Abstract
Background and Purpose
Bariatric surgery is associated with improved cognitive function, but the mechanisms underlying these gains remain poorly understood. Disturbed leptin and ghrelin systems are common in obese individuals and are associated with impaired cognitive function in other samples. Bariatric surgery has been shown to improve serum leptin and ghrelin levels, and these changes may underlie postoperative cognitive improvements.
Methods
Eighty-four patients completed a computerized cognitive test battery prior to bariatric surgery and at 12 months postoperatively. Participants also submitted to an 8-hour fasting blood draw to quantify serum leptin and ghrelin concentrations at these same time points.
Results
Baseline cognitive impairments and disturbed leptin and ghrelin levels improved at the 12-month follow-up compared to presurgery. Higher leptin levels were associated with worse attention/executive function at baseline; no such findings emerged for ghrelin. Regression analyses controlling for baseline factors and demographic characteristics showed that both decreased leptin and increased ghrelin following surgery was associated with better attention/executive function at the 12-month follow-up. These effects diminished after controlling for the postoperative change in body mass index (BMI); however, BMI change did not predict 12-month cognitive function.
Conclusions
Improvements in leptin and ghrelin levels following bariatric surgery appear to contribute to postoperative cognitive benefits. These gains may involve multiple mechanisms, such as reduced inflammation and improved glycemic control. Future studies that employ neuroimaging are needed to clarify the underlying mechanisms and determine whether the effects of bariatric surgery on leptin and ghrelin levels can attenuate adverse brain changes and/or risk of dementia in severely obese individuals.
Keywords: obesity, bariatric surgery, cognitive function, leptin, ghrelin
Introduction
Obesity is a significant health concern worldwide and is now a recognized risk factor for adverse neurological changes, including dementia (e.g., Alzheimer's disease), and abnormalities on neuroimaging.1,2 Neuropsychological testing also shows that obese individuals commonly exhibit more subtle cognitive dysfunction. For instance, an elevated body mass index (BMI) in almost all age groups is inversely associated with reduced cognitive function across multiple domains, including attention, executive function, and memory.3,4,5
There is a growing body of literature suggesting that obesity-associated cognitive deficits are partially reversible through bariatric surgery. Bariatric surgery patients have been shown to exhibit lasting postoperative improvements (i.e., up to 36 months) in nearly all domains of cognitive function.6,7,8 The mechanisms underlying this pattern of improved cognitive function are poorly understood, with past work revealing only minor positive effects from resolution of medical comorbidities and/or the independent effects of substantial weight loss.6
There is reason to believe that the effects of bariatric surgery on key neurohormones may be important contributors to postoperative cognitive improvements. Obese individuals exhibit disturbed leptin and ghrelin systems (i.e., elevated leptin and decreased ghrelin);9 these are both appetitive hormones that are directly involved in the regulation of food intake, energy, and weight.9 Leptin and ghrelin fulfill such functions by binding to receptor sites in brain structures that also play key roles in cognitive function (e.g., hippocampus and hypothalamus).9,10,11,12 Indeed, the existing literature shows that leptin and ghrelin may influence neurocognitive outcomes. For instance, both leptin and ghrelin have been linked with cognitive function in elderly and patient populations, and are speculated to be involved in the pathogenesis of neurodegenerative conditions such as Alzheimer's disease.11,13,14,15,16
Interestingly, serum leptin and ghrelin levels can be improved through bariatric surgery,17,18 although whether such changes translate to better cognitive function has yet to be determined. The present study examined whether changes in leptin and ghrelin levels are associated with improved cognitive function in patients undergoing bariatric surgery. We hypothesized that improvements in serum leptin and ghrelin levels following bariatric surgery would result in better cognitive function at the 12 month follow-up.
Methods
Trial design and participants
Eighty-four patients were recruited into a multisite National Institutes of Health prospective study examining the effects of bariatric surgery on cognitive function. All of these bariatric surgery patients were part of the Longitudinal Assessment of Bariatric Surgery (LABS) parent project and were recruited from existing LABS sites (Columbia University Medical Center, New York; Cornell University Medical Center, New York; and the Neuropsychiatric Research Institute, Fargo).19 All participants were bariatric surgery candidates scheduled for weight-loss surgery and were thus determined eligible for surgery based on a comprehensive preoperative clinical and medical workup that was performed as part of routine weight-loss surgery clinical care; indeed, the participants typically failed to meet the eligibility criteria for bariatric surgery due to medical (e.g., medication status or other health-related concerns), psychiatric (e.g., severe depression that may influence postoperative treatment adherence), and/or cognitive (e.g., altered mental status as based on brief cognitive screening) problems that may either present as safety concerns for surgery or adversely impact postoperative outcomes.
Additional study inclusion/exclusion criteria were also implemented to further minimize potential confounding factors. For study inclusion, bariatric surgery patients were required to be enrolled in LABS, aged 20-70 years, and English-speaking. Exclusion criteria included history of neurological disorder or injury (e.g., dementia or seizures), moderate or severe head injury (defined as loss of consciousness for >10 minutes), past or current history of alcohol or drug abuse [defined by criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV)], history of a learning disorder or developmental disability (defined by DSM-IV criteria), or impairment in any perceptual sensory function. Participants with a past or current history of severe psychiatric illness (e.g., schizophrenia or bipolar disorder) were also excluded. Given the high rates of depressive symptoms among bariatric surgery patients, depression was not applied as an exclusion criterion. It is noteworthy that only three participants met the past-month criteria for major depressive disorder.
Only one bariatric surgery patient underwent a gastric-banding procedure; all of the others underwent a Roux-en-Y gastric bypass surgery, and hence no comparisons for type of surgery were conducted. The study population represents all individuals who had complete baseline and 12-month blood workups and had completed postoperative cognitive testing. Table 1 presents baseline demographic and clinical characteristics of the participants.
Table 1.
Demographic and medical characteristics
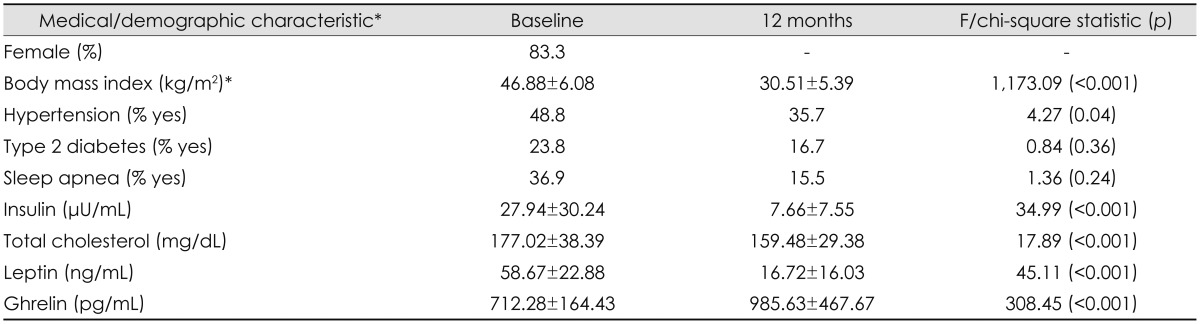
Data are mean±SD values except where indicated otherwise.
*The sample size varied for baseline and 12-month body mass index, and the diagnostic status of hypertension, type 2 diabetes, and sleep apnea; statistics for differences in these variables are based on the total data for each time point.
Interventions and clinical follow-up
The Institutional Review Board approved all procedures and the participants provided written informed consent prior to study involvement. All bariatric surgery patients underwent blood draw after fasting for 8 hours; leptin, ghrelin, and total cholesterol blood assays were analyzed at the LABS Central Laboratory using standardized procedures for the parent project. The participants also completed a computerized cognitive test battery within 30 days prior to the surgery. These same procedures were repeated 12 months after surgery. Participants' heights and weights were measured at each time point and used to calculate their BMIs. Medical and demographic characteristics were ascertained via self-report, and were corroborated by a medical record review performed by trained research staff.
Outcomes
Cognitive function
The IntegNeuro cognitive test battery was administered to assess cognitive function. This is a computerized battery that assesses performance in multiple cognitive domains; it can be completed in 45-60 minutes, involves automated scoring, and has demonstrated excellent validity and reliability.20,21 The cognitive-domain and specific tests that were conducted are described below.
Attention/executive function
Digit span backwards
This task tests auditory working memory. Subjects are presented with a series of digits on the touch screen, separated by a 1-second interval. They are asked to immediately enter the digits on a numeric keypad on the touch screen in the reverse (i.e., backwards) sequence from the order presented. The number of digits in each sequence is gradually increased from three to nine, with two sequences at each level. The total number of correct backward trials was used in the present analyses.
Switching of attention
This test is a computerized adaptation of the Trail-Making Test A and B.22 First, subjects are asked to touch a series of 25 numbers in ascending order as quickly as possible. Then, an array of 13 numbers (1-13) and 12 letters (A-L) is presented, and the subjects must alternately touch numbers and letters in ascending order. The first part of this test assesses attention and psychomotor speed and the second part assesses executive function. Time to completion served as the outcome measure in the present study.
Verbal interference
This task taps into the ability to inhibit automatic and irrelevant responses and has similarities to the Stroop Color Word Test.23 Subjects are presented with colored words one at a time. Below each colored word is a response pad with the four possible words displayed in black and in a fixed format. The subject is required to name the color of each word as quickly as possible; this assesses executive functioning. The total number of words correctly identified was used as the dependent variable in the present study.
Memory
Verbal list learning
In this test the subjects are read a list of 12 words a total of 4 times and then asked to recall as many words as possible after each trial. Following presentation and recall of a distraction list, participants are asked to recall words from the original list. After a 20-minute filled delay (i.e., participants continued to perform other tasks), participants are asked to freely recall the learned list and perform a recognition trial comprising target words and nontarget words. The total long-delay free recall and recognition of these verbal list items were used to assess memory.
Language
Letter fluency
Subjects are asked to generate words beginning with a given letter of the alphabet for 60 seconds. A different letter is used for each of three trials. The total number of correct words generated across the three trials served as the dependent variable in the present study.
Animal fluency
In this task, subjects generate as many animal names as possible in 60 seconds. The total number of correctly named animals served as the dependent variable in the present study.
Statistical analysis
All analyses were performed using SPSS software, version 22 (SPSS Inc., Chicago, IL, USA). Raw scores of all cognitive measures were transformed to T-scores (a distribution with a mean of 50, and a standard deviation of 10) using normative data correcting for age, gender, and estimated intelligence; that is, the participants' scores on each of the cognitive tests were derived through comparison with healthy individuals in the normative population of a similar age, gender, and intellectual function. A T-score of <35 (i.e., 1.5 SDs below the mean of normative standards) was reflective of impairment. Composite scores were computed for attention/executive function, memory, and language that consisted of the mean of T-scores of the respective neuropsychological measures that comprise each domain.
Bivariate correlations were first conducted to examine the association between baseline serum concentrations of leptin and ghrelin, and baseline demographic and medical characteristics. Repeated-measures analysis of variance (ANOVA) was then conducted to examine changes in serum leptin and ghrelin concentration as well as in each cognitive domain (e.g., attention/executive function, memory, and language) between baseline and at 12 months after surgery. Hierarchical regression analyses were then conducted to examine the possible influence of common medical comorbidities in severely obese individuals on the postoperative cognitive function in each domain. Block 1 included baseline cognitive test performance in the respective domain, and block 2 included baseline hypertension and diabetes status (1=positive current diagnostic history; 0=past or no history), as well as insulin and total cholesterol levels.
Regression analyses adjusting for age and sex were performed to determine the baseline associations among leptin and ghrelin with attention/executive function, memory, and language. Separate hierarchical regression analyses were then conducted to investigate the effects of postoperative changes in serum leptin and ghrelin levels on each cognitive domain at the 12-month follow-up. Specifically, the performances in attention/executive function, memory, and language at 12 months postoperatively were entered as dependent variables, yielding a total of three hierarchical regression models for both leptin and ghrelin. For models examining the predictive validity of leptin, age, sex (1=males; 2=females), baseline performance of the respective domain, and baseline leptin were entered into block 1. Serum leptin concentration at 12 months was then entered in block 2 to determine its incremental predictive validity for 12-month cognitive function. These same procedures were followed for models examining the effects of ghrelin on postoperative cognitive function. Finally, the aforementioned analyses were repeated with the addition of changes in pre- to postoperative BMI included as a covariate; the sample size for these analyses was reduced to 78 due to missing data.
Results
Sample characteristics
The participants aged 43.86±10.39 years (mean±SD), 83.3% were female, and they had a baseline BMI of 46.88±6.08 kg/m2, indicating that they were very severely obese (Table 1). Insulin levels were elevated at baseline, and medical conditions such as hypertension, type 2 diabetes, and sleep apnea were also prevalent at that point. Participants exhibited an improved health status at the 12-month follow-up, including lower BMI and decreased prevalence of medical comorbidities.
At baseline, the sample exhibited baseline leptin and ghrelin serum concentrations of 58.67±22.88 ng/mL and 712.28±164.43 pg/mL, respectively. Repeated-measures ANOVA revealed that the serum concentration of leptin decreased [F(1, 83)=308.45, p<0.001] and that ghrelin increased [F(1, 83)= 45.11, p<0.001] between baseline and at 12 months after bariatric surgery. Bivariate correlations (n=78) showed that postoperative decreases in BMI were significantly associated with decreases in leptin [r(76)=0.52, p<0.001] and increases in ghrelin [r(76)=-0.23, p<0.05] concentration.
Bivariate correlations were conducted to examine the association between baseline serum leptin and ghrelin levels, and sample characteristics. Higher baseline leptin concentrations were associated with being female [r(82)=0.34, p<0.01] and having a higher baseline BMI [r(80)=0.38, p<0.001], but not with age, baseline serum insulin or total cholesterol levels, or current diagnostic history of sleep apnea, type 2 diabetes, or hypertension (p>0.05 for all). Higher baseline serum ghrelin levels were associated with decreased baseline status of hypertension [r(82)=-0.25, p=0.02] and type 2 diabetes [r(82)=-0.27, p=0.01]. Serum ghrelin concentration was not correlated with any other baseline medical or demographic factors (p>0.05 for all).
Cognitive function
In terms of baseline cognitive test performance, impairments in memory and language abilities were common (Table 2). Most notably, 15.5% of the sample exhibited impairments in long-delay free recall and 17.9% on the recognition task. For language, 16.7% of the sample demonstrated impairments in the letter fluency task. Fewer than 10.0% of the subjects exhibited impairments for each of the attention/executive function tasks.
Table 2.
Neuropsychological test performance (mean±SD or T-score) among the bariatric surgery patients (n=84)
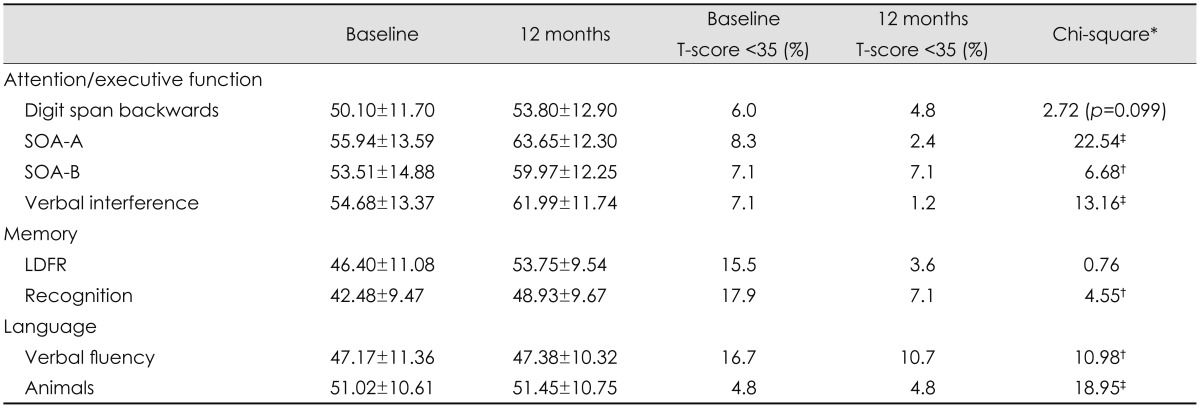
*Chi-square analyses compared impairment at baseline and at 12 months postoperatively, †p<0.05, ‡p<0.001.
LDFR: long-delay free recall, SOA-A/-B: switching of attention A/B.
Repeated-measures ANOVA revealed a significant and positive effect on cognitive function over the 12 months following surgery with respect to attention/executive function [F(1, 83)=36.20, p<0.001] and memory [F(1, 83)=54.90, p<0.001]. In contrast, performance on tests of language abilities remained stable over time [F(1, 83)=0.19, p=0.67]. The prevalence of impairments in many measures of attention/executive function, memory, and language was lower at 12 months postoperatively than preoperatively. Regression analyses controlling for baseline cognitive test performance revealed that a medical comorbidity model that included baseline history of hypertension and sleep apnea, and serum total cholesterol and insulin levels was not predictive of postoperative cognitive changes in any domain (p>0.05 for all).
Leptin, ghrelin, and cognitive function
Table 3 provides a summary of regression models examining the baseline associations between serum leptin and ghrelin levels, and cognitive function. After controlling for age and sex, higher baseline serum leptin concentrations were associated with worse attention/executive function (β=-0.26, p=0.02). No such pattern emerged for memory or language abilities (p>0.05 for all). In terms of baseline ghrelin, there was only a nonsignificant trend between lower serum ghrelin levels and worse language abilities (β=0.20, p=0.08). Baseline serum ghrelin concentration was not associated with attention/executive function or memory (p>0.05 for all).
Table 3.
Baseline associations between serum leptin and ghrelin concentrations and cognitive function (n=84)
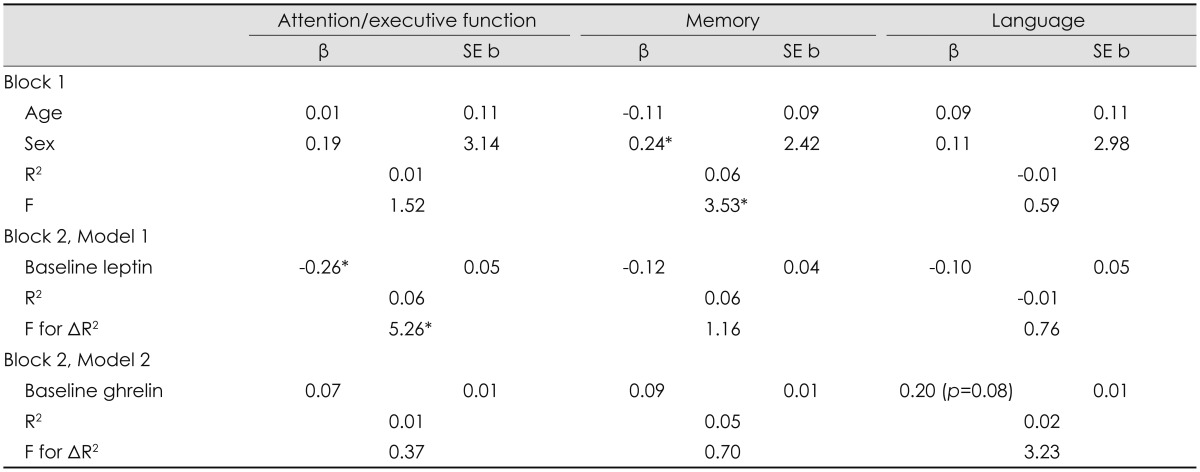
*p<0.05.
After controlling for age, sex, and baseline factors, 12-month serum leptin (β=-0.19, p=0.03) and ghrelin (β=0.25, p=0.03) levels predicted 12-month attention/executive function. Specifically, the reduction in serum leptin level and increase in serum ghrelin level following bariatric surgery was accompanied by improved attention/executive function. This pattern did not emerge for either 12-month memory or language abilities (p>0.05 for all) (Table 4 and 5).
Table 4.
Predictive validity of the influence of postoperative changes in serum leptin concentration for cognitive function
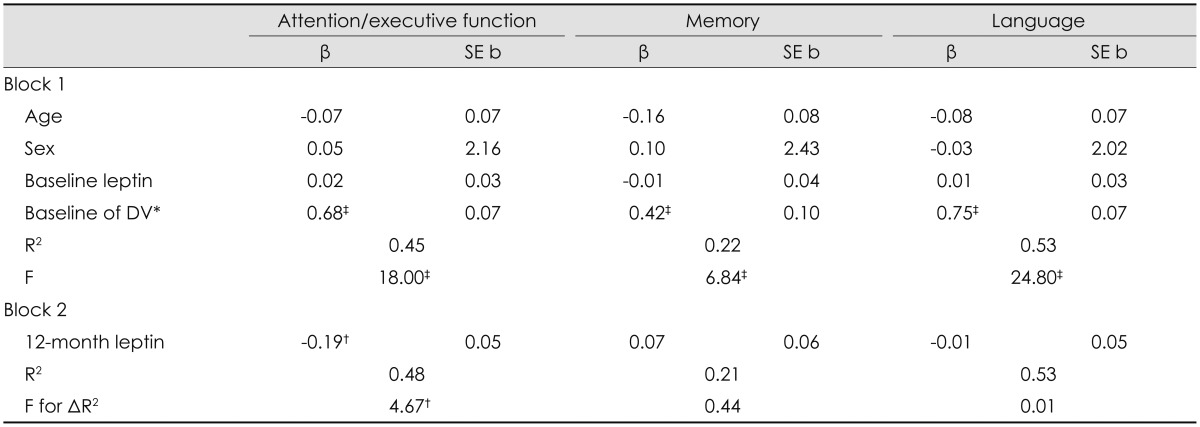
*Baseline test performance of the 12-month DV was entered as a covariate, †p<0.05, ‡p<0.01.
DV: dependent variable.
Table 5.
Predictive validity of the influence of postoperative changes in serum ghrelin concentration for cognitive function
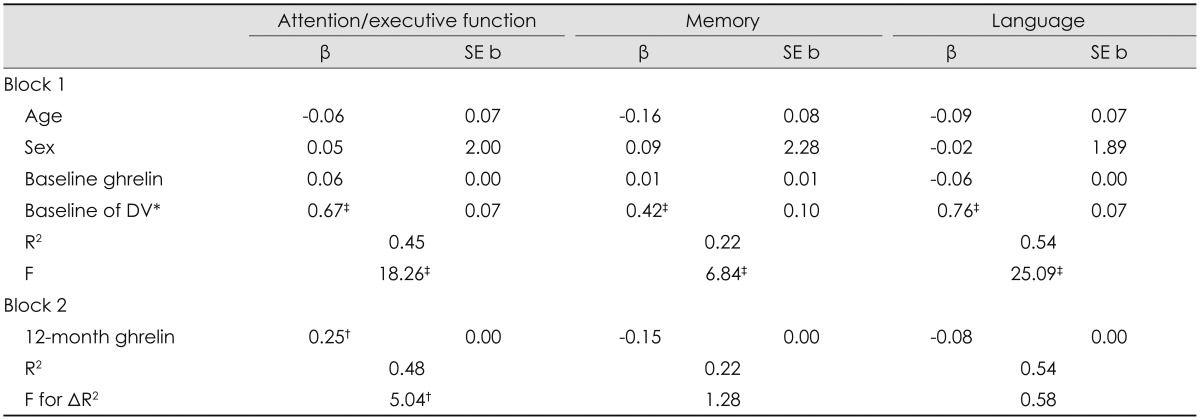
*Baseline test performance of the 12-month DV was entered as a covariate, †p≤0.05, ‡p<0.01.
DV: dependent variable.
BMI changes, leptin, ghrelin, and cognitive function
Follow-up analyses were conducted to determine whether the effects of leptin and ghrelin on attention/executive function remained after accounting for pre- to postoperative changes in BMI. After taking the change in BMI into account, the serum levels of leptin and ghrelin no longer exhibited significant effects on attention/executive function (p>0.05 for all). However, partial correlations controlling for baseline cognitive function also showed that changes in BMI were not associated with 12-month cognitive function in any of the domains (p>0.05 for all).
Discussion
Cognitive function improved across multiple domains at 12 months following bariatric surgery. While the mechanisms underlying this phenomenon remain poorly understood, the findings of this study extend those in the existing literature by showing that changes in serum leptin and ghrelin levels predict postoperative cognitive function. Several aspects of these findings warrant brief discussion.
Baseline serum leptin concentration adversely impacted attention/executive function, and postoperative decreases in serum leptin were associated with improved performance in this domain. Serum leptin is elevated in obese individuals and can be improved through bariatric surgery.17,24 It has been suggested that such changes not only help to sustain weight loss following surgery,18 but also underlie postoperative improvements in neurocognitive function. Higher plasma leptin levels have been shown to attenuate cognitive decline, reduce dementia risk, and slow brain atrophy in nonobese elderly subjects,25,26 possibly through the role of leptin in the modulation of synaptic plasticity and beta-amyloid regulation. However, severely obese individuals can become resistant to the effects of leptin, and elevated serum levels are often viewed as being an indicator of leptin resistance.27 This results in a reduction of the effects of leptin on brain receptors and a subsequent decrease in the aforementioned neuroprotective effects.28,29,30,31,32 For example, past work has shown that leptin resistance in the hippocampus is associated with Alzheimer's disease pathology;16 leptin receptor dysfunction has also been linked with worse cognitive function.33 It has also been suggested that leptin enters the brain via a saturable transport system, and that the capacity of this mechanism is reduced in obese individuals, in turn increasing leptin resistance in the brain.28 Since serum leptin levels improved (i.e., decreased) in the present sample after surgery, it is possible that bariatric surgery increases the brain's sensitivity to leptin (i.e., decreased leptin resistance) in obese persons, perhaps via increased brain permeability to leptin, to improve cognition and possibly reduce the risk of cognitive decline; future studies should explore this possibility.
A particularly interesting finding was the association of postoperative increases (i.e., improvements) in serum ghrelin levels with better attention/executive function at the 12-month follow-up. The effects of ghrelin are often described as being opposite to those of leptin. Ghrelin functions as an appetite stimulant, and modulates food intake, energy homeostasis, and body weight largely through interactions with receptors in the hypothalamus.9 The serum ghrelin concentration is inversely associated with BMI, but can also be modified through bariatric surgery.9,17 Such changes likely promote cognition in severely obese individuals via ghrelin's neuroprotective functions. For example, ghrelin has been shown to reduce cell death and increase neuronal survival following cerebral ischemia in rodents.34,35,36 This is noteworthy in light of the elevated risk of brain hypoperfusion and vascular injury (e.g., stroke) in severely obese people.37,38 Ghrelin is indeed closely involved in the manifestation of vascular comorbidities (e.g., metabolic syndrome, diabetes)39 and may interact with such conditions to influence pre- and postoperative cognitive function in bariatric surgery patients. Some studies have linked higher serum ghrelin levels to better cognitive function,40 and plasma concentrations of this hormone are reduced in aging individuals and patients with Alzheimer's disease.41,42 Together these findings suggest that future studies should determine whether increases in ghrelin after bariatric surgery reduce the risk of cognitive decline and dementia in obese individuals via neuroprotective effects against vascular (e.g., ischemic) injury.
No associations between serum leptin and ghrelin levels and pre- or postoperative memory emerged in the current study. This finding was unexpected given the proposed role of these hormones in Alzheimer's disease and hippocampal function.16,40,42,43 In fact, recent work shows that leptin and ghrelin may actually prevent hippocampal dysfunction through neuroprotective effects against beta amyloid and tau pathologies.44 Thus, despite the current findings, it is likely that these hormones play a key role in learning and memory, and further research is needed to clarify this relationship. It is possible that the methodological limitations of the current study limited the ability to detect this association, including the use of a single memory assessment and its possible lack of sensitivity to neurohormonal changes. Alternatively, the effects of leptin and ghrelin on cognitive function may be dependent on the population examined. For example, past work in obese individuals with diabetes also found an association between leptin and executive function but not memory;15 in contrast, an association between leptin and reduced dementia risk was found among nonobese people.26 Nonetheless, future studies that include both controls and comparison groups are needed to determine the differential effects of leptin and ghrelin on cognitive function, particularly in the context of bariatric surgery.
Finally, the findings of the present study also show that, after accounting for the pre- to postoperative change in BMI, leptin and ghrelin no longer exhibited significant effects on attention/executive function, even though the postoperative change in BMI was also not a predictor of cognitive function at 12 months postoperatively. This pattern of findings is unexpected, but may be attributable to several possible processes. The most likely explanation is that the improved cognitive function following bariatric surgery is attributable to multiple physiological changes. For instance, recent work shows that reduced inflammatory processes following post-bariatric-surgery weight loss is associated with reduced amyloid precursor protein expression.45 Similarly, weight loss after bariatric surgery reverses insulin abnormalities in the brain in severely obese individuals, and such processes may also translate to better cognitive function.46 It is clear that future work involving larger study populations is much needed to fully elucidate the most important postoperative contributors to cognitive improvement, including the possible mediating role of leptin and ghrelin.
The generalizability of the current findings is limited in several ways. First, a healthy control group was not included; future studies employing healthy and patient comparison groups are needed to clarify the cognitive effects of leptin and ghrelin changes in bariatric surgery patients. In addition, we examined serum levels of leptin and ghrelin, and it is possible that assessment of these biomarkers using cerebrospinal fluid (CSF) may be more sensitive to neurocognitive outcomes. Past work shows that a lower CSF leptin is a key contributor to learning and memory processes, possibly offering another explanation for the lack of memory findings in the present study.47 Likewise, appetite hormones represent just one possible contributor to postoperative cognitive changes, and additional work that utilizes objective medical assessments is needed to better clarify other mechanisms that lead to cognitive gains after bariatric surgery, such as resolution of medical conditions (e.g., hypertension, sleep apnea, and type 2 diabetes), improved medication status, and/or psychiatric disorders (e.g., depression). Future studies should also examine changes in neural activity in response to food stimuli between pre- and postbariatric surgery, particularly since this involves changes in leptin and ghrelin levels. In addition, future studies that employ advanced neuroimaging are needed to provide insight into the effects of severe obesity and bariatric surgery on the brain, as well as the pre- and postoperative effects of leptin and ghrelin levels on the structure and function of the brain. For instance, elevations in plasma leptin concentration have been found to predict a larger total brain volume while simultaneously reducing the incident dementia risk.26 Through the use of an extended follow-up (e.g., 10 years) and more comprehensive neuropsychological testing, prospective studies should determine whether continued improvements in leptin and ghrelin levels following bariatric surgery result in reduced risk of cognitive decline and dementia in this high-risk population. Similarly, mild cognitive impairment is common in bariatric surgery patients, and larger longitudinal studies with cognitively diverse bariatric surgery candidates are needed to ascertain the effectiveness of weight-loss surgery in 1) delaying the onset of mild cognitive impairment and 2) preventing conversion from mild cognitive impairment to dementia.
In summary, while there is a growing body of evidence in the literature demonstrating that bariatric surgery is associated with improved cognition, the underlying mechanisms have not been well understood. The results of the present study suggest that improved serum leptin and ghrelin concentrations following bariatric surgery are independent contributors to the observed postoperative cognitive improvements. Future studies that employ advanced neuroimaging and extended follow-ups are needed to elucidate whether bariatric surgery can attenuate adverse brain changes and/or the risk of dementia over time.
Acknowledgements
The data collection was supported by National Institutes of Health grant no. DK075119, and the manuscript was supported in part by National Institutes of Health grant no. HL089311.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT, Jr, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolzenius JD, Laidlaw DH, Cabeen RP, Conturo TE, McMichael AR, Lane EM, et al. Impact of body mass index on neuronal fiber bundle lengths among healthy older adults. Brain Imaging Behav. 2013;7:300–306. doi: 10.1007/s11682-013-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanek KM, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, et al. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology. 2013;27:141–151. doi: 10.1037/a0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lokken KL, Boeka AG, Austin HM, Gunstad J, Harmon CM. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surg Obes Relat Dis. 2009;5:547–552. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Gunstad J, Strain G, Devlin MJ, Wing R, Cohen RA, Paul RH, et al. Improved memory function 12 weeks after bariatric surgery. Surg Obes Relat Dis. 2011;7:465–472. doi: 10.1016/j.soard.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, et al. Improved memory function two years after bariatric surgery. Obesity (Silver Spring) 2014;22:32–38. doi: 10.1002/oby.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller LA, Crosby RD, Galioto R, Strain G, Devlin MJ, Wing R, et al. Bariatric surgery patients exhibit improved memory function 12 months postoperatively. Obes Surg. 2013;23:1527–1535. doi: 10.1007/s11695-013-0970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alosco ML, Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, et al. Cognitive function after bariatric surgery: evidence for improvement 3 years after surgery. Am J Surg. 2014;207:870–876. doi: 10.1016/j.amjsurg.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 10.Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Dos Santos VV, Rodrigues AL, De Lima TC, de Barioglio SR, Raisman-Vozari R, Prediger RD. Ghrelin as a neuroprotective and palliative agent in Alzheimer's and Parkinson's disease. Curr Pharm Des. 2013;19:6773–6790. doi: 10.2174/13816128113199990411. [DOI] [PubMed] [Google Scholar]
- 12.McNay EC. Insulin and ghrelin: peripheral hormones modulating memory and hippocampal function. Curr Opin Pharmacol. 2007;7:628–632. doi: 10.1016/j.coph.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Spitznagel MB, Benitez A, Updegraff J, Potter V, Alexander T, Glickman E, et al. Serum ghrelin is inversely associated with cognitive function in a sample of non-demented elderly. Psychiatry Clin Neurosci. 2010;64:608–611. doi: 10.1111/j.1440-1819.2010.02145.x. [DOI] [PubMed] [Google Scholar]
- 14.Zeki Al, Haan MN, Whitmer RA, Yaffe K, Neuhaus J. Central obesity, leptin and cognitive decline: the Sacramento Area Latino Study on Aging. Dement Geriatr Cogn Disord. 2012;33:400–409. doi: 10.1159/000339957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labad J, Price JF, Strachan MW, Deary IJ, Seckl JR, Sattar N, et al. Serum leptin and cognitive function in people with type 2 diabetes. Neurobiol Aging. 2012;33:2938–2941.e2. doi: 10.1016/j.neurobiolaging.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Bonda DJ, Stone JG, Torres SL, Siedlak SL, Perry G, Kryscio R, et al. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem. 2014;128:162–172. doi: 10.1111/jnc.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terra X, Auguet T, Guiu-Jurado E, Berlanga A, Orellana-Gavaldà JM, Hernàndez M, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23:1790–1798. doi: 10.1007/s11695-013-1033-9. [DOI] [PubMed] [Google Scholar]
- 18.Dimitriadis E, Daskalakis M, Kampa M, Peppe A, Papadakis JA, Melissas J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257:647–654. doi: 10.1097/SLA.0b013e31826e1846. [DOI] [PubMed] [Google Scholar]
- 19.Belle SH, Berk PD, Courcoulas AP, Flum DR, Miles CW, Mitchell JE, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of "integneuro": a new computerized battery of neurocognitive tests. Int J Neurosci. 2005;115:1549–1567. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- 21.Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: "neuromarker". Int J Neurosci. 2005;115:1605–1630. doi: 10.1080/00207450590958475. [DOI] [PubMed] [Google Scholar]
- 22.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 23.Golden CJ. Stroop color and word test : cat. no. 30150M; a manual for clinical and experimental uses. Wood Dale, IL: Stoelting; 1978. [Google Scholar]
- 24.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 25.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K, et al. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Reed DR, Price RA. Leptin resistance is associated with extreme obesity and aggregates in families. Int J Obes Relat Metab Disord. 2001;25:1471–1473. doi: 10.1038/sj.ijo.0801736. [DOI] [PubMed] [Google Scholar]
- 28.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 29.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 30.Arch JR. Central regulation of energy balance: inputs, outputs and leptin resistance. Proc Nutr Soc. 2005;64:39–46. doi: 10.1079/pns2004407. [DOI] [PubMed] [Google Scholar]
- 31.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg GR, McAinch AJ, Chen MB, O'Brien PE, Dixon JB, Cameron-Smith D, et al. The suppressor of cytokine signaling 3 inhibits leptin activation of AMP-kinase in cultured skeletal muscle of obese humans. J Clin Endocrinol Metab. 2006;91:3592–3597. doi: 10.1210/jc.2006-0638. [DOI] [PubMed] [Google Scholar]
- 33.Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Wang PS, Xie D, Liu K, Chen L. Ghrelin reduces injury of hippocampal neurons in a rat model of cerebral ischemia/reperfusion. Chin J Physiol. 2006;49:244–250. [PubMed] [Google Scholar]
- 35.Chung H, Kim E, Lee DH, Seo S, Ju S, Lee D, et al. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology. 2007;148:148–159. doi: 10.1210/en.2006-0991. [DOI] [PubMed] [Google Scholar]
- 36.Miao Y, Xia Q, Hou Z, Zheng Y, Pan H, Zhu S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2007;359:795–800. doi: 10.1016/j.bbrc.2007.05.192. [DOI] [PubMed] [Google Scholar]
- 37.Selim M, Jones R, Novak P, Zhao P, Novak V. The effects of body mass index on cerebral blood flow velocity. Clin Auton Res. 2008;18:331–338. doi: 10.1007/s10286-008-0490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song YM, Sung J, Davey Smith G, Ebrahim S. Body mass index and ischemic and hemorrhagic stroke: a prospective study in Korean men. Stroke. 2004;35:831–836. doi: 10.1161/01.STR.0000119386.22691.1C. [DOI] [PubMed] [Google Scholar]
- 39.Pulkkinen L, Ukkola O, Kolehmainen M, Uusitupa M. Ghrelin in diabetes and metabolic syndrome. Int J Pept. 2010;2010 doi: 10.1155/2010/248948. pii: 248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 41.Rigamonti AE, Pincelli AI, Corrà B, Viarengo R, Bonomo SM, Galimberti D, et al. Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol. 2002;175:R1–R5. doi: 10.1677/joe.0.175r001. [DOI] [PubMed] [Google Scholar]
- 42.Gahete MD, Rubio A, Córdoba-Chacón J, Gracia-Navarro F, Kineman RD, Avila J, et al. Expression of the ghrelin and neurotensin systems is altered in the temporal lobe of Alzheimer's disease patients. J Alzheimers Dis. 2010;22:819–828. doi: 10.3233/JAD-2010-100873. [DOI] [PubMed] [Google Scholar]
- 43.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martins I, Gomes S, Costa RO, Otvos L, Oliveira CR, Resende R, et al. Leptin and ghrelin prevent hippocampal dysfunction induced by Aβ oligomers. Neuroscience. 2013;241:41–51. doi: 10.1016/j.neuroscience.2013.02.062. [DOI] [PubMed] [Google Scholar]
- 45.Ghanim H, Monte SV, Sia CL, Abuaysheh S, Green K, Caruana JA, et al. Reduction in inflammation and the expression of amyloid precursor protein and other proteins related to Alzheimer's disease following gastric bypass surgery. J Clin Endocrinol Metab. 2012;97:E1197–E1201. doi: 10.1210/jc.2011-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuulari JJ, Karlsson HK, Hirvonen J, Hannukainen JC, Bucci M, Helmiö M, et al. Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese. Diabetes. 2013;62:2747–2751. doi: 10.2337/db12-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang JS, Letendre S, Marquie-Beck J, Cherner M, McCutchan JA, Grant I, et al. Low CSF leptin levels are associated with worse learning and memory performance in HIV-infected men. J Neuroimmune Pharmacol. 2007;2:352–358. doi: 10.1007/s11481-007-9093-z. [DOI] [PubMed] [Google Scholar]


