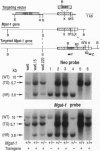
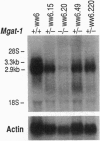
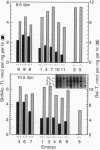
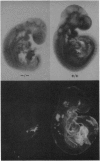
Abstract
Eukaryotic cells require N-linked carbohydrates for survival. However, the biosynthetic intermediate Man5GlcNAc2Asn, in place of mature N-linked structures, allows glycoprotein synthesis and somatic cell growth to proceed normally. To determine whether the same would be true in a complex biological situation, the gene Mgat-1 was disrupted by homologous recombination in embryonic stem cells and transmitted to the germ line. The Mgat-1 gene encodes N-acetylglucosaminyltransferase I [GlcNAc-TI; alpha-1,3-mannosyl-glycoprotein beta-1,2-N-acetylglucosaminyltransferase; UDP-N-acetyl-D-glucosamine:glycoprotein (N-acetyl-D-glucosamine to alpha-D-mannosyl-1,3-(R1)-beta-D-mannosyl-R2) beta-1,2-N-acetyl-D-glucosaminyltransferase, EC 2.4.1.101], the transferase that initiates synthesis of hybrid and complex N-linked carbohydrates from Man5GlcNAc2Asn. Mice lacking GlcNAc-TI activity did not survive to term. Biochemical and morphological analyses of embryos from 8.5 to 13.5 days of gestation showed that Mgat-1-/-embryos are developmentally retarded, most noticeably in neural tissue, and die between 9.5 and 10.5 days of development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adra C. N., Boer P. H., McBurney M. W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60(1):65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney W., Stanley P. Lec1A Chinese hamster ovary cell mutants appear to arise from a structural alteration in N-acetylglucosaminyltransferase I. J Biol Chem. 1986 Aug 15;261(23):10551–10557. [PubMed] [Google Scholar]
- Chaney W., Sundaram S., Friedman N., Stanley P. The Lec4A CHO glycosylation mutant arises from miscompartmentalization of a Golgi glycosyltransferase. J Cell Biol. 1989 Nov;109(5):2089–2096. doi: 10.1083/jcb.109.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron J., Malynn B. A., Fisher P., Stewart V., Jeannotte L., Goff S. P., Robertson E. J., Alt F. W. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992 Dec;6(12A):2248–2257. doi: 10.1101/gad.6.12a.2248. [DOI] [PubMed] [Google Scholar]
- Chen J., Lansford R., Stewart V., Young F., Alt F. W. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Davis A. C., Wims M., Spotts G. D., Hann S. R., Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993 Apr;7(4):671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Dell A., Scartezzini P. Primary defect of congenital dyserythropoietic anemia type II. Failure in glycosylation of erythrocyte lactosaminoglycan proteins caused by lowered N-acetylglucosaminyltransferase II. J Biol Chem. 1987 May 25;262(15):7195–7206. [PubMed] [Google Scholar]
- Fukuda M. N., Masri K. A., Dell A., Luzzatto L., Moremen K. W. Incomplete synthesis of N-glycans in congenital dyserythropoietic anemia type II caused by a defect in the gene encoding alpha-mannosidase II. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7443–7447. doi: 10.1073/pnas.87.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Spooncer E., Oates J. E., Dell A., Klock J. C. Structure of sialylated fucosyl lactosaminoglycan isolated from human granulocytes. J Biol Chem. 1984 Sep 10;259(17):10925–10935. [PubMed] [Google Scholar]
- Fukushi Y., Nudelman E., Levery S. B., Hakomori S., Rauvala H. Novel fucolipids accumulating in human adenocarcinoma. III. A hybridoma antibody (FH6) defining a human cancer-associated difucoganglioside (VI3NeuAcV3III3Fuc2nLc6). J Biol Chem. 1984 Aug 25;259(16):10511–10517. [PubMed] [Google Scholar]
- Gleeson P. A., Feeney J., Hughes R. C. Structures of N-glycans of a ricin-resistant mutant of baby hamster kidney cells. Synthesis of high-mannose and hybrid N-glycans. Biochemistry. 1985 Jan 15;24(2):493–503. doi: 10.1021/bi00323a037. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Hammarström S., Hammarström M. L., Sundblad G., Arnarp J., Lönngren J. Mitogenic leukoagglutinin from Phaseolus vulgaris binds to a pentasaccharide unit in N-acetyllactosamine-type glycoprotein glycans. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1611–1615. doi: 10.1073/pnas.79.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C., Feeney J. Ricin-resistant mutants of baby-hamster-kidney cells deficient in alpha-mannosidase-II-catalyzed processing of asparagine-linked oligosaccharides. Eur J Biochem. 1986 Jul 15;158(2):227–237. doi: 10.1111/j.1432-1033.1986.tb09742.x. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Nozaki M. Effects of tunicamycin on preimplantation mouse embryos: prevention of molecular differentiation during blastocyst formation. Dev Biol. 1985 Nov;112(1):135–144. doi: 10.1016/0012-1606(85)90127-7. [DOI] [PubMed] [Google Scholar]
- Kimber S. J. Glycoconjugates and cell surface interactions in pre- and peri-implantation mammalian embryonic development. Int Rev Cytol. 1990;120:53–167. doi: 10.1016/s0074-7696(08)61599-5. [DOI] [PubMed] [Google Scholar]
- Kojima N., Handa K., Newman W., Hakomori S. Inhibition of selectin-dependent tumor cell adhesion to endothelial cells and platelets by blocking O-glycosylation of these cells. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1288–1295. doi: 10.1016/0006-291x(92)91872-n. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kumar R., Stanley P. Transfection of a human gene that corrects the Lec1 glycosylation defect: evidence for transfer of the structural gene for N-acetylglucosaminyltransferase I. Mol Cell Biol. 1989 Dec;9(12):5713–5717. doi: 10.1128/mcb.9.12.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Yang J., Eddy R. L., Byers M. G., Shows T. B., Stanley P. Cloning and expression of the murine gene and chromosomal location of the human gene encoding N-acetylglucosaminyltransferase I. Glycobiology. 1992 Aug;2(4):383–393. doi: 10.1093/glycob/2.4.383. [DOI] [PubMed] [Google Scholar]
- Larsen G. R., Sako D., Ahern T. J., Shaffer M., Erban J., Sajer S. A., Gibson R. M., Wagner D. D., Furie B. C., Furie B. P-selectin and E-selectin. Distinct but overlapping leukocyte ligand specificities. J Biol Chem. 1992 Jun 5;267(16):11104–11110. [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Dowbenko D., Imai Y., Henzel W. J., Grimley C., Fennie C., Gillett N., Watson S. R., Rosen S. D. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992 Jun 12;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Lo C. W. Localization of low abundance DNA sequences in tissue sections by in situ hybridization. J Cell Sci. 1986 Mar;81:143–162. doi: 10.1242/jcs.81.1.143. [DOI] [PubMed] [Google Scholar]
- Maemura K., Fukuda M. Poly-N-acetyllactosaminyl O-glycans attached to leukosialin. The presence of sialyl Le(x) structures in O-glycans. J Biol Chem. 1992 Dec 5;267(34):24379–24386. [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Stults N. L., Diaz S., Smith D. F., Cummings R. D., Varki A., McEver R. P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992 Jul;118(2):445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen R. M., Conner D. A., Chao S., Geisterfer-Lowrance A. A., Seidman J. G. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992 May;12(5):2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Pownall S., Kozak C. A., Schappert K., Sarkar M., Hull E., Schachter H., Marth J. D. Molecular cloning and characterization of the mouse UDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I gene. Genomics. 1992 Apr;12(4):699–704. doi: 10.1016/0888-7543(92)90297-6. [DOI] [PubMed] [Google Scholar]
- Rudnicki M. A., Braun T., Hinuma S., Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992 Oct 30;71(3):383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Rudnicki M. A., Ruben M., McBurney M. W. Regulated expression of a transfected human cardiac actin gene during differentiation of multipotential murine embryonal carcinoma cells. Mol Cell Biol. 1988 Jan;8(1):406–417. doi: 10.1128/mcb.8.1.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Caillibot V., Siminovitch L. Stable alterations at the cell membrane of Chinese hamster ovary cells resistant to the cytotoxicity of phytohemagglutinin. Somatic Cell Genet. 1975 Jan;1(1):3–26. doi: 10.1007/BF01538729. [DOI] [PubMed] [Google Scholar]
- Stanley P. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol Cell Biol. 1989 Feb;9(2):377–383. doi: 10.1128/mcb.9.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Narasimhan S., Siminovitch L., Schachter H. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine--glycoprotein N-acetylglucosaminyltransferase activity. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3323–3327. doi: 10.1073/pnas.72.9.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surani M. A. Glycoprotein synthesis and inhibition of glycosylation by tunicamycin in preimplantation mouse embryos: compaction and trophoblast adhesion. Cell. 1979 Sep;18(1):217–227. doi: 10.1016/0092-8674(79)90370-2. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Polsky F., Edgell M. H., Seidman J. G., Leder A., Enquist L. W., Norman B., Leder P. Cloning specific segments of the mammalian genome: bacteriophage lambda containing mouse globin and surrounding gene sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4406–4410. doi: 10.1073/pnas.74.10.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]