Abstract
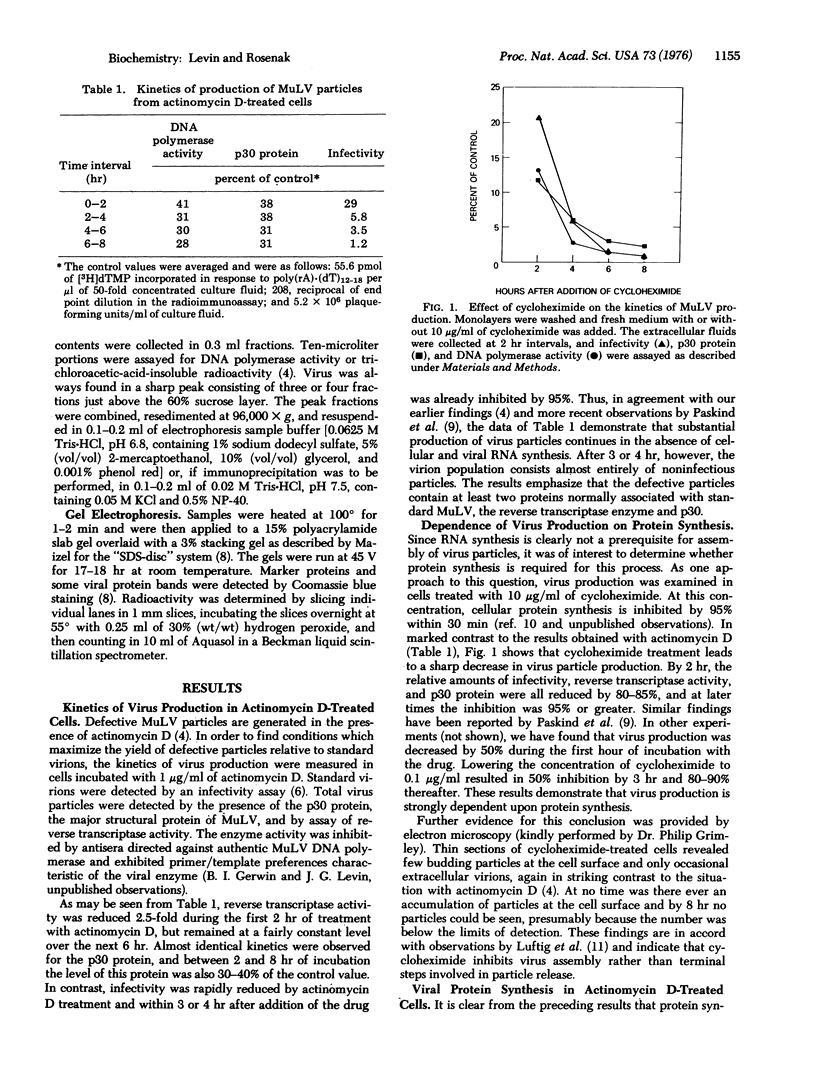
Murine leukemia virus particles assembled in actinomycin D-treated cells were detected by determination of reverse transcriptase [RNA-dependent DNA polymerase (nucleotidyltransferase)] activity and by radioimmunoassay of the major virion protein, p30. The levels of enzyme activity and p30 protein were both 30-40% relative to the control over an 8 hr period, whereas after 3 or 4 hr infectivity was reduced by 95%. Thus, virions produced in the absence of RNA synthesis represent a fairly homogeneous population of defective particles. Although RNA synthesis is not necessary for virus assembly, protein synthesis is required. Treatment of cells with 10 mug/ml of cycloheximide reduced virus production by 80-85% within 2 hr, and by greater than 95% at later times. As might be expected from this finding, viral protein synthesis accompanies virus assembly in actinomycin D-treated cells. Newly synthesized proteins associated with the defective particles were identical with those found in standard virions and were present in the correct proportions. The results demonstrate that viral mRNA persists in cells in which RNA synthesis is blocked and continues to direct viral protein synthesis with a functional half-life of approximately 6-8 hr. Since viral mRNA is not packaged in virions even when viral RNA synthesis is shut off [Levin et al. (1974) J. Virol. 14, 152-161], we propose that murine leukemia virus-infected cells contain two nonequilibrating pools of intracellular viral RNA molecules, one associated with polyribosomes and one which is encapsidated into extracellular particles.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcement L. J., Karshin W. L., Naso R. B., Jamjoom G., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins: presence of p30 and envelope p15 sequences in precursor polypeptides. Virology. 1976 Feb;69(2):763–774. doi: 10.1016/0042-6822(76)90504-3. [DOI] [PubMed] [Google Scholar]
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Metabolic requirements for infection by Rous sarcoma virus. I. The transient requirement for DNA synthesis. Virology. 1966 Jul;29(3):444–451. doi: 10.1016/0042-6822(66)90220-0. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Synthesis of the RNA of RNA-containing tumor viruses. I. The interval between synthesis and envelopment. Virology. 1970 Mar;40(3):494–504. doi: 10.1016/0042-6822(70)90192-3. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., Sheinin R. Selective measurement of the synthesis and metabolic stability of messenger RNA in 3T3 mouse cells. Biochim Biophys Acta. 1970 Apr 15;204(2):449–461. doi: 10.1016/0005-2787(70)90165-6. [DOI] [PubMed] [Google Scholar]
- Craig N., Kelley D. E., Perry R. P. Lifetime of the messenger RNA's which code for ribosomal proteins in L-cells. Biochim Biophys Acta. 1971 Sep 24;246(3):493–498. doi: 10.1016/0005-2787(71)90786-6. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fischinger P. F., Tuttle-Fuller N., Hüper G., Bolognesi D. P. Mitosis is required for production of murine leukemia virus and structural proteins during de novo infection. J Virol. 1975 Aug;16(2):267–274. doi: 10.1128/jvi.16.2.267-274.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner E., Ikeda H., Tung J. S., Vitetta E. S., Tress E., Hardly W., Jr, Stockert E., Boyse E. A., Pincus T., O'Donnell P. Characterization of murine leukemia virus-specific proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1057–1066. doi: 10.1101/sqb.1974.039.01.121. [DOI] [PubMed] [Google Scholar]
- Gielkens A. L., Salden M. H., Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. S., Penman S. Regulation of protein synthesis in mammalian cells. V. Further studies on the effect of actinomycin D on translation control in HeLa cells. J Mol Biol. 1973 Oct 25;80(2):243–254. doi: 10.1016/0022-2836(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Jr, Hanna M. G., Schafer W., Hunsmann G., Bolognesi D. P., HUPER G. Polypeptides of mammalian oncornaviruses. III. Localization of p 15 and reactivity with natural antibody. Virology. 1975 Jan;63(1):60–67. doi: 10.1016/0042-6822(75)90370-0. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Hardy W., Jr, Tress E., Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. V. Identification of a new murine viral protein, p15(E). J Virol. 1975 Jul;16(1):53–61. doi: 10.1128/jvi.16.1.53-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom G., Karshin W. L., Naso R. B., Arcement L. J., Arlinghaus R. B. Proteins of Rauscher murine leukemia virus: resolution of a 70,000-dalton, Nonglycosylated polypeptide containing p30 peptide sequences. Virology. 1975 Nov;68(1):135–145. doi: 10.1016/0042-6822(75)90155-5. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Grimley P. M., Ramseur J. M., Berezesky I. K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974 Jul;14(1):152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Arlinghaus R. B. Biosynthesis of Rauscher leukemia viral proteins. Cell. 1975 Jan;4(1):31–36. doi: 10.1016/0092-8674(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Paskind M. P., Weinberg R. A., Baltimore D. Dependence of Moloney murine leukemia virus production on cell growth. Virology. 1975 Sep;67(1):242–248. doi: 10.1016/0042-6822(75)90421-3. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Gielen J., Packman S., Ikawa Y., Leder P. Globin gene expression in cultured erythroleukemic cells. J Mol Biol. 1974 Aug 25;87(4):697–714. doi: 10.1016/0022-2836(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- STAEHELIN T., WETTSTEIN F. O., NOLL H. Breakdown of rat-liver ergosomes in vivo after actinomycin inhibition of messenger RNA synthesis. Science. 1963 Apr 12;140(3563):180–183. doi: 10.1126/science.140.3563.180. [DOI] [PubMed] [Google Scholar]
- Schafer W., Hunsmann G., Moennig V., Noranha F., Bolognesi D. P., Green R. W., Hüper G. Polypeptides of mammalian oncornaviruses. II Characterization of murine leukemia virus polypeptide (p 15) bearing interspecies reactivity. Virology. 1975 Jan;63(1):48–59. doi: 10.1016/0042-6822(75)90369-4. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Livingston D. M. Radioimmunoassay of mammalian type C viral proteins. I. Species specific reactions of murine and feline viruses. J Immunol. 1972 Sep;109(3):570–577. [PubMed] [Google Scholar]
- Shanmugam G., Bhaduri S., Green M. The virus-specific RNA species in free and membrane-bound polyribosomes of transformed cells replicating murine sarcoma-leukemia viruses. Biochem Biophys Res Commun. 1974 Feb 4;56(3):697–702. doi: 10.1016/0006-291x(74)90661-5. [DOI] [PubMed] [Google Scholar]
- Shanmugam G., Vecchio G., Attardi D., Green M. Immunological studies on viral polypeptide synthesis in cells replicating murine sarcoma-leukemia virus. J Virol. 1972 Sep;10(3):447–455. doi: 10.1128/jvi.10.3.447-455.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L. Polypeptides of Moloney sarcoma-leukemia virions: their resolution and incorporation into extracellular virions. Virology. 1974 Oct;61(2):575–587. doi: 10.1016/0042-6822(74)90291-8. [DOI] [PubMed] [Google Scholar]
- Zaanie D., Gielkens A. L., Dekker-michielsen M. J., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus. Virology. 1975 Oct;67(2):544–552. doi: 10.1016/0042-6822(75)90454-7. [DOI] [PubMed] [Google Scholar]


