Abstract
Purpose
Medicare beneficiaries with cancer bear a greater portion of their health care costs, because cancer treatment costs have increased. Beneficiaries have supplemental insurance to reduce out-of-pocket costs; those without supplemental insurance may face barriers to care. This study examines the association between type of supplemental insurance coverage and receipt of chemotherapy among Medicare patients with cancer who, per National Comprehensive Cancer Network treatment guidelines, should generally receive chemotherapy.
Patients and Methods
This retrospective, observational study included 1,200 Medicare patients diagnosed with incident cancer of the breast (stage IIB to III), colon (stage III), rectum (stage II to III), lung (stage II to IV), or ovary (stage II to IV) from 2000 to 2005. Using the National Cancer Institute Patterns of Care Studies and linked SEER-Medicare data, we determined each Medicare patient's supplemental insurance status (private insurance, dual eligible [ie, Medicare with Medicaid], or no supplemental insurance), consultation with an oncologist, and receipt of chemotherapy. Using adjusted logistic regression, we evaluated the association of type of supplemental insurance with oncologist consultation and receipt of chemotherapy.
Results
Dual-eligible patients were significantly less likely to receive chemotherapy than were Medicare patients with private insurance. Patients with Medicare only who saw an oncologist had comparable rates of chemotherapy compared with Medicare patients with private insurance.
Conclusion
Dual-eligible Medicare beneficiaries received recommended cancer chemotherapy less frequently than other Medicare beneficiaries. With the increasing number of Medicaid patients under the Affordable Care Act, there will be a need for patient navigators and sufficient physician reimbursement so that low-income patients with cancer will have access to oncologists and needed treatment.
INTRODUCTION
The Medicare program provides insurance coverage to elderly Americans, reducing patient-borne expenses associated with health care. However, rising health care costs and the transition of health care from inpatient to outpatient settings have increased Medicare coinsurance payments and deductibles for beneficiaries.1 For patients with cancer, medical costs have risen over the past decade, driven in part by ongoing development of expensive chemotherapy drugs.2 In a recent report, cumulative 2-year out-of-pocket spending per Medicare beneficiaries with cancer averaged $4,727 compared with $3,209 for those without cancer.3 This cost sharing from Medicare beneficiaries supports the financial stability of the Medicare program but places a substantial burden on elderly patients.
To reduce the impact of out-of-pocket costs, most Medicare beneficiaries have supplemental health insurance. Fee-for-service beneficiaries can obtain supplemental insurance from the purchase of a private (ie, Medigap) policy or from employer-sponsored plans for their retirees. Low-income Medicare beneficiaries may not be able to afford supplemental policies. For some low-income beneficiaries, financial assistance from Medicaid can offset these costs. Approximately 20% of Medicare beneficiaries are dually eligible for Medicare and Medicaid, with Medicaid serving as the supplemental coverage.4 Dual-eligible beneficiaries may encounter challenges in accessing care. Prior studies have demonstrated that Medicaid recipients of all ages are less likely to obtain recommended care compared with those who are privately insured.5–7 Medicare patients who do not have Medicaid or supplemental private insurance face significant out-of-pocket expenses. Medicare patients with no supplemental insurance have been reported to receive less cancer treatment.8
In this study, we examined the relationship between supplemental insurance coverage and the receipt of chemotherapy among Medicare beneficiaries. The study included patients with selected cancers who, based on their cancer site and stage, should generally receive chemotherapy per National Comprehensive Cancer Network (NCCN) treatment guidelines.9–13 We sought to determine whether the type of supplemental insurance was related to disparities in the receipt of chemotherapy. We hypothesized that Medicare patients with no supplemental insurance or those dually eligible for Medicaid may receive less recommended chemotherapy than those with private supplemental insurance.
PATIENTS AND METHODS
Data Sources
The National Cancer Institute (NCI) SEER cancer registries collect information on all incident cancers occurring in 17 defined geographic regions and are generally representative of the US population. The population-based SEER data, collected primarily from hospital records, include date of diagnosis, tumor site and stage, initial treatment, and selected demographic characteristics for each patient. Chemotherapy is under-reported in SEER data, because most systemic therapies are provided in the outpatient setting. To obtain information on chemotherapy use, NCI annually conducts Patterns of Care (POC) studies on a subset of SEER patients with selected cancer sites. The specific cancers vary by year. POC studies collect information from the medical records of patients with cancer about treatment, comorbidities, and type of health insurance.14 Each SEER registry obtains institutional review board approval as required before initiating the study.
NCI also sponsors the SEER-Medicare data, a linkage of patients in the SEER data with their Medicare enrollment and claims files. Of patients in SEER registries age ≥ 65 years, 94% have been linked to the Medicare master enrollment file.15 Medicare enrollment data include monthly indicators about health maintenance organization enrollment or whether the beneficiary receives state buy-in (SBI) assistance, a proxy for Medicaid enrollment. For beneficiaries with fee-for-service coverage, a Medicare claim includes a variable indicating if Medicare is the secondary payer to a primary insurer for that claim. Medicare physician and outpatient claims include procedure codes that describe the services billed on each claim, each physician's unique provider identification number (UPIN), and the physician's specialty. The NCI POC data and SEER-Medicare data include a unique SEER case number for each patient that was used to match persons in the POC data to the SEER-Medicare data.
Study Population
The POC study population included a subset of patients reported in the SEER data with an incident diagnosis. Cancer sites varied, because the POC studies include different cancer sites each year. Our analysis included patients with stage IIB to III breast, stage III colon, and stage II to III rectal cancers in 2000 and 2005; stage II to IV non–small-cell lung cancer in 2005; and stage II to IV ovarian cancer in 2002. We included the most recent years of data available for the specific sites.
Patients with a prior cancer diagnosis, a simultaneous second cancer diagnosis, or cancer reported only by autopsy or death certificate were ineligible for the POC study. Eligible patients were stratified by registry, sex, and racial/ethnic group and then randomly sampled within strata. Sampling weights varied based on the sex and race/ethnicity of the patient. Sampling fractions were used to calculate weighted percentages, reflecting the SEER populations from which the data were obtained. Women, non-Hispanic blacks, Hispanics, Asians/Pacific Islanders, American Indians, and Native Alaskans were oversampled to obtain more stable estimates. From the POC data, we identified persons age ≥ 65 years who also appeared in the SEER-Medicare data. To ensure complete data from the Medicare files, patients were required to have had continuous Medicare Part A (hospital insurance) and Part B (medical insurance) and fee-for-service coverage in the 6 months after diagnosis. Patients were not required to have undergone surgery for their cancer, because surgery was not indicated for all of the cancers.
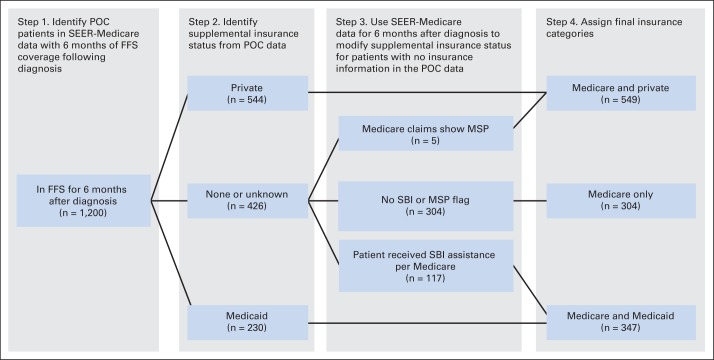
Medicare Supplemental Insurance Definitions
Supplemental insurance status was determined from the POC and SEER-Medicare data, as shown in Figure 1. We classified each patient's insurance information into one of three categories: Medicare with private insurance, Medicare only, or Medicare and Medicaid, also known as dual-eligible patients. From the POC data, private insurance was defined as any insurance provided by a private insurance company, government insurance other than Medicare or Medicaid (eg, Tricare, Veterans Affairs, other military), Blue Cross/Blue Shield, or other similar insurance types. Medicaid eligibility was assigned if the patient was identified as having Medicaid. The Medicare-only designation included patients who were reported as having no insurance other than Medicare.
Fig 1.
Steps to determine if Medicare beneficiaries had private insurance or Medicaid. FFS, fee for service; MSP, Medicare is secondary payer to private insurance; POC, Patterns of Care; SBI, state buy-in.
For patients who had no supplement insurance identified in the POC data, we used the SEER-Medicare data to search for additional information about insurance. Medicare claims include a variable—primary payer amount—noting if there is a primary payer other than Medicare. This occurs when Medicare beneficiaries or their spouses are still working and are covered through an employer's health insurance plan. Patients were considered to have a private insurer in addition to Medicare if the primary payer amount variable on any Medicare claims during the 6 months after the date of diagnosis included any value > $0. We used the SBI variable from the Medicare data. Medicare buy-in benefits, operated by state Medicaid programs, help low-income Medicare beneficiaries pay their Medicare premiums, deductibles, and copayments. If the SBI variable was flagged during the calendar year of diagnosis, the patient was assigned as having Medicaid in addition to having Medicare.
Assessment of Receipt of Chemotherapy and Consultation With Oncologist
Information about whether a patient had received chemotherapy was collected in the POC study from a patient's treating physician. Chemotherapy receipt was limited to initial treatment, generally regarded as treatment planned or administered before progression or disease recurrence.
Our focus was on the receipt of chemotherapy. However, oncologists are key decision makers about chemotherapy administration. Therefore, we evaluated whether consultation with an oncologist varied by supplemental insurance status. Oncologists were identified by matching UPINs for the physicians from the SEER-Medicare claims to American Medical Association (AMA) data on physician specialty. If the patient had a claim from a physician whose primary or secondary specialty was hematology/oncology or medical oncology in the AMA data, the patient was classified as having had an oncology consultation. If the physician's UPIN was not matched to the AMA data, the physician specialty on each patient's Medicare claim was used to determine if the patient had seen an oncologist.
Statistical Analysis
Multivariable logistic regression models were used to assess the association between receipt of oncologist consultation and chemotherapy by supplemental insurance type. Two models were used with binary dependent variables (yes v no) for oncologist consultation and receipt of chemotherapy, respectively. Oncologist consultation was hypothesized to modify the effect of insurance type on receipt of chemotherapy, and this was tested with an interaction term in the second model and included in the second model along with the main effects. The results of the logistic regression analyses were presented as standardized percentages (predictive margins), representing the average percent of patients consulting with an oncologist or receiving chemotherapy.16 Cancer sites were combined to increase sample size. To control for differences in patients in each of the three insurance categories, the standardized percentages and SEs were adjusted for supplemental insurance type, cancer site and stage, race, age, marital status, Charlson comorbidity score, and whether the patient was in a nursing home during the 3 months before the cancer diagnosis, using an established algorithm based on procedure codes for nursing home visits reported in the physician claims.17 Information about income and educational level for the US Census tract where the patient lived was assessed in earlier models but not included in the final model, because the variables were not significantly associated with oncologist consultation or receipt of chemotherapy. We used SUDAAN statistical software (Research Triangle Institute, Research Triangle Park, NC) to account for the sampling design in all analyses.
RESULTS
Of the 1,200 patients in our study, 45.8% had Medicare with private insurance, 25.3% had Medicare only, and 28.9% had Medicare and Medicaid (Table 1). Supplemental insurance status varied by age, race, and marital status. The oldest beneficiaries—those age ≥ 85 years—accounted for 13.7% of patients with Medicare only, although they accounted for 10.6% of all patients with cancer. Non-Hispanic black patients composed 22.2% of dual-eligible patients, although they accounted for 9.1% of all patients. Dual-eligible patients had more physician nursing home visits (7.5%) than did Medicare-only patients (3.3%) or those with Medicare and private insurance (1.5%).
Table 1.
Patient Characteristics by Type of Supplement Insurance Among Medicare Beneficiaries With Cancer in Selected Years
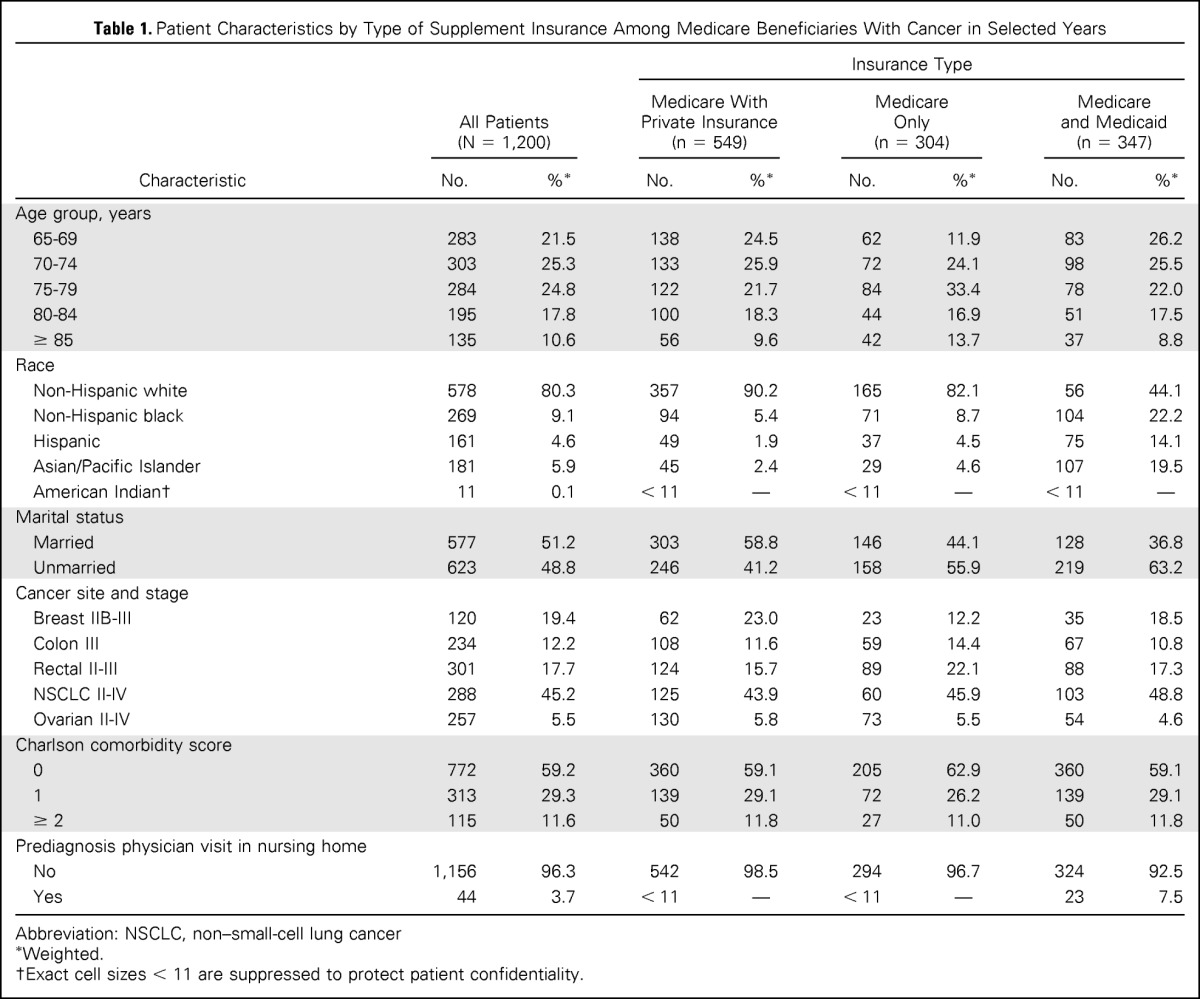
| Characteristic | All Patients (N = 1,200) |
Insurance Type |
||||||
|---|---|---|---|---|---|---|---|---|
| Medicare With Private Insurance (n = 549) |
Medicare Only (n = 304) |
Medicare and Medicaid (n = 347) |
||||||
| No. | %* | No. | %* | No. | %* | No. | %* | |
| Age group, years | ||||||||
| 65-69 | 283 | 21.5 | 138 | 24.5 | 62 | 11.9 | 83 | 26.2 |
| 70-74 | 303 | 25.3 | 133 | 25.9 | 72 | 24.1 | 98 | 25.5 |
| 75-79 | 284 | 24.8 | 122 | 21.7 | 84 | 33.4 | 78 | 22.0 |
| 80-84 | 195 | 17.8 | 100 | 18.3 | 44 | 16.9 | 51 | 17.5 |
| ≥ 85 | 135 | 10.6 | 56 | 9.6 | 42 | 13.7 | 37 | 8.8 |
| Race | ||||||||
| Non-Hispanic white | 578 | 80.3 | 357 | 90.2 | 165 | 82.1 | 56 | 44.1 |
| Non-Hispanic black | 269 | 9.1 | 94 | 5.4 | 71 | 8.7 | 104 | 22.2 |
| Hispanic | 161 | 4.6 | 49 | 1.9 | 37 | 4.5 | 75 | 14.1 |
| Asian/Pacific Islander | 181 | 5.9 | 45 | 2.4 | 29 | 4.6 | 107 | 19.5 |
| American Indian† | 11 | 0.1 | < 11 | — | < 11 | — | < 11 | — |
| Marital status | ||||||||
| Married | 577 | 51.2 | 303 | 58.8 | 146 | 44.1 | 128 | 36.8 |
| Unmarried | 623 | 48.8 | 246 | 41.2 | 158 | 55.9 | 219 | 63.2 |
| Cancer site and stage | ||||||||
| Breast IIB-III | 120 | 19.4 | 62 | 23.0 | 23 | 12.2 | 35 | 18.5 |
| Colon III | 234 | 12.2 | 108 | 11.6 | 59 | 14.4 | 67 | 10.8 |
| Rectal II-III | 301 | 17.7 | 124 | 15.7 | 89 | 22.1 | 88 | 17.3 |
| NSCLC II-IV | 288 | 45.2 | 125 | 43.9 | 60 | 45.9 | 103 | 48.8 |
| Ovarian II-IV | 257 | 5.5 | 130 | 5.8 | 73 | 5.5 | 54 | 4.6 |
| Charlson comorbidity score | ||||||||
| 0 | 772 | 59.2 | 360 | 59.1 | 205 | 62.9 | 360 | 59.1 |
| 1 | 313 | 29.3 | 139 | 29.1 | 72 | 26.2 | 139 | 29.1 |
| ≥ 2 | 115 | 11.6 | 50 | 11.8 | 27 | 11.0 | 50 | 11.8 |
| Prediagnosis physician visit in nursing home | ||||||||
| No | 1,156 | 96.3 | 542 | 98.5 | 294 | 96.7 | 324 | 92.5 |
| Yes | 44 | 3.7 | < 11 | — | < 11 | — | 23 | 7.5 |
Abbreviation: NSCLC, non–small-cell lung cancer
Weighted.
Exact cell sizes < 11 are suppressed to protect patient confidentiality.
All patients in the sample had a cancer site and stage where chemotherapy would generally be indicated per NCCN guidelines. Only 52.5% of all patients received chemotherapy (Table 2). Although > 80% of the sample had a consultation with an oncologist, < 60% of those who saw an oncologist received chemotherapy. Chemotherapy use varied by cancer site; patients with rectal cancer having the greatest use of chemotherapy (63.6%), whereas those with lung cancer had the lowest use of chemotherapy (48.7%).
Table 2.
Patient Comorbidity, Consultation With Oncologist, and Receipt of Chemotherapy by Cancer Type
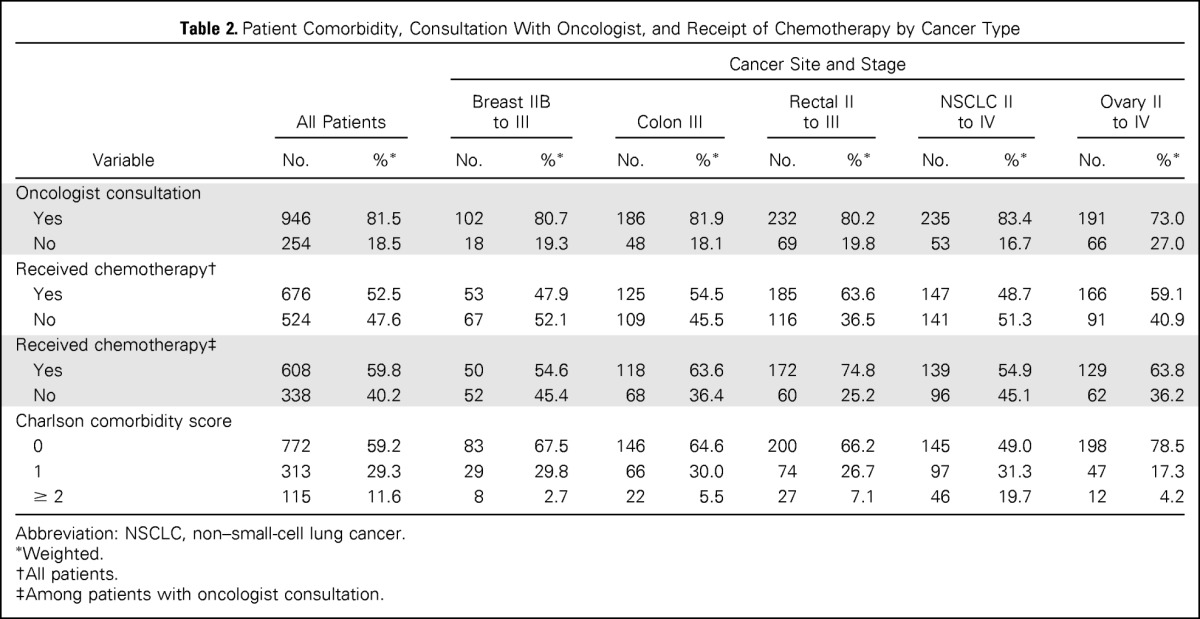
| Variable | All Patients |
Cancer Site and Stage |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast IIB to III |
Colon III |
Rectal II to III |
NSCLC II to IV |
Ovary II to IV |
||||||||
| No. | %* | No. | %* | No. | %* | No. | %* | No. | %* | No. | %* | |
| Oncologist consultation | ||||||||||||
| Yes | 946 | 81.5 | 102 | 80.7 | 186 | 81.9 | 232 | 80.2 | 235 | 83.4 | 191 | 73.0 |
| No | 254 | 18.5 | 18 | 19.3 | 48 | 18.1 | 69 | 19.8 | 53 | 16.7 | 66 | 27.0 |
| Received chemotherapy† | ||||||||||||
| Yes | 676 | 52.5 | 53 | 47.9 | 125 | 54.5 | 185 | 63.6 | 147 | 48.7 | 166 | 59.1 |
| No | 524 | 47.6 | 67 | 52.1 | 109 | 45.5 | 116 | 36.5 | 141 | 51.3 | 91 | 40.9 |
| Received chemotherapy‡ | ||||||||||||
| Yes | 608 | 59.8 | 50 | 54.6 | 118 | 63.6 | 172 | 74.8 | 139 | 54.9 | 129 | 63.8 |
| No | 338 | 40.2 | 52 | 45.4 | 68 | 36.4 | 60 | 25.2 | 96 | 45.1 | 62 | 36.2 |
| Charlson comorbidity score | ||||||||||||
| 0 | 772 | 59.2 | 83 | 67.5 | 146 | 64.6 | 200 | 66.2 | 145 | 49.0 | 198 | 78.5 |
| 1 | 313 | 29.3 | 29 | 29.8 | 66 | 30.0 | 74 | 26.7 | 97 | 31.3 | 47 | 17.3 |
| ≥ 2 | 115 | 11.6 | 8 | 2.7 | 22 | 5.5 | 27 | 7.1 | 46 | 19.7 | 12 | 4.2 |
Abbreviation: NSCLC, non–small-cell lung cancer.
Weighted.
All patients.
Among patients with oncologist consultation.
The standardized percentage of patients who saw an oncologist or received chemotherapy varied by type of supplemental insurance. A lower percentage of dual-eligible Medicare and Medicaid patients saw oncologists than did patients who had Medicare with private insurance (78.4% and 83.6%, respectively; Table 3). However, patients of any insurance type who saw an oncologist received chemotherapy more often compared with those with no oncology consultation, although this difference was not statistically significant (interaction P = .55). Dual-eligible patients received chemotherapy significantly less often than did patients who had Medicare with private insurance, regardless of whether they had an oncologist consultation. Only 44.2% of dual-eligible patients who had an oncology consultation received chemotherapy, compared with 60.8% of those with Medicare and private insurance. Patient age was also a significant factor for receipt of chemotherapy, with patients age ≥ 75 years significantly less likely to receive chemotherapy than younger patients. Receipt of chemotherapy varied significantly by cancer site and stage, with patients with stage II to IV lung cancer or stage IIB to III breast cancer significantly less likely to receive chemotherapy than those with colon, rectal, or ovarian cancer. Patients with a prediagnosis nursing home claim were significantly less likely (15.9%) to receive chemotherapy than were patients with no nursing home claims (53.0%).
Table 3.
Standardized Percentages of Patients Receiving Oncologist Consultation or Chemotherapy
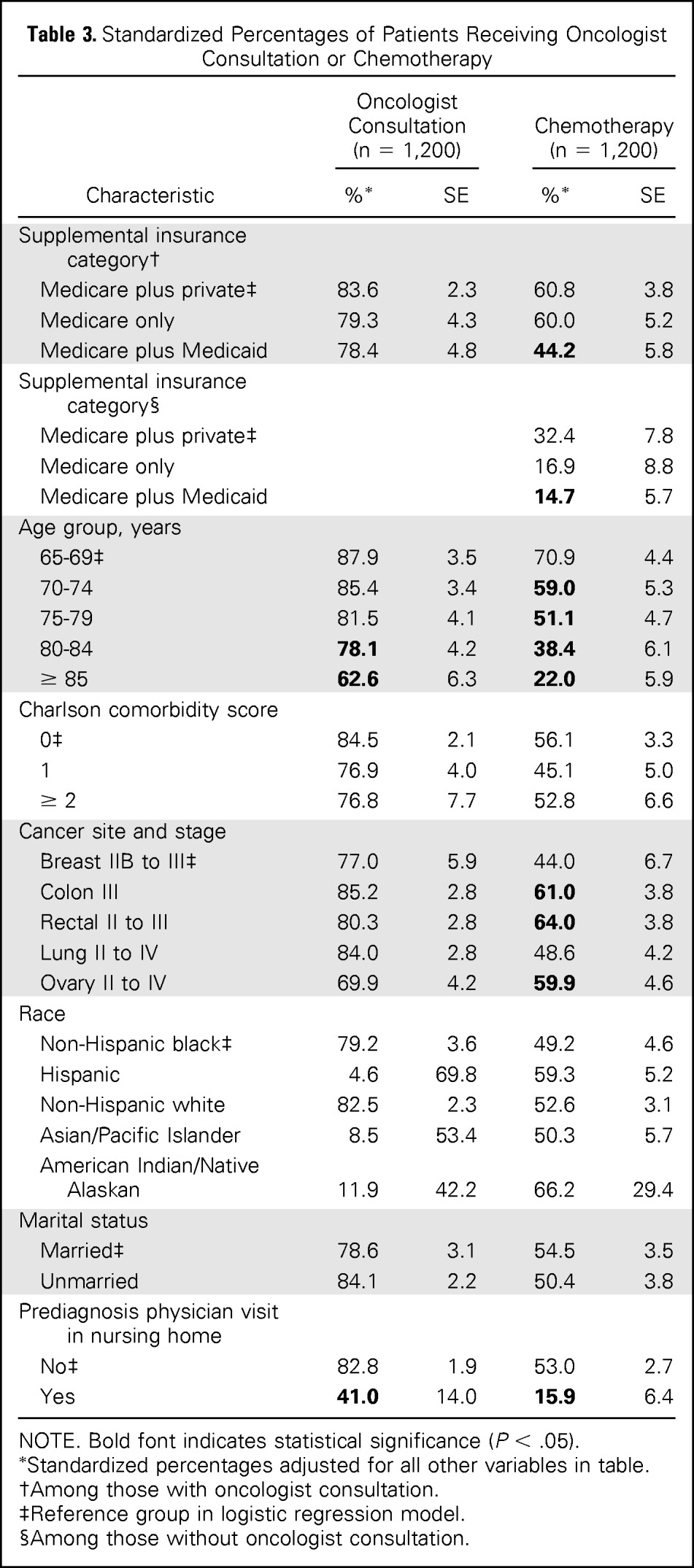
| Characteristic | Oncologist Consultation (n = 1,200) |
Chemotherapy (n = 1,200) |
||
|---|---|---|---|---|
| %* | SE | %* | SE | |
| Supplemental insurance category† | ||||
| Medicare plus private‡ | 83.6 | 2.3 | 60.8 | 3.8 |
| Medicare only | 79.3 | 4.3 | 60.0 | 5.2 |
| Medicare plus Medicaid | 78.4 | 4.8 | 44.2 | 5.8 |
| Supplemental insurance category§ | ||||
| Medicare plus private‡ | 32.4 | 7.8 | ||
| Medicare only | 16.9 | 8.8 | ||
| Medicare plus Medicaid | 14.7 | 5.7 | ||
| Age group, years | ||||
| 65-69‡ | 87.9 | 3.5 | 70.9 | 4.4 |
| 70-74 | 85.4 | 3.4 | 59.0 | 5.3 |
| 75-79 | 81.5 | 4.1 | 51.1 | 4.7 |
| 80-84 | 78.1 | 4.2 | 38.4 | 6.1 |
| ≥ 85 | 62.6 | 6.3 | 22.0 | 5.9 |
| Charlson comorbidity score | ||||
| 0‡ | 84.5 | 2.1 | 56.1 | 3.3 |
| 1 | 76.9 | 4.0 | 45.1 | 5.0 |
| ≥ 2 | 76.8 | 7.7 | 52.8 | 6.6 |
| Cancer site and stage | ||||
| Breast IIB to III‡ | 77.0 | 5.9 | 44.0 | 6.7 |
| Colon III | 85.2 | 2.8 | 61.0 | 3.8 |
| Rectal II to III | 80.3 | 2.8 | 64.0 | 3.8 |
| Lung II to IV | 84.0 | 2.8 | 48.6 | 4.2 |
| Ovary II to IV | 69.9 | 4.2 | 59.9 | 4.6 |
| Race | ||||
| Non-Hispanic black‡ | 79.2 | 3.6 | 49.2 | 4.6 |
| Hispanic | 4.6 | 69.8 | 59.3 | 5.2 |
| Non-Hispanic white | 82.5 | 2.3 | 52.6 | 3.1 |
| Asian/Pacific Islander | 8.5 | 53.4 | 50.3 | 5.7 |
| American Indian/Native Alaskan | 11.9 | 42.2 | 66.2 | 29.4 |
| Marital status | ||||
| Married‡ | 78.6 | 3.1 | 54.5 | 3.5 |
| Unmarried | 84.1 | 2.2 | 50.4 | 3.8 |
| Prediagnosis physician visit in nursing home | ||||
| No‡ | 82.8 | 1.9 | 53.0 | 2.7 |
| Yes | 41.0 | 14.0 | 15.9 | 6.4 |
NOTE. Bold font indicates statistical significance (P < .05).
Standardized percentages adjusted for all other variables in table.
Among those with oncologist consultation.
Reference group in logistic regression model.
Among those without oncologist consultation.
DISCUSSION
This analysis assessed the association between supplemental health insurance and receipt of chemotherapy among Medicare patients with cancer for whom chemotherapy is generally recommended per guidelines. We found that dual-eligible patients had significantly lower receipt of chemotherapy than did Medicare patients with cancer who had private supplemental coverage, whether or not they had an oncology consultation and accounting for differences between the two groups, including cancer site, stage, age, race, comorbidities, and whether the patient was in a nursing home before diagnosis. The reasons for these lower rates of treatment are not entirely apparent, because both groups had Medicare and additional insurance to cover copayments and deductibles.
There may be health care system and patient factors that influenced treatment decisions. We examined consultation with an oncologist as one factor related to treatment. Oncology consultations among dual-eligible patients were 8% lower than those for Medicare patients with private supplemental insurance. This difference may reflect challenges encountered by dual-eligible patients in finding an oncologist to care for them. Reduced government payments to physicians have resulted in an increasing number of physicians declining to treat Medicare and Medicaid patients. A 2012 survey of physicians reported that 25% were not accepting new Medicare patients, and 36% were not accepting new Medicaid patients.18 For dual-eligible patients, states have been required to cover their Medicare copayments. However, states have been allowed to limit their obligation to the Medicaid rate of the state. As a result, many physicians are not reimbursed for patient cost sharing, affecting physicians' financial ability to accept dual-eligible patients. Physicians' willingness to accept dual-eligible patients is a particular concern, given the projected shortage of oncologists.19 With patient demand exceeding supply, oncologists may limit the types of insurance they are willing to accept.
The expansion of Medicaid under the Affordable Care Act (ACA) will increase the number of Medicare patients with cancer who are eligible for Medicaid. Under the ACA, state Medicaid programs will be required to pay medical oncologists any cost sharing for Medicare patients receiving evaluation and management physician services.20 Physicians have reported willingness to take more Medicaid patients if reimbursement were increased, although they have been found to perceive Medicaid patients as being needier and more likely to be noncompliant.21 Future evaluation is needed to determine whether coverage by Medicaid of cost sharing for dual-eligible patients will alter physicians' acceptance of such patients.
There are other possible explanations for the lower rate in chemotherapy use found between dual-eligible patients and patients who had Medicare with private insurance. Dual-eligible patients may not be good candidates for chemotherapy; they have been reported to have more chronic conditions and cognitive or mental impairment when compared with other Medicare beneficiaries.4 In our study, comorbidity scores were comparable across insurance groups. Nursing home visits were higher among dual-eligible patients, although in the adjusted models, this did not explain the lower use of chemotherapy among the dual-eligible population. The type of hospital where patients were treated may have influenced the lower rate of chemotherapy among dual-eligible patients. Patients treated in larger teaching hospitals have been found to receive more adjuvant therapy, regardless of type of insurance.22 However, Medicaid patients with cancer have been reported to be more likely to receive care in low-volume hospitals,23 where there may be less use of chemotherapy. Lower rates of chemotherapy use in the dual-eligible patients may reflect Medicaid patients having different perspectives about chemotherapy than patients with Medicare with private insurance. Our data do not have detailed information about patient or provider treatment preferences.
We hypothesized that patients with Medicare only would be less likely to see an oncologist and receive chemotherapy because of concerns about out-of-pocket payments. We found lower consultation with an oncologist for patients with Medicare only compared with patients with Medicare with private insurance. However, receipt of chemotherapy was similar for patients with Medicare only and those with Medicare with private insurance. The growing use of oral chemotherapeutic agents will greatly increase the copayment burden for patients with Medicare only.24 There needs to be ongoing assessment of the use of chemotherapy among patients with Medicare only.
Among patients in our study, approximately half were not receiving chemotherapy, despite NCCN guidelines generally recommending chemotherapy for these patients and > 80% having a consultation with an oncologist. The rate of nontreatment in our study was higher than the 9% to 12% nontreatment rate reported from a study using data from the National Cancer Data Base and Iowa Cancer Registry.25 The earlier study included patients of all ages. In contrast, our analysis was limited to elderly persons. Our findings, especially that persons age ≥ 75 years and those in nursing homes were significantly less likely to receive treatment, suggest that patients and physicians are considering life expectancy when deciding about whether to refer a patient to an oncologist or to recommend chemotherapy.
This study of the Medicare population is novel in that it examined how type of supplemental insurance is associated with cancer treatment. Although the question addresses a gap in the literature, there are limitations to our analysis. The findings from the SEER population may not be generalized to the entire US population. Our study group included a higher percentage of dual-eligible patients and patients with Medicare only than reported in the general Medicare population.26 We relied on information reported in the medical records, augmented with information from Medicare, to determine type of supplemental coverage. If this information was missing or inaccurately reported, this would influence our findings. For example, the sensitivity of the Medicare SBI variable is suboptimal.27 We also relied on identification of oncologists based on physician number from Medicare data. Patients may have received chemotherapy from other medical specialists, such as gynecologists or surgeons. This may explain why patients with ovarian cancer had a lower percentage of oncology consultations but a similar rate of chemotherapy use.
In conclusion, this study demonstrates that for elderly patients with cancer, being eligible for Medicare does not guarantee receipt of cancer treatment. The most vulnerable of the Medicare population—low-income dual-eligible patient—are receiving less cancer treatment than are other Medicare beneficiaries. Programs to provide patient navigators for dual-eligible patients with cancer may help them access the system. In addition, ensuring adequate physician reimbursement so that there are a sufficient number of physicians willing to treat Medicaid patients is an important consideration, especially because the number of Medicaid patients will increase under the ACA.
Footnotes
See accompanying editorial on page 297
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Joan L. Warren, Eboneé N. Butler, Christopher S. Lathan, Linda C. Harlan
Collection and assembly of data: Eboneé N. Butler, Jennifer Stevens, Kevin Ward, Linda C. Harlan
Data analysis and interpretation: Joan L. Warren, Eboneé N. Butler, Jennifer Stevens, Anne-Michelle Noone, Linda C. Harlan
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Receipt of Chemotherapy Among Medicare Patients With Cancer by Type of Supplemental Insurance
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Joan L. Warren
No relationship to disclose
Eboneé N. Butler
No relationship to disclose
Jennifer Stevens
No relationship to disclose
Christopher S. Lathan
No relationship to disclose
Anne-Michelle Noone
No relationship to disclose
Kevin Ward
No relationship to disclose
Linda C. Harlan
No relationship to disclose
REFERENCES
- 1.Riley GF. Trends in out-of-pocket healthcare costs among older community-dwelling Medicare beneficiaries. Am J Manag Care. 2008;14:692–696. [PubMed] [Google Scholar]
- 2.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidoff AJ, Erten M, Shaffer T, et al. Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer. 2013;119:1257–1265. doi: 10.1002/cncr.27848. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson G, Neuman T, Damico A. Medicare's Role for Dual Eligible Beneficiaries. http://kff.org/medicaid/slide/dually-eligible-beneficiaries-comprise-20-of-the-medicare-population-and-15-of-the-medicaid-population-2008/
- 5.Harlan LC, Greene AL, Clegg LX, et al. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol. 2005;23:9079–9088. doi: 10.1200/JCO.2004.00.1297. [DOI] [PubMed] [Google Scholar]
- 6.Warren JL, Harlan LC, Stevens J, et al. Multiple myeloma treatment transformed: A population-based study of changes in initial management approaches in the United States. J Clin Oncol. 2013;31:1984–1989. doi: 10.1200/JCO.2012.46.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley CJ, Dahman B, Given CW. Inadequate access to surgeons: Reason for disparate cancer care? Med. 2009;47:758–764. doi: 10.1097/MLR.0b013e31819e1f17. [DOI] [PubMed] [Google Scholar]
- 8.Davidoff AJ, Shaffer T, Erten MZ, et al. Use and spending on antineoplastic therapy for Medicare beneficiaries with cancer. Med Care. 2013;51:351–360. doi: 10.1097/MLR.0b013e3182726ceb. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Colon Cancer (version 2. 2005) [Google Scholar]
- 10.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer (version 1. 2005) [Google Scholar]
- 11.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Ovarian Cancer (version 1. 2002) [Google Scholar]
- 12.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Rectal Cancer (version 3. 2005) [Google Scholar]
- 13.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Breast Cancer (version 1. 2005) [Google Scholar]
- 14.National Cancer Institute. Applied Research Cancer Control and Population Sciences: Patterns of Care/Quality of Care Studies. http://healthservices.cancer.gov/surveys/poc/
- 15.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl):IV3–IV18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 17.Koroukian SM, Xu F, Murray P. Ability of Medicare claims data to identify nursing home patients: A validation study. Med Care. 2008;46:1184–1187. doi: 10.1097/MLR.0b013e31817925d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson Healthcare. A Tough Time for Physicians: 2012 Medical Practice and Attitude Report. http://www.jacksonhealthcare.com/media/137811/physiciantrendsreport_ebook0712-final.pdf.
- 19.Erikson C, Salsberg E, Forte G, et al. Future supply and demand for oncologists: Challenges to assuring access to oncology services. J Oncol Pract. 2007;3:79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Medicare and Medicaid Services. Qs & As on the Increased Medicaid Payment for Primary Care. http://www.medicaid.gov/affordablecareact/provisions/downloads/qanda-set-ii-increased-payments-for-pcps.pdf.
- 21.Washington University Center for Health Policy. Health Care Access for Medicaid Patients: Physicians and Dentists Interview Study. http://healthpolicy.wustl.edu/Content/HealthCareAccess.html?OpenDocument.
- 22.Richardson LC, Tian L, Voti L, et al. The roles of teaching hospitals, insurance status, and race/ethnicity in receipt of adjuvant therapy for regional-stage breast cancer in Florida. Am J Public Health. 2006;96:160–166. doi: 10.2105/AJPH.2004.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JH, Zingmond DS, McGory ML, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296:1973–1980. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 24.Raborn ML, Pelletier EM, Smith DB, et al. Patient out-of-pocket payments for oral oncolytics: Results from a 2009 US claims data analysis. Am J Manag Care. 2012;18:SP57–SP64. [PubMed] [Google Scholar]
- 25.Ward MM, Ullrich F, Matthews K, et al. Who does not receive treatment for cancer? J Oncol Pract. 2013;9:20–26. doi: 10.1200/JOP.2012.000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser Family Foundation. Medigap: Spotlight on Enrollment, Premiums, and Recent Trends. http://kaiserfamilyfoundation.files.wordpress.com/2013/04/8412-2.pdf.
- 27.Koroukian SM, Dahman B, Copeland G, et al. The utility of the state buy-in variable in the Medicare denominator file to identify dually eligible Medicare-Medicaid beneficiaries: A validation study. Health Serv Res. 2010;45:265–282. doi: 10.1111/j.1475-6773.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]