published studies in order to identify the efficacy of and patient compliance with the KD and its main subtypes in adults with intractable epilepsy. The review of Payne et al.20 published in 2011 focused on the KD and related diets in both adolescents and adults; the results of the included studies were simply listed, without being analyzed statistically. Since then, several studies on KD in adult epileptics have been published. These studies were subjected to meta-analysis herein using statistical methods in an attempt to quantitatively determine the efficacy of and patient compliance with this dietary treatment and its main variants in adults.
Efficacy of and patient compliance with the KD
The present meta-analysis of 12 studies yielded a combined efficacy rate of the KD of 42%, with significant heterogeneity. As mentioned in the Introduction, a high effectiveness (a reduction in seizures of at least 50%) of 30-60% can be achieved when applying the KD in pediatric epilepsy.6 Thus, the efficacy of the KD in adults was comparable with that in pediatric patients. In fact, therapeutic success rates of up to 70% were reported at the third International Symposium on Dietary Therapies by several groups who have been applying the KD (including both the CKD and MAD) in their adult epilepsy diet centers.12 Therefore, the KD could be a promising complementary therapy in adult intractable epilepsy.
The significant heterogeneity found on meta-analysis of the combined efficacy rate of the KD could be at least partly attributable to the different subtypes of KD used in the various studies (i.e., CKD versus MAD). Subgroup meta-analysis revealed a reduced heterogeneity. Other factors that might cause heterogeneity are discussed in Section 4.3.
The meta-analysis of 11 studies (i.e., excluding the study of Barborka8) revealed a combined rate of patient compliance with the KD of 45%. This relatively low compliance rate, which is consistent with previous studies,20 was caused mainly by ineffectiveness or lack of compliance (Table 1). Lack of compliance could be attributed to intolerability caused by severe side effects or discontinuation caused by either psychosocial factors or the restrictiveness of the diet.12,13,15 With few reports of severe or fatal side effects,6,10,11,15,16,19,20 it is possible for neurologists and dietitians to make efforts to increase patient compliance, which could in turn increase the efficacy of the dietary therapy.
Comparison of the CKD and the MAD
The present meta-analysis revealed that the combined efficacy rate was significantly better for the CKD (52%) than for the MAD (34%, p=0.036), whereas the combined compliance rate was significantly higher for the MAD (56% vs. 38%, p=0.006).Thus, the subgroup analysis of the existing studies suggests that while the CKD is more effective, patients with intractable epilepsy are less likely to comply with it than with the MAD.
In pediatric applications, two case-control studies comparing the CKD and MAD yielded inconsistent results: Miranda et al.21 demonstrated a similar effectiveness of the two KD subtypes, while El-Rashidy et al.22 found a higher efficacy for the CKD and a similar tolerability for both. However, Kossoff et al.23 observed additional seizure control following a switch from the MAD to the CKD, indicating that the latter likely represents a "higher dose" of dietary therapy than the MAD. There have been no direct comparisons of these two KD subtypes in adults. The present study performed an indirect comparison of the CKD and MAD, shedding a little light on the issue. It can perhaps be concluded that, as reported in a previously published review pertaining to childhood epilepsy,24 dietary therapy for adult patients with intractable epilepsy should be initiated with the MAD, with a switch to the CKD being considered if greater seizure control is required. Further case-control studies in adults are needed to confirm this conclusion.
Limitations
This meta-analysis should be interpreted in the light of certain limitations. First, the number of patients was small; ethical factors limited the enrollment of patients, there were practical difficulties, and the included studies were all observational and without control groups; thus, the conclusions provide only class III evidence assessing the efficacy and compliance of the KD in adults. Second, the long follow-up duration (3-118 months), different distributions of age, gender, and seizure type, and other uncontrolled confounding factors might add to the bias of the meta-analysis. Third, the included patients undertook dietary therapies based on the practices of individual treating centers and providers, which might also have introduced some bias.
In conclusion, this meta-analysis provides formal statistical support for the efficacy and compliance of the KD and its two variants (CKD and MAD) in the treatment of adult intractable epilepsy. The results indicate that this diet is a promising complementary therapy in adult intractable epilepsy, with an overall efficacy of 42%. The overall compliance rate was relatively low, at 45%. Subgroup analysis revealed that the CKD might be more effective but less tolerable than the MAD in adults. Together these findings indicate that previous studies have provided valuable statistical evidence supporting the application of the KD in adults with refractory epilepsy. Controlled studies with large samples should be conducted to confirm these conclusions.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Bull. 1921;2:307–308. [Google Scholar]
- 2.Cervenka MC, Kossoff EH. Dietary treatment of intractable epilepsy. Continuum (Minneap Minn) 2013;19(3 Epilepsy):756–766. doi: 10.1212/01.CON.0000431396.23852.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47:421–424. doi: 10.1111/j.1528-1167.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 4.Kossoff EH, Hartman AL. Ketogenic diets: new advances for metabolism-based therapies. Curr Opin Neurol. 2012;25:173–178. doi: 10.1097/WCO.0b013e3283515e4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kossoff EH, Cervenka MC, Henry BJ, Haney CA, Turner Z. A decade of the modified Atkins diet (2003-2013): Results, insights, and future directions. Epilepsy Behav. 2013;29:437–442. doi: 10.1016/j.yebeh.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Lee PR, Kossoff EH. Dietary treatments for epilepsy: management guidelines for the general practitioner. Epilepsy Behav. 2011;21:115–121. doi: 10.1016/j.yebeh.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365:919–926. doi: 10.1056/NEJMra1004418. [DOI] [PubMed] [Google Scholar]
- 8.Barborka CJ. Epilepsy in adults: results of treatment by ketogenic diet in one hundred cases. Arch Neurol Psychiatry. 1930;23:904–914. [Google Scholar]
- 9.Sirven J, Whedon B, Caplan D, Liporace J, Glosser D, O'Dwyer J, et al. The ketogenic diet for intractable epilepsy in adults: preliminary results. Epilepsia. 1999;40:1721–1726. doi: 10.1111/j.1528-1157.1999.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 10.Mosek A, Natour H, Neufeld MY, Shiff Y, Vaisman N. Ketogenic diet treatment in adults with refractory epilepsy: a prospective pilot study. Seizure. 2009;18:30–33. doi: 10.1016/j.seizure.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Klein P, Janousek J, Barber A, Weissberger R. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav. 2010;19:575–579. doi: 10.1016/j.yebeh.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Cervenka MC, Henry B, Nathan J, Wood S, Volek JS. Worldwide dietary therapies for adults with epilepsy and other disorders. J Child Neurol. 2013;28:1034–1040. doi: 10.1177/0883073813488671. [DOI] [PubMed] [Google Scholar]
- 13.Nei M, Ngo L, Sirven JI, Sperling MR. Ketogenic diet in adolescents and adults with epilepsy. Seizure. 2014;23:439–442. doi: 10.1016/j.seizure.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Carrette E, Vonck K, de Herdt V, Dewaele I, Raedt R, Goossens L, et al. A pilot trial with modified Atkins' diet in adult patients with refractory epilepsy. Clin Neurol Neurosurg. 2008;110:797–803. doi: 10.1016/j.clineuro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Kossoff EH, Rowley H, Sinha SR, Vining EP. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008;49:316–319. doi: 10.1111/j.1528-1167.2007.01256.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith M, Politzer N, Macgarvie D, McAndrews MP, Del Campo M. Efficacy and tolerability of the modified Atkins diet in adults with pharmacoresistant epilepsy: a prospective observational study. Epilepsia. 2011;52:775–780. doi: 10.1111/j.1528-1167.2010.02941.x. [DOI] [PubMed] [Google Scholar]
- 17.Cervenka MC, Terao NN, Bosarge JL, Henry BJ, Klees AA, Morrison PF, et al. E-mail management of the modified Atkins Diet for adults with epilepsy is feasible and effective. Epilepsia. 2012;53:728–732. doi: 10.1111/j.1528-1167.2012.03406.x. [DOI] [PubMed] [Google Scholar]
- 18.Kossoff EH, Henry BJ, Cervenka MC. Efficacy of dietary therapy for juvenile myoclonic epilepsy. Epilepsy Behav. 2013;26:162–164. doi: 10.1016/j.yebeh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Lambrechts DA, Wielders LH, Aldenkamp AP, Kessels FG, de Kinderen RJ, Majoie MJ. The ketogenic diet as a treatment option in adults with chronic refractory epilepsy: efficacy and tolerability in clinical practice. Epilepsy Behav. 2012;23:310–314. doi: 10.1016/j.yebeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Payne NE, Cross JH, Sander JW, Sisodiya SM. The ketogenic and related diets in adolescents and adults--a review. Epilepsia. 2011;52:1941–1948. doi: 10.1111/j.1528-1167.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- 21.Miranda MJ, Mortensen M, Povlsen JH, Nielsen H, Beniczky S. Danish study of a modified Atkins diet for medically intractable epilepsy in children: can we achieve the same results as with the classical ketogenic diet? Seizure. 2011;20:151–155. doi: 10.1016/j.seizure.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 22.El-Rashidy OF, Nassar MF, Abdel-Hamid IA, Shatla RH, Abdel-Hamid MH, Gabr SS, et al. Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol Scand. 2013;128:402–408. doi: 10.1111/ane.12137. [DOI] [PubMed] [Google Scholar]
- 23.Kossoff EH, Bosarge JL, Miranda MJ, Wiemer-Kruel A, Kang HC, Kim HD. Will seizure control improve by switching from the modified Atkins diet to the traditional ketogenic diet? Epilepsia. 2010;51:2496–2499. doi: 10.1111/j.1528-1167.2010.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auvin S. Should we routinely use modified Atkins diet instead of regular ketogenic diet to treat children with epilepsy? Seizure. 2012;21:237–240. doi: 10.1016/j.seizure.2012.02.005. [DOI] [PubMed] [Google Scholar]