Abstract
Purpose
To determine whether prolonged androgen suppression (AS) duration before radiotherapy improves survival and disease control in prostate cancer.
Patients and Methods
One thousand five hundred seventy-nine men with intermediate-risk prostate cancer were randomly assigned to 8 weeks of AS followed by radiotherapy with an additional 8 weeks of concurrent AS (16 weeks total) or to 28 weeks of AS followed by radiotherapy with an additional 8 weeks of AS (36 weeks total). The trial sought primarily to detect a 33% reduction in the hazard of prostate cancer death in the 28-week assignment. Time-to-event end points are reported for up to 10 years of follow-up.
Results
There were no between-group differences in baseline characteristics of 1,489 eligible patients with follow-up. For the 8- and 28-week assignments, 10-year disease-specific survival rates were 95% (95% CI, 93.3% to 97.0%) and 96% (95% CI, 94.6% to 98.0%; hazard ratio [HR], 0.81; P = .45), respectively, and 10-year overall survival rates were 66% (95% CI, 62.0% to 69.9%) and 67% (95% CI, 63.0% to 70.8%; HR, 0.95; P = .62), respectively. For the 8- and 28-week assignments, 10-year cumulative incidences of locoregional progression were 6% (95% CI, 4.3% to 8.0%) and 4% (95% CI, 2.5% to 5.7%; HR, 0.65; P = .07), respectively; 10-year distant metastasis cumulative incidences were 6% (95% CI, 4.0% to 7.7%) and 6% (95% CI, 4.0% to 7.6%; HR, 1.07; P = .80), respectively; and 10-year prostate-specific antigen–based recurrence cumulative incidences were 27% (95% CI, 23.1% to 29.8%) and 27% (95% CI, 23.4% to 30.3%; HR, 0.97; P = .77), respectively.
Conclusion
Extending AS duration from 8 weeks to 28 weeks before radiotherapy did not improve outcomes. A lower than expected prostate cancer death rate reduced ability to detect a between-group difference in disease-specific survival. The schedule of 8 weeks of AS before radiotherapy plus 8 weeks of AS during radiotherapy remains a standard of care in intermediate-risk prostate cancer.
INTRODUCTION
The Radiation Therapy Oncology Group (RTOG) has a nearly four-decade history of prostate cancer research, largely conducted through large-scale randomized clinical trials. Investigation of various androgen suppression (AS) regimens with radiotherapy is a prominent endeavor of the RTOG,1–5 having established standards of care used worldwide. Luteinizing hormone–releasing hormone analog (LHRHa) agents are the principal means of inducing the potentially reversible hypogonadal state of AS. An antiandrogen is often added to block the effects of residual androgens. Early studies demonstrated the value of adding AS to radiotherapy in locally advanced prostate cancer,1–3 and short-term AS was found later to benefit some men with localized disease as well.4
AS typically starts before radiotherapy,2–5 because exploratory analyses of its use in this way suggested a promising line of research. Early studies chose an 8-week duration of preradiotherapy AS,2–5 and AS was continued for an additional 8 weeks during radiotherapy. Research in animal models showed subsequently that extending the duration of AS improved cancer control when radiotherapy was then given.6 The RTOG sought to translate this observation into a clinical trial testing the hypothesis that prolonged-duration AS used before radiotherapy improves outcomes in intermediate-risk prostate cancer.
PATIENTS AND METHODS
Trial Design and Participants
Men age ≥ 18 years with prostate adenocarcinoma were eligible if they met one of the following criteria sets: clinical classification T1b-4 (according to American Joint Committee on Cancer staging system, fifth edition),7 Gleason score 2 to 6, and prostate-specific antigen (PSA) more than 10 but ≤ 100 ng/mL; T1b-4, Gleason score 7, and PSA less than 20 ng/mL; or T1b-1c, Gleason score 8 to 10, and PSA less than 20 ng/mL. Additional criteria were no nodal or distant metastatic disease, expected comorbidity survival duration of ≥ 10 years, Zubrod performance status less than 2, serum ALT level ≤ 2× upper normal limit, and no prior bilateral orchiectomy, chemotherapy, radiotherapy, cryosurgery, or definitive surgery for prostate cancer. Prior AS was not allowed unless an LHRHa was started ≤ 30 days before random assignment and bicalutamide or flutamide was started within 14 days of the LHRHa; any finasteride was discontinued. Patients with another invasive cancer, other than localized basal or squamous cell skin carcinoma, were not eligible unless continually free of that cancer for ≥ 5 years. Before study entry, evaluation required history and physical examination (including digital rectal examination), serum PSA (within 90 days before random assignment and before AS), CBC, serum chemistry, bone scan, and assessment of Zubrod performance status and regional lymph nodes with surgical sampling, computed tomography, or magnetic resonance imaging.
After institutional review board approval at each center, participants were recruited at 152 community-based and tertiary medical site members of the RTOG. Membership was established and maintained through a quality-control system compliant with National Cancer Institute (NCI) guidelines. All participants provided written informed consent before registration and were to receive protocol-specified care and follow-up at a member site. Participants did not receive compensation for joining the study, and no commercial support was provided.
Random Assignment
This was a multicenter, stratified, open-label, parallel-group study with 1:1 random assignment approved and sponsored by the NCI. The RTOG was responsible for design, administration, data collection, quality assurance, statistical analysis, interpretation of findings, and article preparation. Participants were stratified according to PSA level (≤ 10 v > 10 to 20 v > 20 to ≤ 100 ng/mL), Gleason score (2 to 4 v 5 to 6 v 7 to 10), tumor stage (T1b-2 v T3-4), and whether AS had been started (no v yes). Participants were then randomly assigned using the permuted-block method8 either to AS for 8 weeks (short duration) followed by radiotherapy with an additional 8 weeks of AS concurrently or to 28 weeks of AS (prolonged duration) followed by radiotherapy with an additional 8 weeks of AS.
Treatment
Participants started AS within 6 weeks of random assignment. AS consisted of a commercially available LHRHa (eg, goserelin, leuprolide) given by intramuscular or subcutaneous injection according to formulary instructions. Various dosing formulations were allowed to achieve the required total treatment duration as assigned. Bicalutamide 50 mg once a day orally or flutamide 250 mg three times a day orally was started within 14 days of the first LHRHa injection. Bicalutamide or flutamide was continued until the last day of radiotherapy, which was for 16 weeks (maximum of 112 days) for the short-duration group or 36 weeks (maximum of 252 days) for the prolonged-duration group. These agents were discontinued if ALT increased to more than twice the upper limit of normal.
Radiotherapy started 8 weeks after the first LHRHa injection in the short-duration group and after 28 weeks in the prolonged-duration group. Radiotherapy used two-dimensional or three-dimensional conformal external technique (intensity modulation and brachytherapy were not allowed) without requirement for image guidance. Computed tomography identified the prostate, any extraprostatic tumor extensions, and pelvic anatomy. The radiotherapy target was the prostate and any extraprostatic tumor extensions, with a 1.0- to 1.5-cm margin. When lymph node assessment was based on imaging, external and internal iliac lymph nodes and seminal vesicles were also targeted if one of the following existed: T3-4, Gleason score 7, and PSA more than 4 but ≤ 20 ng/mL; T3-4, Gleason score 6, and PSA more than 10 but ≤ 20 ng/mL; T2a, Gleason score 7, and PSA more than 10 but ≤ 20 ng/mL; T1b-1c, Gleason score 8 to 10, and PSA more than 10 but ≤ 20 ng/mL; or T2a-4, Gleason score 6, and PSA more than 20 ng/mL.
Radiotherapy doses were prescribed according to International Commission on Radiation Units and Measurements reference points. The prostate and any extraprostatic tumor extensions were to receive 70.2 Gy in 39 daily fractions. The iliac lymph nodes were to receive 46.8 Gy in 26 fractions, when included.
Patient Assessment and End Points
After starting AS, patients were evaluated every 2 months before radiotherapy with history and examination, assessment of adverse events, and serum PSA. Serum ALT and bilirubin were monitored monthly until AS was completed. Adverse event monitoring occurred weekly during radiotherapy. History and examination, assessment of adverse events, and PSA level measurement were performed at the end of radiotherapy, every 3 months for the first year, every 6 months the next 4 years, and then annually thereafter. Any findings noted between scheduled evaluations were also recorded. Prostate biopsy, radionuclide bone scan, computed tomography, or magnetic resonance imaging was used to investigate clinical findings or serum PSA elevation. These same tests with history, examination, and serum PSA were to be done if there was disease progression.
The study was designed primarily to compare disease-specific survival rate between assignments. Death was attributed to prostate cancer if certified primarily as such, disease progressed on secondary AS, or death resulted from an adverse effect of therapy. The cause of all deaths was reviewed by the study chair, who was blinded to assignment.
Overall and disease-free survival, time to locoregional progression or distant metastatic progression, time to biochemical failure and to biochemical failure on secondary AS, and adverse treatment events were additional between-group planned comparisons. Disease-free survival required the patient to be alive and without locoregional or metastatic disease or biochemical failure. Biochemical failure was defined initially as serum PSA increasing on three consecutive occasions from the nadir value.9 The study allowed analysis with another definition if adopted through consensus subsequently, so the criterion of PSA increasing ≥ 2 ng/mL above the nadir was also used.10 Biochemical failure on secondary AS was defined as a PSA increase of more than 1 ng/mL on two or more consecutive measurements. Early adverse events were defined by NCI Common Toxicity Criteria (version 2), and late events were scored using the RTOG/European Organisation for Research and Treatment of Cancer Late Radiation Morbidity Scoring schema.11
Statistical Methods
Sample size determination was based on disease-specific mortality, assuming 40% of deaths in the 8-week assignment would be from prostate cancer and that 8-year disease-specific survival would be 79%.2 Two-hundred seventy prostate cancer deaths were required to detect a 33% hazard reduction in the 28-week assignment with 90% power using the log-rank test12 with a two-sided significance level of P = .05. Under assumed failure rates, 1,540 patients accrued over 4 years and observed for an additional 6 years were expected to provide the requisite events. This sample accounted for a 10% ineligible or lack-of-data rate and three interim analyses. Interim reports were provided to the Data Monitoring Committee every 6 months, with interim efficacy and futility analyses performed at specific event number landmarks, the first specified at 50 prostate cancer deaths. The boundary for early stopping for efficacy was based on the O'Brien-Fleming α spending function approach,13 with low conditional power providing a basis for early stopping for lack of efficacy.14
All eligible patients with follow-up were included and analyzed according to assignment in this modified intent-to-treat analysis,15 with time-to-event duration originating at random assignment and ending with data received through December 2012. Overall and disease-free survival distributions were calculated using the Kaplan-Meier method.16 The cumulative incidence estimator17 was used for all other end points to account for competing risks. Treatment efficacy for disease-specific mortality was tested by comparing cause-specific hazards with the log-rank statistic.12 Hazard ratios with 95% CIs were computed using Cox proportional hazards18 or Fine-Gray competing risks hazards regression19 as appropriate.
RESULTS
Demographic Characteristics
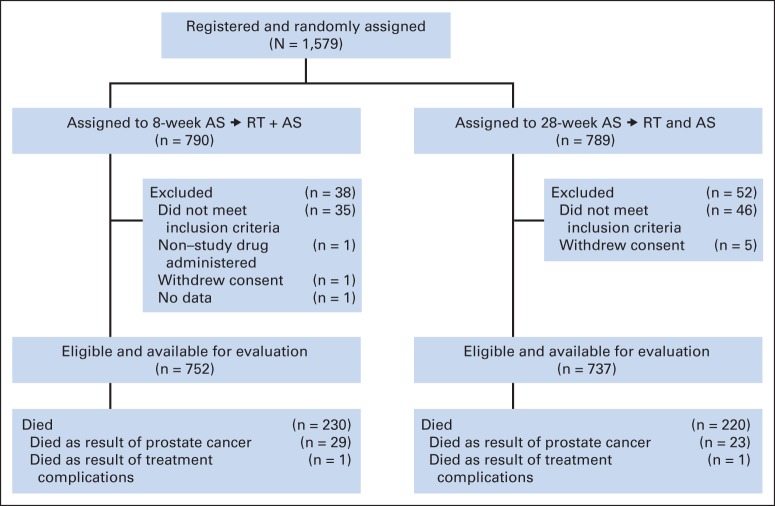
Between February 2000 and May 2004, 1,579 patients were enrolled (Fig 1). Exclusions were mainly for the following reasons: AS started early (47%), entry characteristics not met (24%), or testing performed outside specified time frame (18%). Baseline characteristics of 1,489 eligible participants with follow-up are listed in Table 1. Ninety percent of patients had no physical limitation (Zubrod performance score, 0), and 10% were limited in strenuous activity only (Zubrod performance score, 1). Baseline characteristics were balanced well, with no significant between-group differences.
Fig 1.
Enrollment, random assignment, and follow-up of the study participants. AS, androgen suppression; RT, radiotherapy.
Table 1.
Baseline Demographics and Clinical Characteristics
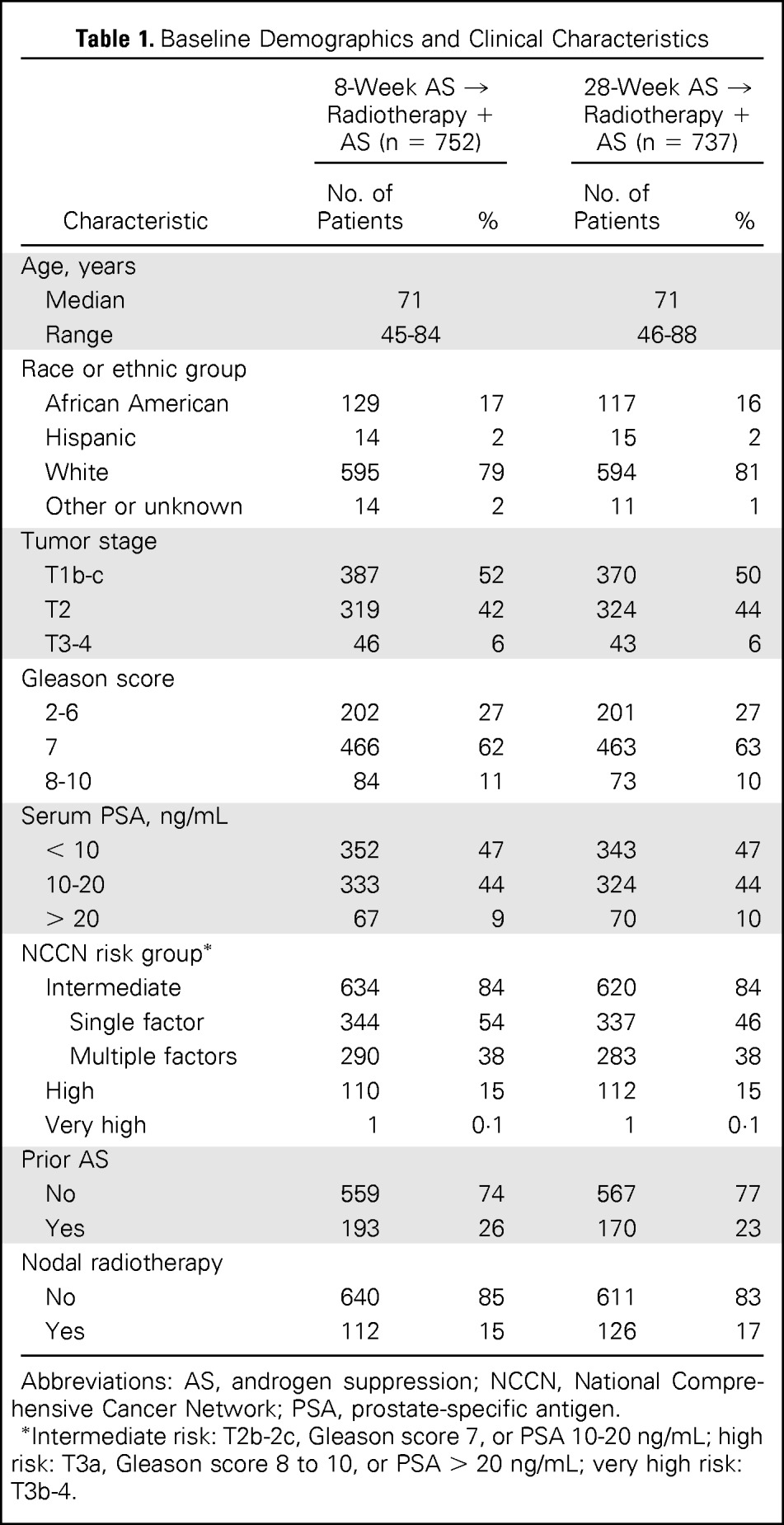
| Characteristic | 8-Week AS → Radiotherapy + AS (n = 752) |
28-Week AS → Radiotherapy + AS (n = 737) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 71 | 71 | ||
| Range | 45-84 | 46-88 | ||
| Race or ethnic group | ||||
| African American | 129 | 17 | 117 | 16 |
| Hispanic | 14 | 2 | 15 | 2 |
| White | 595 | 79 | 594 | 81 |
| Other or unknown | 14 | 2 | 11 | 1 |
| Tumor stage | ||||
| T1b-c | 387 | 52 | 370 | 50 |
| T2 | 319 | 42 | 324 | 44 |
| T3-4 | 46 | 6 | 43 | 6 |
| Gleason score | ||||
| 2-6 | 202 | 27 | 201 | 27 |
| 7 | 466 | 62 | 463 | 63 |
| 8-10 | 84 | 11 | 73 | 10 |
| Serum PSA, ng/mL | ||||
| < 10 | 352 | 47 | 343 | 47 |
| 10-20 | 333 | 44 | 324 | 44 |
| > 20 | 67 | 9 | 70 | 10 |
| NCCN risk group* | ||||
| Intermediate | 634 | 84 | 620 | 84 |
| Single factor | 344 | 54 | 337 | 46 |
| Multiple factors | 290 | 38 | 283 | 38 |
| High | 110 | 15 | 112 | 15 |
| Very high | 1 | 0·1 | 1 | 0·1 |
| Prior AS | ||||
| No | 559 | 74 | 567 | 77 |
| Yes | 193 | 26 | 170 | 23 |
| Nodal radiotherapy | ||||
| No | 640 | 85 | 611 | 83 |
| Yes | 112 | 15 | 126 | 17 |
Abbreviations: AS, androgen suppression; NCCN, National Comprehensive Cancer Network; PSA, prostate-specific antigen.
Intermediate risk: T2b-2c, Gleason score 7, or PSA 10-20 ng/mL; high risk: T3a, Gleason score 8 to 10, or PSA > 20 ng/mL; very high risk: T3b-4.
Adherence to Assignment
Criteria for adherence to assigned therapy were established a priori. AS was compliant if LHRHa total dosage was 80% to 120% of protocol specification. Six hundred forty-four participants (86%) in the 8-week assignment and 647 participants (88%) in the 28-week assignment were adherent; 56 participants (7%) in the 8-week assignment received more than 120% of dosage, and 70 participants (10%) in the 28-week assignment received less than 80% of dosage. Median radiotherapy dose to the prostate was 70.2 Gy (interquartile range, 70.2 to 70.2 Gy) in each group. Radiotherapy quality assurance was performed in 29% of randomly selected patients from each group, with no between-group difference in the 3% overall unacceptable deviation rate.
Outcomes
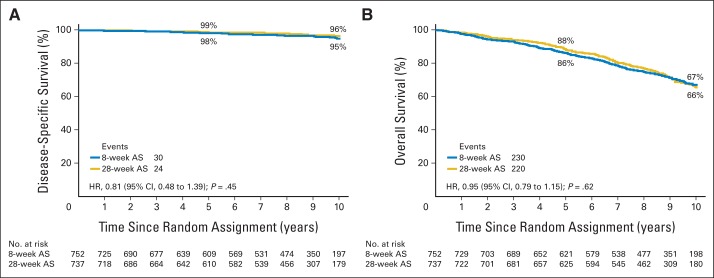
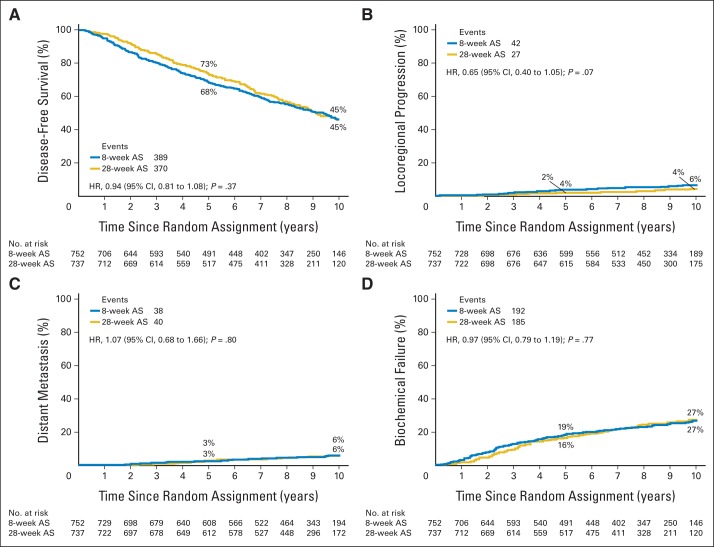
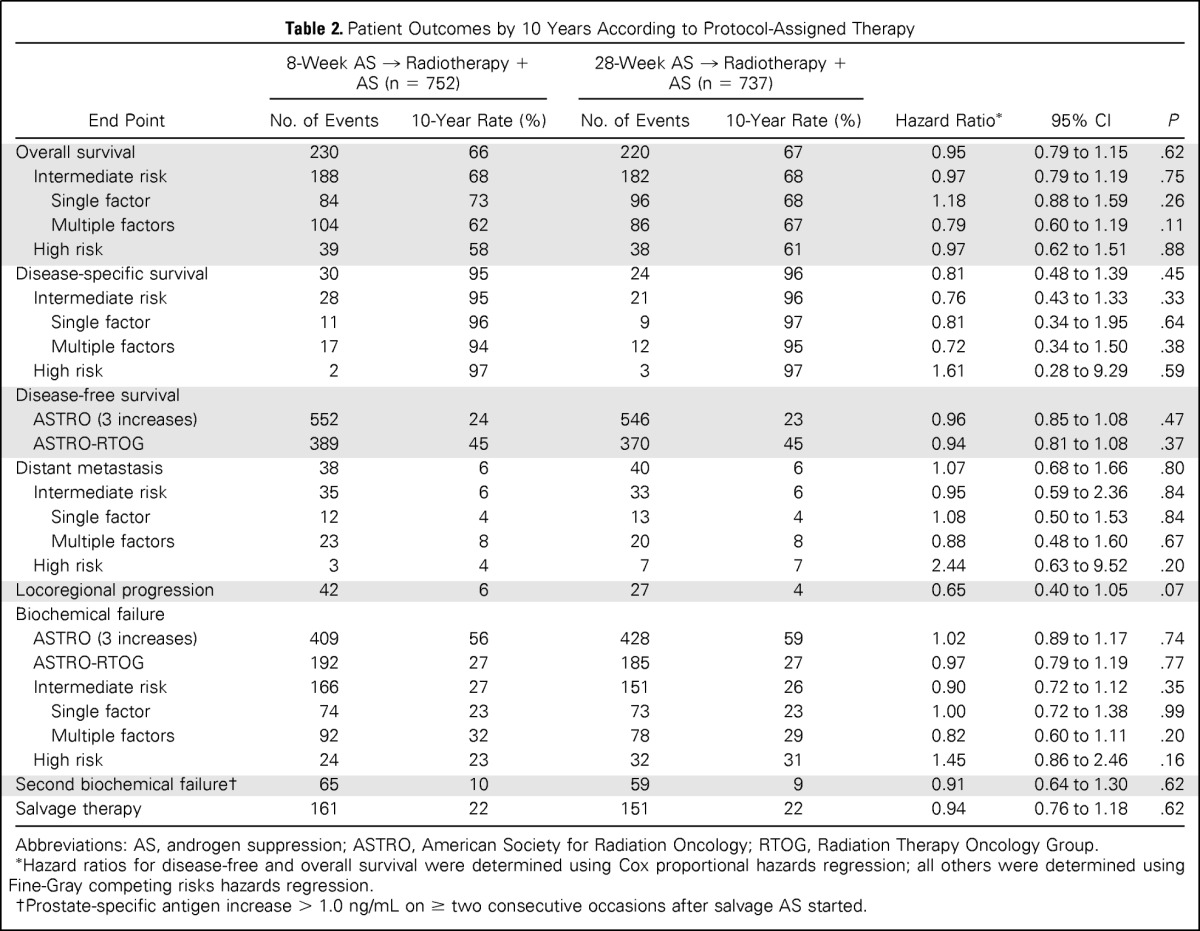
Study end points and between-group comparisons are listed in Table 2. Median follow-up duration was 9.4 years. Four hundred fifty participants died, with prostate cancer accounting for 54 deaths (12%), with 30 deaths (13%) in the 8-week group and 24 deaths (11%) in the 28-week group. Disease-specific and overall survival differences (log-rank P = .42 for disease-specific survival) between assignments were not observed (Fig 2). The cumulative incidence of disease-specific mortality within 10 years was 5% (95% CI, 3.0% to 6.7%) in the 8-week group and 4% (95% CI, 2.0% to 5.4%) in the 28-week group (hazard ratio, 0.81; 95% CI, 0.48 to 1.39; P = .45). The most frequent causes of death were cardiovascular (23%), other cancerous (21%), and pulmonary (9%) diseases; cause was entirely unknown in two patients (0.4%). A difference in cause of death was not apparent between groups. Extending AS to 28 weeks did not affect disease-free survival, locoregional or distant metastatic disease progression, or the incidence of biochemical failure (Fig 3).
Table 2.
Patient Outcomes by 10 Years According to Protocol-Assigned Therapy
| End Point | 8-Week AS → Radiotherapy + AS (n = 752) |
28-Week AS → Radiotherapy + AS (n = 737) |
Hazard Ratio* | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| No. of Events | 10-Year Rate (%) | No. of Events | 10-Year Rate (%) | ||||
| Overall survival | 230 | 66 | 220 | 67 | 0.95 | 0.79 to 1.15 | .62 |
| Intermediate risk | 188 | 68 | 182 | 68 | 0.97 | 0.79 to 1.19 | .75 |
| Single factor | 84 | 73 | 96 | 68 | 1.18 | 0.88 to 1.59 | .26 |
| Multiple factors | 104 | 62 | 86 | 67 | 0.79 | 0.60 to 1.19 | .11 |
| High risk | 39 | 58 | 38 | 61 | 0.97 | 0.62 to 1.51 | .88 |
| Disease-specific survival | 30 | 95 | 24 | 96 | 0.81 | 0.48 to 1.39 | .45 |
| Intermediate risk | 28 | 95 | 21 | 96 | 0.76 | 0.43 to 1.33 | .33 |
| Single factor | 11 | 96 | 9 | 97 | 0.81 | 0.34 to 1.95 | .64 |
| Multiple factors | 17 | 94 | 12 | 95 | 0.72 | 0.34 to 1.50 | .38 |
| High risk | 2 | 97 | 3 | 97 | 1.61 | 0.28 to 9.29 | .59 |
| Disease-free survival | |||||||
| ASTRO (3 increases) | 552 | 24 | 546 | 23 | 0.96 | 0.85 to 1.08 | .47 |
| ASTRO-RTOG | 389 | 45 | 370 | 45 | 0.94 | 0.81 to 1.08 | .37 |
| Distant metastasis | 38 | 6 | 40 | 6 | 1.07 | 0.68 to 1.66 | .80 |
| Intermediate risk | 35 | 6 | 33 | 6 | 0.95 | 0.59 to 2.36 | .84 |
| Single factor | 12 | 4 | 13 | 4 | 1.08 | 0.50 to 1.53 | .84 |
| Multiple factors | 23 | 8 | 20 | 8 | 0.88 | 0.48 to 1.60 | .67 |
| High risk | 3 | 4 | 7 | 7 | 2.44 | 0.63 to 9.52 | .20 |
| Locoregional progression | 42 | 6 | 27 | 4 | 0.65 | 0.40 to 1.05 | .07 |
| Biochemical failure | |||||||
| ASTRO (3 increases) | 409 | 56 | 428 | 59 | 1.02 | 0.89 to 1.17 | .74 |
| ASTRO-RTOG | 192 | 27 | 185 | 27 | 0.97 | 0.79 to 1.19 | .77 |
| Intermediate risk | 166 | 27 | 151 | 26 | 0.90 | 0.72 to 1.12 | .35 |
| Single factor | 74 | 23 | 73 | 23 | 1.00 | 0.72 to 1.38 | .99 |
| Multiple factors | 92 | 32 | 78 | 29 | 0.82 | 0.60 to 1.11 | .20 |
| High risk | 24 | 23 | 32 | 31 | 1.45 | 0.86 to 2.46 | .16 |
| Second biochemical failure† | 65 | 10 | 59 | 9 | 0.91 | 0.64 to 1.30 | .62 |
| Salvage therapy | 161 | 22 | 151 | 22 | 0.94 | 0.76 to 1.18 | .62 |
Abbreviations: AS, androgen suppression; ASTRO, American Society for Radiation Oncology; RTOG, Radiation Therapy Oncology Group.
Hazard ratios for disease-free and overall survival were determined using Cox proportional hazards regression; all others were determined using Fine-Gray competing risks hazards regression.
Prostate-specific antigen increase > 1.0 ng/mL on ≥ two consecutive occasions after salvage AS started.
Fig 2.
Estimates of (A) disease-specific survival and (B) overall survival. AS, androgen suppression; HR, hazard ratio.
Fig 3.
Estimates of (A) disease-free survival and cumulative incidences of (B) locoregional progression, (C) distant metastasis, and (D) biochemical failure (nadir + 2 ng/mL). AS, androgen suppression; HR, hazard ratio.
Secondary Analyses
Post hoc subgroup analysis according to whether patients had intermediate-risk (tumor stage T2b-2c, Gleason score 7, or PSA 10 to 20 ng/mL) or high-risk (clinical stage T3a, Gleason score 8 to 10, or PSA > 20 ng/mL) disease20 is shown in Table 2. The intermediate-risk subgroup was stratified further into single intermediate-risk factor or multiple-factor groupings, with outcomes provided similarly.20 There were no differences in treatment effect (8 weeks v 28 weeks) for disease-specific survival, cumulative incidence of distant metastasis, or biochemical failure according to risk group, and no difference between risk groups (intermediate v high risk) was evident within assignment.
Adverse Effects
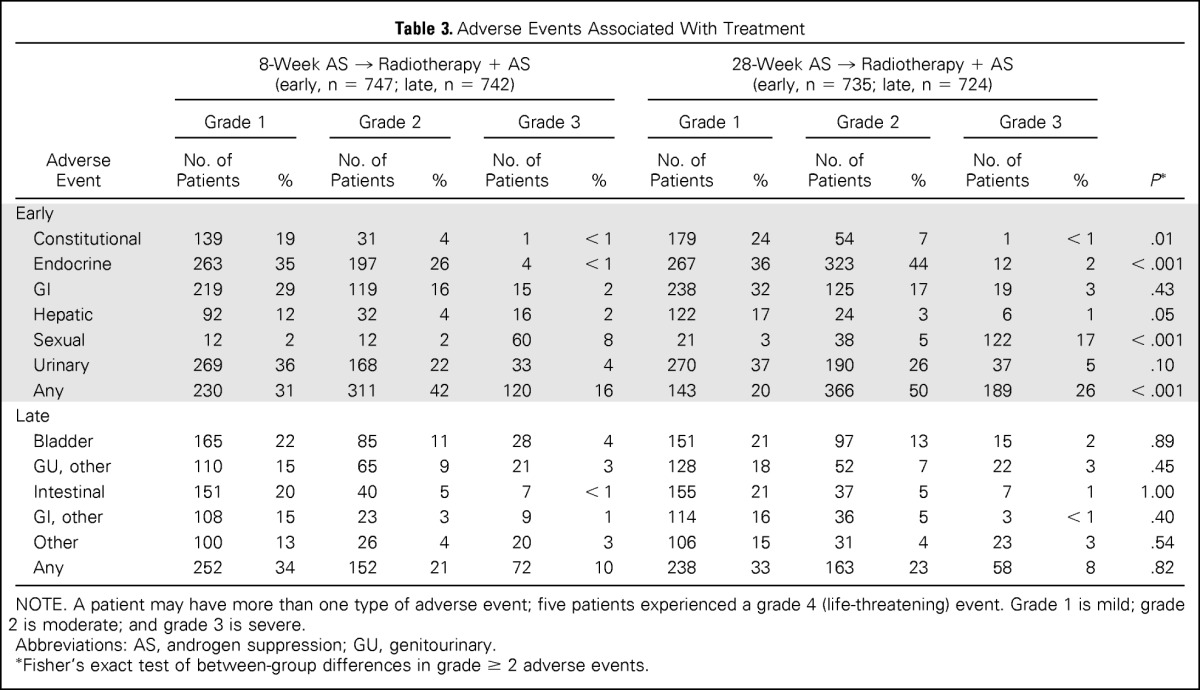
The greatest severity of an adverse event at any time was reported irrespective of its persistence or resolution subsequently. Early events occurred from the start of AS through 90 days after radiotherapy, and late events occurred thereafter. The higher incidence of early grade ≥ 2 adverse events in the 28-week assignment (P < .001) was attributed to AS, with no between-group differences observed thereafter (Table 3).
Table 3.
Adverse Events Associated With Treatment
| Adverse Event | 8-Week AS → Radiotherapy + AS (early, n = 747; late, n = 742) |
28-Week AS → Radiotherapy + AS (early, n = 735; late, n = 724) |
P* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 1 |
Grade 2 |
Grade 3 |
||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| Early | |||||||||||||
| Constitutional | 139 | 19 | 31 | 4 | 1 | < 1 | 179 | 24 | 54 | 7 | 1 | < 1 | .01 |
| Endocrine | 263 | 35 | 197 | 26 | 4 | < 1 | 267 | 36 | 323 | 44 | 12 | 2 | < .001 |
| GI | 219 | 29 | 119 | 16 | 15 | 2 | 238 | 32 | 125 | 17 | 19 | 3 | .43 |
| Hepatic | 92 | 12 | 32 | 4 | 16 | 2 | 122 | 17 | 24 | 3 | 6 | 1 | .05 |
| Sexual | 12 | 2 | 12 | 2 | 60 | 8 | 21 | 3 | 38 | 5 | 122 | 17 | < .001 |
| Urinary | 269 | 36 | 168 | 22 | 33 | 4 | 270 | 37 | 190 | 26 | 37 | 5 | .10 |
| Any | 230 | 31 | 311 | 42 | 120 | 16 | 143 | 20 | 366 | 50 | 189 | 26 | < .001 |
| Late | |||||||||||||
| Bladder | 165 | 22 | 85 | 11 | 28 | 4 | 151 | 21 | 97 | 13 | 15 | 2 | .89 |
| GU, other | 110 | 15 | 65 | 9 | 21 | 3 | 128 | 18 | 52 | 7 | 22 | 3 | .45 |
| Intestinal | 151 | 20 | 40 | 5 | 7 | < 1 | 155 | 21 | 37 | 5 | 7 | 1 | 1.00 |
| GI, other | 108 | 15 | 23 | 3 | 9 | 1 | 114 | 16 | 36 | 5 | 3 | < 1 | .40 |
| Other | 100 | 13 | 26 | 4 | 20 | 3 | 106 | 15 | 31 | 4 | 23 | 3 | .54 |
| Any | 252 | 34 | 152 | 21 | 72 | 10 | 238 | 33 | 163 | 23 | 58 | 8 | .82 |
NOTE. A patient may have more than one type of adverse event; five patients experienced a grade 4 (life-threatening) event. Grade 1 is mild; grade 2 is moderate; and grade 3 is severe.
Abbreviations: AS, androgen suppression; GU, genitourinary.
Fisher's exact test of between-group differences in grade ≥ 2 adverse events.
DISCUSSION
Men with intermediate- or high-risk prostate cancer are not typically candidates for active surveillance.21 Prior studies in these men showed that radiotherapy with AS reduced the risk of death from prostate cancer compared with radiotherapy alone,2,4,22,23 establishing evidence-based guidelines for best medical practice. This study is part of a long-range plan to build on prior research efforts, to confirm and refine the findings of prior studies,4 and to improve outcomes with new treatments, if possible. The RTOG noted previously that 8 weeks of AS given before and also during radiotherapy reduced disease-specific mortality.4 A post hoc exploratory analysis in that study suggested that a subgroup of men with intermediate-risk disease derived the greatest benefit from AS and that the 10-year disease-specific survival was 97% compared with 90% with radiotherapy alone. Similar 10-year disease-specific survival (95%) was observed in this study, with a nearly identical incidence of biochemical failure (27% in this study v 28% in previous RTOG study) noted between studies. Thus, this study provides confirmatory testing and affirmation of the exploratory analysis reported by Jones et al4 and also provides reproducibility to enhance confidence in the outcomes achieved with this treatment approach.
Basic research suggested that locoregional control of prostate cancer is improved when prolonged-duration AS is given before radiotherapy.6 This generated a hypothesis tested in this large-scale parallel-group clinical study, in which a 28-week duration of preradiotherapy AS was tested against the 8-week standard established previously.2,4 Prolongation of AS from 8 to 28 weeks before radiotherapy and extending the total duration of AS from 16 to 36 weeks did not further benefit patient outcomes across a broad measure of clinically important end points. It may be reasoned that more treatment at diagnosis may be warranted if cure is affected and secondary therapies are used less frequently, but no evidence of such was apparent in this study. The additional 20 weeks of AS led only to more medication use (with the associated costs) and more endocrine (mainly hot flushing) and sexual adverse effects.
This study has significant and broad implications for best medical practices. Healthcare providers may use this treatment approach with confidence that its outcomes are accurate, reproducible, and generalizable to their patients. Current guidelines recommend up to 6 months of AS with radiotherapy for intermediate-risk prostate cancer based on evidence available before this report.22,23 A single meta-analysis of 761 patients,24 which included some of these other trials,22,23,25 concluded that 6 months of AS reduced prostate cancer mortality compared with 3 or 4 months of AS. Other studies sought also to determine the preferred duration of short-term AS,23,25,26 with these investigations reaching different conclusions. However, the risk profile of participants in these studies differed, as they did in our study also, and this may account for varying observations and conflicting conclusions. This study provides the strongest evidence available to guide short-term AS use with radiotherapy for intermediate-risk disease.
This study has several strengths. Patients were racially diverse, varied in age, and recruited across a wide geographic distribution and spectrum of medical practices, reflective of the population for whom this intervention was eventually intended, providing generalizability of study results. Patient accrual was robust and accomplished within the predetermined time frame, with follow-up extended beyond that originally required. The study also included several safeguards to assure accuracy of the data and impartiality of its analysis, interpretation, and reporting.
Nonetheless, some uncertainty in application of the findings remains. The radiotherapy method prescribed more than a decade ago is no longer recommended.20 It is likely that higher doses would reduce biochemical failure and possibly salvage therapy,27 but studies of higher doses have yet to demonstrate reduced prostate cancer–specific mortality, which was the principal measure of efficacy in this study. Advances in dose delivery have also occurred, allowing more precise application of the prescribed dose to the intended target with reduced exposure of adjacent normal organs. Thus, results of this study may overestimate the incidence of biochemical failure, salvage therapy, and adverse events expected today.
Consensus-based risk group definitions were largely introduced (then changed) after our study was designed, so some study participants are now considered high risk.20 However, different outcomes between the intermediate- and high-risk groups were not evident in our study, irrespective of treatment assignment. Some investigators recently proposed modifications to the intermediate-risk group definition28–30 and postulated that certain patients may not benefit from AS. Although our study cannot address this issue, the RTOG is currently conducting a randomized clinical trial to formally test this hypothesis.
This study provided the opportunity for incidental observations important for interpreting the outcomes of radiotherapy with short-term AS and for development and design of future studies. The method used to define biochemical failure yielded substantial differences in estimating some aspects of treatment outcome. The method of three consecutive serum PSA increases was the first consensus-based definition of biochemical failure9 and the only available method during conduct of this study. It is now recognized that serum PSA often increases when short-term AS ends and testosterone recovery ensues.10 Slight increases in serum PSA have high sensitivity for biochemical failure, but specificity is lacking. Although historically important, the successive consensus-based definition (nadir + 2 ng/mL) should take its place.10
Disease-specific survival was favorable for both treatment groups and much better than historical outcomes used in study design. Early reporting of study results at the first planned interim analysis was based on this, inspection of annualized cause-specific death rates, the follow-up duration needed to reach this and subsequent planned analyses, and greatly diminished prospects for observing the requisite prostate cancer deaths for final analysis. This has significant implications for any future study that tests an alternative treatment strategy using this outcome in intermediate-risk prostate cancer. These studies must assume that the standard regimen of 8-week AS followed by radiotherapy with 8 additional weeks of AS will yield a disease-specific survival of 95% at 10 years. Reducing the hazard of death from prostate cancer by 33%, as sought in this study, will require large study cohorts (approximately 7,000 participants) with prolonged follow-up requirements to a level that has yet to be even closely approximated. Feasibility may demand a change in focus to research outcomes that are less dependent on survival end points, a transition that has occurred already in other cancer types.
Acknowledgment
We thank the following substantial contribution by nonauthors: Asha George, MS, and Wendy Seirerheld, Supporting Statisticians; Kathryn A. Winter, MS, Director of Statistics; Roseann Bonanni, Senior Research Associate; Elaine Motyka-Welsh, MSN, Research Associate; Dana Robinson, MEd, Protocol Associate, Protocol Development and Regulatory Compliance; and Joanne Hunter, BS, Dosimetry.
Glossary Terms
- locoregional failure:
failure at the primary site or the regional lymphatics.
- prostate-specific antigen (PSA):
a protein produced by cells of the prostate gland. The blood level of prostate-specific antigen (PSA) is used as a tumor marker for men who may be suspected of having prostate cancer. Most physicians consider 0 to 4.0 ng/mL to be the normal range. Levels of 4 to 10 and 10 to 20 ng/mL are considered slightly and moderately elevated, respectively. PSA levels have to be complemented with other tests to make a firm diagnosis of prostate cancer.
Footnotes
See accompanying editorial on page 301
Supported by Radiation Therapy Oncology Group Grant No. U10 CA21661, Community Clinical Oncology Program Grant No. U10 CA37422, and Grant No. U10 CA180822 from the National Cancer Institute.
Presented, in part, at the 55th Annual Meeting of the American Society for Radiation Oncology, September 22-25, 2013, Atlanta GA.
Clinical trial information: NCT00005044.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
The Radiation Therapy Oncology Group is solely responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the article. The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Thomas M. Pisansky, Leonard G. Gomella, Mahul B. Amin, William U. Shipley, Howard M. Sandler
Provision of study materials or patients: Thomas M. Pisansky, Alexander G. Balogh, Daniel M. Chinn, Michael J. Seider, Marie Duclos, Seth A. Rosenthal, Glenn S. Bauman, Elizabeth M. Gore, Marvin Z. Rotman, Himanshu R. Lukka, William U. Shipley, Howard M. Sandler
Collection and assembly of data: Daniel Hunt, Alexander G. Balogh, Daniel M. Chinn, Michael J. Seider, Marie Duclos, Seth A. Rosenthal, Glenn S. Bauman, Elizabeth M. Gore, Marvin Z. Rotman, Himanshu R. Lukka, James J. Dignam
Data analysis and interpretation: Thomas M. Pisansky, Daniel Hunt, Leonard G. Gomella, Mahul B. Amin, William U. Shipley, James J. Dignam, Howard M. Sandler
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Duration of Androgen Suppression Before Radiotherapy for Localized Prostate Cancer: Radiation Therapy Oncology Group Randomized Clinical Trial 9910
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Thomas M. Pisansky
No relationship to disclose
Daniel Hunt
Employment: Puma Biotechnology
Stock or Other Ownership: Puma Biotechnology
Research Funding: Puma Biotechnology
Travel, Accommodations, Expenses: Puma Biotechnology (I)
Leonard G. Gomella
Consulting or Advisory Role: Astellas Pharma, Bayer, Dendreon, Janssen Pharmaceuticals
Research Funding: Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Cell Detection
Mahul B. Amin
Employment: Consultants for Pathology & Laboratory Medicine
Stock or Other Ownership: OncoDx
Honoraria: The Doctors Company, Maximum Medical Solutions, International Scientific Symposiums
Consulting or Advisory Role: Foundation Medicine, Amgen
Patents, Royalties, Other Intellectual Property: Wolters Kluwer, Elsevier
Alexander G. Balogh
Honoraria: Seattle Genetics, Spectrum Pharmaceuticals, Amgen/Xofigo
Daniel M. Chinn
No relationship to disclose
Michael J. Seider
No relationship to disclose
Marie Duclos
No relationship to disclose
Seth A. Rosenthal
No relationship to disclose
Glenn S. Bauman
Research Funding: BiPar/sanofi-aventis
Elizabeth M. Gore
No relationship to disclose
Marvin Z. Rotman
No relationship to disclose
Himanshu R. Lukka
Honoraria: Abbott Laboratories, AstraZeneca, Ferring, Paladin, BiPar/sanofi-aventis
Consulting or Advisory Role: Abbott Laboratories, AstraZeneca, Ferring, Paladin, BiPar/sanofi-aventis
Research Funding: Abbott Laboratories, AstraZeneca, Paladin, BiPar/sanofi-aventis
Other Relationship: Abbott Laboratories, AstraZeneca, Paladin, BiPar/sanofi-aventis
William U. Shipley
Stock or Other Ownership: Pfizer
James J. Dignam
No relationship to disclose
Howard M. Sandler
Consulting or Advisory Role: Medivation-Astellas, AstraZeneca, Janssen Pharmaceuticals, Bayer, Eviti
Research Funding: Myriad Genetics
Other Relationship: Caribou Publishing
REFERENCES
- 1.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: Long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of Radiation Therapy Oncology Group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 4.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 5.Lawton CA, DeSilvio M, Roach M, 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: Updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. doi: 10.1016/j.ijrobp.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zietman AL, Nakfoor BM, Prince EA, et al. The effect of androgen deprivation and radiation therapy on an androgen-sensitive murine tumor: An in vitro and in vivo study. Cancer J Sci Am. 1997;3:31–36. [PubMed] [Google Scholar]
- 7.Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC Cancer Staging Manual. ed 5. Philadelphia, PA: Lippincott-Raven Publishers; 1997. Prostate; pp. 219–224. [Google Scholar]
- 8.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 9.American Society for Therapeutic Radiology and Oncology Consensus Panel. Consensus statement: Guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 10.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 12.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 13.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 14.Lan KKG, Simon R, Halperin M. Stochastically curtailed tests in long-term clinical trials theory. Commun Stat Simul C. 1982;1:207–219. [Google Scholar]
- 15.Green S, Benedetti J, Crowley J. Clinical Trials in Oncology. London, United Kingdom: Chapman and Hall/CRC; 2002. [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 18.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: Prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 21.Ganz PA, Barry JM, Burke W, et al. National Institutes of Health State-of-the-Science Conference: Role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012;156:591–595. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen PL, Chen MH, Beard CJ, et al. Radiation with or without 6 months of androgen suppression therapy in intermediate- and high-risk clinically localized prostate cancer: A postrandomization analysis by risk group. Int J Radiat Oncol Biol Phys. 2010;77:1046–1052. doi: 10.1016/j.ijrobp.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 23.Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12:451–459. doi: 10.1016/S1470-2045(11)70063-8. [DOI] [PubMed] [Google Scholar]
- 24.D'Amico AV, Chen MH, Crook J, et al. Duration of short-course androgen suppression therapy and the risk of death as a result of prostate cancer. J Clin Oncol. 2011;29:4682–4687. doi: 10.1200/JCO.2011.37.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong JG, Gillham CM, Dunne MT, et al. A randomized trial (Irish Clinical Oncology Research Group 97-01) comparing short versus protracted neoadjuvant hormonal therapy before radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:35–45. doi: 10.1016/j.ijrobp.2010.04.065. [DOI] [PubMed] [Google Scholar]
- 26.Crook J, Ludgate C, Malone S, et al. Final report of multicenter Canadian phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:327–333. doi: 10.1016/j.ijrobp.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 27.Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 28.Castle KO, Hoffman KE, Levy LB, et al. Is androgen deprivation therapy necessary in all intermediate-risk prostate cancer patients treated in the dose escalation era? Int J Radiat Oncol Biol Phys. 2013;85:693–699. doi: 10.1016/j.ijrobp.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues G, Lukka H, Warde P, et al. The prostate cancer risk stratification (ProCaRS) project: Recursive partitioning risk stratification analysis. Radiother Oncol. 2013;109:204–210. doi: 10.1016/j.radonc.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]