Abstract
Introduction
Extracellular matrix proteins play a crucial role in influencing the invasion of trophoblast cells. However the role of collagens and collagen type IV (col-IV) in particular at the implantation site is not clear.
Methods
Immunohistochemistry was used to determine the distribution of collagen types I, III, IV and VI in endometrium and decidua during the menstrual cycle and the first trimester of pregnancy. Expression of col-IV alpha chains during the reproductive cycle was determined by qPCR and protein localisation by immunohistochemistry. The structure of col-IV in placenta was examined using transmission electron microscopy. Finally, the expression of col-IV alpha chain NC1 domains and collagen receptors was localised by immunohistochemistry.
Results
Col-IV alpha chains were selectively up-regulated during the menstrual cycle and decidualisation. Primary extravillous trophoblast cells express collagen receptors and secrete col-IV in vitro and in vivo, resulting in the increased levels found in decidua basalis compared to decidua parietalis. A novel expression pattern of col-IV in the mesenchyme of placental villi, as a three-dimensional network, was found. NC1 domains of col-IV alpha chains are known to regulate tumour cell migration and the selective expression of these domains in decidua basalis compared to decidua parietalis was determined.
Discussion
Col-IV is expressed as novel forms in the placenta. These findings suggest that col-IV not only represents a structural protein providing tissue integrity but also influences the invasive behaviour of trophoblast cells at the implantation site.
Keywords: Alpha(IV) NC1 domains, Extracellular matrix, Placental bed, Reproductive cycle, Trophoblast
Highlights
-
•
Our data suggest that progesterone might regulate collagen type IV.
-
•
Primary extravillous trophoblast cells secrete collagen type IV.
-
•
A novel three-dimensional network of collagen type IV in placenta is presented.
-
•
Trophoblast cells express integrin alpha 10, integrin alpha 11 and collagen receptors DDR-1 and DDR-2.
1. Introduction
The cycling endometrium undergoes extensive tissue remodelling in preparation for the implantation of the blastocyst. This process, known as decidualisation, occurs in response to changing levels of estrogen and progesterone [1], [2]. After embryo attachment, trophoblast, derived from trophectoderm, differentiates into two cellular layers. Villous cytotrophoblast cells form the inner layer surrounding the placental villi and these fuse to form overlying syncytiotrophoblast. Proliferation of villous cytotrophoblast results in cell columns, which attach to the mucosal lining and invade decidual tissue as extravillous trophoblast cells (EVT) [3], [4]. These play a key role in early placentation by remodelling maternal spiral arteries to ensure sufficient blood flow to the developing fetus [5]. Insufficient trophoblast invasion during early pregnancy is associated with pregnancy complications such as pre-eclampsia, fetal growth restriction and recurrent miscarriage [6], [7]. Interactions of components of decidua such as the extracellular matrix (ECM) are known to regulate the correct degree of EVT invasion [3], [8].
Decidualisation and trophoblast invasion are accompanied by extensive remodelling of the ECM. Collagens are a major component of the ECM scaffold, regulating cellular behaviour and determining the structural integrity of human tissue. To date, the collagen family comprises 28 members, each assembling into supramolecular structures to form fibrils (collagen types I and III), networks (collagen type IV) or beaded filaments (collagen type VI) [9].
Collagen type IV (col-IV) is abundant in the lamina densa of all basement membranes. Identified as the first non-fibre-forming collagen, it provides structural support regulating adhesion, migration and survival of cells [10]. Unlike other collagen types, the polypeptide chains of human col-IV are encoded by six different genes (COL4A1, COL4A2, COL4A3, COL4A4, COL4A5 and COL4A6) [11], [12], [13]. The polypeptide chains consist of a cysteine- and lysine-rich 7S domain at the N-terminus, a collagenous domain and a C-terminal, glomerular non collagenous (NC1) domain [14]. The NC1 domain facilitates the assembly of three polypeptide chains into trimers that further assemble into multidomains via their NC1 and 7S domains eventually giving rise to a two-dimensional network organised in sheets. Networks of col-IV can be composed of three distinct heterotrimeric molecules: [(α1(IV))2α2(IV)], [α3(IV)α4(IV)α5(IV)] and [(α5(IV))2α6(IV)] of which [(α1(IV))2α2(IV)] is the predominant form present in most embryonic and adult basement membranes [15]. There is increasing evidence that NC1 domains of collagen molecules play important roles in cell migration, proliferation and apoptosis [16], [17], [18]. Although col-IV NCI domains might also affect the invasion of trophoblast, their expression in the placental bed is not yet known.
The effects of ECM on cell behaviour are mediated by receptors that specifically bind ECM components. At least six different groups of collagen receptors have been identified: Integrins, discoidin domain receptors (DDR), leukocyte-associated immunoglobulin like receptors (LAIR), glycoprotein VI (GPVI), mannose-receptor family and osteoclast-associated receptor (OSCAR) [19]. Among these collagen receptors only the integrin heterodimers α1β1 and α2β1 and soluble receptor LAIR-2 are known to be expressed by trophoblast [20], [21], [22]. LAIR-2 is exclusively expressed by invading trophoblast cells and is significantly down-regulated in chorionic villous samples from women who subsequently develop pre-eclampsia [23], [24]. Integrins alpha 10 (α10) and alpha 11 (α11) and collagen receptors DDR-1 and DDR-2 that also bind to col-IV and fibril-forming collagens are linked with the progression of various tumours via mediating cell migration [25], [26], [27], [28]. The expression of these collagen receptors at the fetal–maternal interface has not been described.
We have now studied the distribution of collagen types I, III, IV and VI during the endometrial cycle and in the first trimester of pregnancy. Alpha(IV) chains are significantly up-regulated in secretory compared to proliferative endometrium. In vitro studies also revealed secretion of col-IV by invading EVT resulting in elevated levels of this collagen type at the implantation site compared to decidua parietalis. We show a novel expression pattern and the three-dimensional structure of this molecule in placenta. Finally, the expression of col-IV NC1 domains and collagen receptors in trophoblast is described. This study suggests that col-IV does not simply function as a component of the basement membrane but also regulates the migration of trophoblast cells.
2. Methods
2.1. Tissue samples
Samples of first trimester placental and decidual tissue were taken from routine vaginal terminations of pregnancy (8–10 weeks gestation) as previously described (King et al., 1989). Ethical approval for placenta and decidua was obtained from the Cambridgeshire 2 Research Committee (reference no. 04/Q0108/23). For quantitative real time PCR samples of proliferative and secretory endometrium were collected at the University of Edinburgh. All patients provided written informed consent and ethical approval was obtained from the Research Ethical Committee (reference no. REC 07/S1103/29 and REC 10/S1402/59).
Human adult kidney was used as a positive control for antibodies against NC1 domains of alpha(IV) chains. Decidual and villous tissue fragments (1 cm2) were snap frozen in O.C.T. (VWR chemicals) in liquid nitrogen. For paraffin-embedded sections, tissue samples were embedded in 4% paraformaldehyde (PFA) overnight. Tissue sections of 5 μm and 100 μm were cut and stored at −20 °C (frozen sections) or room temperature (RT, paraffin-embedded sections) until required for immunohistology.
2.2. Isolation of primary trophoblast cells
Trophoblast cells were isolated from placental samples from normal pregnancies between 8 and 10 weeks of gestation using our well-established protocol. Following overnight culture of the isolated cells on fibronectin coated wells, this typically yields cultures containing 70–90% HLA-G+ trophoblast as determined by flow cytometry [29]. To determine collagen production, EVT were re-plated onto uncoated BD Falcon cultures slides (BD, Becton Dickinson) and cultured for 48 h in Ham's F12 medium (Biosera) supplemented with 20% fetal calf serum (FCS), 2 mM L-glutamine, 10units/ml penicillin and 100 μg/ml streptomycin and 2 mg/ml gentamycin.
2.3. Immunohistochemistry
Frozen tissue sections were fixed in acetone for 5 min before staining. Paraffin-embedded sections were de-paraffinized, dehydrated in a series of decreasing concentrations of ethanol and re-hydrated in phosphate buffered saline (PBS). Incubation with Proteinase K (Dako) was used for antigen retrieval on paraffin-embedded sections to detect collagen IV. Non-specific binding was blocked by incubating sections with 2.5% serum of the species in which the secondary antibody was raised. Sections were incubated with mouse antibodies to human collagen I (COL-I, Abcam), collagen III (FH-7A, Abcam), collagen IV (CIV-22, Abcam), collagen VI (3C4, Abcam), cytokeratin 7 (OV-TL 12/30, Dako), HLA-G (G233-216, Quantum Biosystems) and isotype control mouse IgG1 or mouse IgG2a (Biolegend and R&D, respectively) for 30 min. These anti collagen antibodies have previously been shown to recognize only the native triple helical forms of each of these collagens [30].
For the detection of collagen receptors, paraffin-embedded tissue sections were subjected to heat-induced epitope retrieval (HIER) in either citrate or high pH TRIS buffer using a pressure cooker (Menarini Diagnostics). Following blocking as above, sections were incubated with anti-human integrin α10 (Millipore), anti-human integrin α11 (Sigma), anti-human DDR-1 and DDR-2 (clones N1N3 and N2N3, respectively, Genetex) and isotype control rabbit IgG (Sigma). Protein expression was detected with biotinylated secondary antibodies followed by incubation with streptavidin–biotin peroxidise complex (ABC reagent – Vector Laboratories) for 30 min. Peroxidase was visualized using 3′diaminobenzidine (DAB, Sigma). Slides were counterstained in Carazzi's haematoxylin. Images were taken with Leica DC500 using the Leica IM50 software.
The intensity of collagen immunostaining in tissue sections was quantified using five representative view fields of each slide and calculating the area of staining as a percentage of the total area using a macro written in ImageJ (1.46r).
2.4. Immunofluorescence staining
Cultured EVT were fixed with 4% PFA for 15 min at RT. Fixed cells were blocked with 1% fetal bovine serum and 2% normal goat serum in PBS. Following incubation with fluorescein isothiocyanate-labelled anti HLA-G (MEMG9-FITC, Serotec) overnight at 4 °C, fixed cells and slides were incubated with anti collagen IV for 2 h at RT. Alexa Fluor 568-conjugated secondary antibody together with DAPI (Sigma) were applied for 1 h. Slides were mounted in Vectashield (Hard Set mounting medium, Vector Laboratories). To determine the structure of col-IV in placenta and fetal amniotic membrane, sections of 100 μm were cut and stained as above. All other sections stained were 8 μm. To stain for alpha1-alpha6(IV) NC1 domains, antigen retrieval was performed on frozen tissue sections by incubating sections with 6 M urea, 0.1 M glycine, pH 3.3 for 30 min before blocking. Sections were incubated with rat anti-human alpha1 – 6(IV) NC1 (clones H11, H21, H31, H43, H52, H63, Chondrex, Inc.) followed by incubation with Alexa Fluor 568- or FITC-conjugated secondary antibody together with DAPI for 1 h and samples were mounted in Vectashield. The anti NC1 domain antibodies recognise the cryptic NC1 domains of the single alpha(IV) chain. The specificity and characterization of these antibodies was shown previously [31], [32], [33]. Adult kidney was used as positive control tissue. Staining for the collagen receptors was carried out by incubating sections with MEMG9-FITC as described above, followed by the incubation with anti DDR-1 (R&D) and anti DDR-2 (R&D) overnight at 4 °C. Sections were then incubated with Alexa Fluor 568-conjugated secondary antibody to visualise DDR-1 or DDR-2 immunoreactivity and mounted with DAPI as above. Images and z-stacks were acquired using a Zeiss LSM 700 confocal microscope. Unless stated otherwise, all fluorescence images represent individual optical sections.
2.5. Transmission electron microscopy
Placental biopsies were fixed in 4% glutaraldehyde in 0.1 M hydroxyethyl piperazineethanesulfonic acid (HEPES) buffer at pH 7.4 for 12h at 4 °C. The tissue was rinsed in 0.1 M HEPES buffer followed by treatment with 1% osmium ferricyanide at RT for 2 h. Tissue was rinsed in water and treated with 2% uranyl acetate in 0.05 M maleate buffer at pH 5.5 for 2 h at RT. Subsequently tissue was rinsed in water and dehydrated in an ascending series of ethanol solutions from 70% to 100%, followed by treatment with two changes of dry acetonitrile and infiltration with Quetol 651 epoxy resin. Images were taken in an FEI Tecnai G2 operated at 120 kV using an AMT XR60B digital camera running Deben software.
2.6. Quantitative real-time RT-PCR
Ten proliferative endometrium, 13 secretory endometrium and 7 decidual samples were used to investigate the transcript levels of alpha1-6(IV) chains. These samples were dated as mid proliferative and mid secretory using Noyes' criteria and confirmed as consistent with the participant's reported last menstrual period and serum levels of progesterone and estradiol collected at the time of biopsy.
The samples were flash frozen in liquid nitrogen and total RNA was extracted using Qiagen RNeasy Kit according to manufacturer's protocol. RNA samples were reverse transcribed to cDNA with the SuperScript VILO cDNA synthesis kit (Life Technologies, Invitrogen) using a mix of oligo(dT) and random hexamer primers according to manufacturer's instructions. qPCR experiments were carried out within three months after the synthesis of cDNA. Primers were designed using Geneious (Biomatters Ltd.) and the Primer-Blast program (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Melting temperatures and potential formation of secondary structures were tested using OligoCalc (http://www.basic.northwestern.edu/biotools/OligoCalc.html). The primer specificity was confirmed by sequencing PCR products. Validated sequences are shown in Table 1. RT-PCR was carried out using Fast SYBR green mastermix and run on an ABI PRISM 7700 sequence detection system according to the manufacturer's instructions (Applied Biosystems, Warrington, UK) with following cycle conditions: 95 °C 20 s (1 cycle), 95 °C 3 s and 60 °C 30 s (40 cycles). A dissociation curve was derived at the end of each run to check specificity. Controls lacking template or reverse transcriptase were included to ensure no foreign or genomic DNA contamination during preparations. Reference genes hRPL-19 and 18s rRNA were used to normalise for the relative mRNA levels between samples. The normalisation factor was gained by calculating the arithmetic mean of the relative quantities of both reference genes [34]. Samples were run in duplicates and standard curves for each target were prepared from pooled decidual samples. qPCR data was analysed using the Kruskal–Wallis and Dunn's multiple comparisons test.
Table 1.
Primer sequences used for RT-PCR.
| Gene | Accession number | Primer sequence |
|
|---|---|---|---|
| Sense 5′–3′ | Antisense 5′–3′ | ||
| COL4A1 | NM_001845.4 | GTG CTG TGT GTG AGG CGC | GCC GAT CCA CAG CGA GGA |
| COL4A2 | NM_001846.2 | GCC CAG AGA GCC CAG CAA G | CAG TCC CAC TTA GCC TCG G |
| COL4A3 | NM_000091.4 | TCC CAG GAA GAC AAG GCG C | GGC ACC TGG GAA ACC TGG A |
| COL4A4 | NM_000092.4 | TGA AGG GAA ATC CCG GTG TG | CAG GTG GCT CTA CCA ACA GG |
| COL4A5 | NM_000495.4 | GCC TGG GCT AAA GGG TCT AC | CAA ACC ACG GGT ACC TGG C |
| COL4A6 | NM_001847.2 | TTC GGG ATG CCT GGA ATG CC | GCT TTC TCT TGC CCC TCC AC |
| hRPL-19 | NM_000981.3 | CAA GCC TGT GAC GGT CCA TT | CTT CTC TGG CAT TCG GGC AT |
| 18s | NR_003286.2 | GCC TGC GGC TTA ATT TGA CTC | CAT GCC AGA GTC TCG TTC GTT |
3. Results
3.1. Col-IV expression is up-regulated during the menstrual cycle and early pregnancy
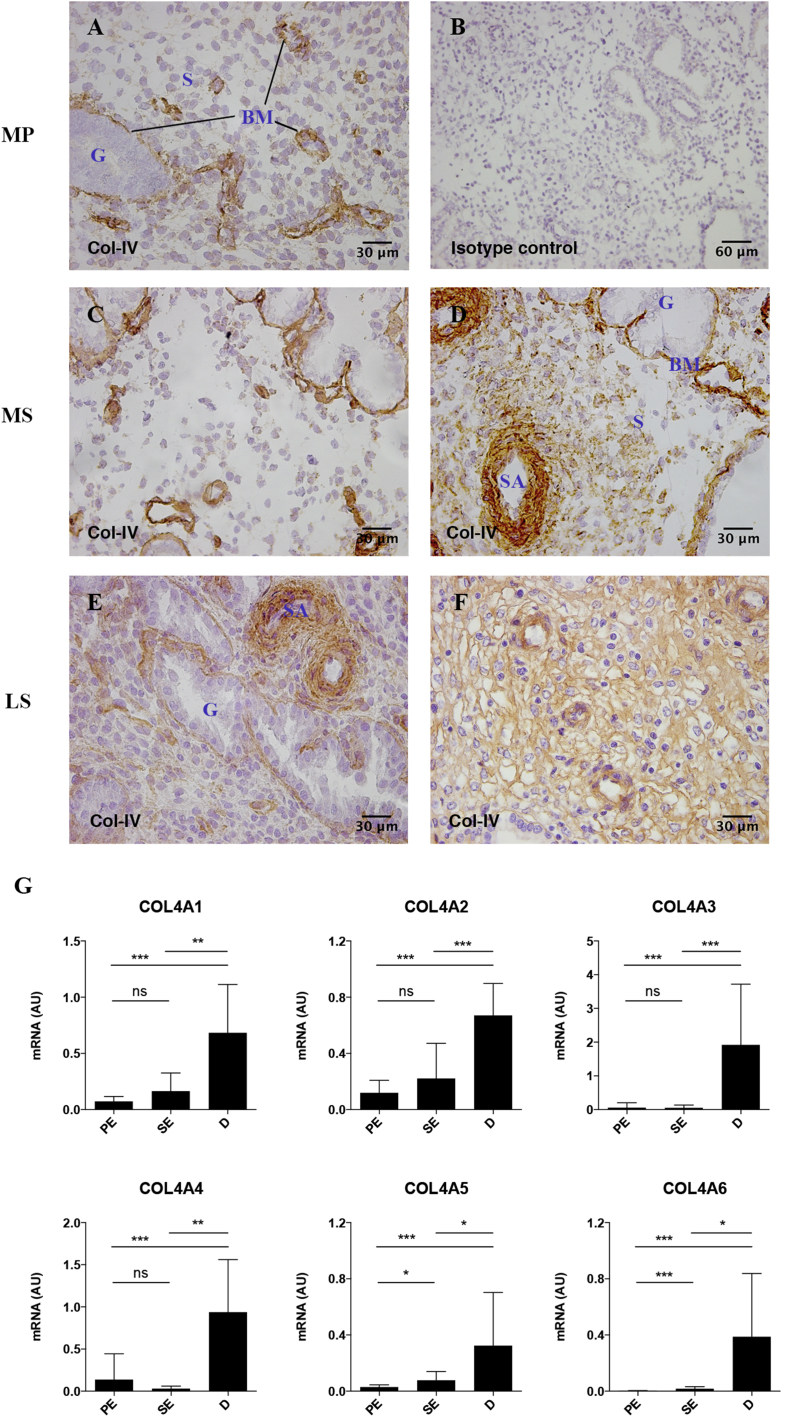
Immunohistochemistry was carried out to determine the localisation of collagen types I, III, IV and VI during the menstrual cycle and in the first trimester of pregnancy using antibodies detecting the native triple helical conformation of each collagen (Table 2). Collagen types I, III, and VI are widely distributed throughout the tissue (data not shown) with immunoreactivity in vessel walls, myometrium and stroma of decidua basalis and decidua parietalis and more intense staining in decidual compared to endometrial stroma. In contrast, strong col-IV immunoreactivity is confined to basement membranes in mid-proliferative and mid-secretory endometrium (Fig. 1). During the mid secretory phase, col-IV immunostaining also appears around arteries in the sheaths known as Streeter's columns where the first changes of decidualisation are seen (Fig. 1D). During the late secretory phase col-IV is more evenly distributed in the stroma appearing as pericellular staining around stromal cells (Fig. 1E, F).
Table 2.
Immunostaining of collagen types I, III, IV and VI within uterus and placenta.a
| Collagen type |
||||||
|---|---|---|---|---|---|---|
| I | III | IV | VI | |||
| Endometrium | Proliferative | Stroma | + | + | − | + |
| Basement membrane | − | − | + | − | ||
| Vessel wall | + | + | + | + | ||
| Myometrium | + | + | + | + | ||
| Secretory | Stroma | + | + | + | ++ | |
| Basement membrane | − | − | + | − | ||
| Vessel wall | + | + | ++ | + | ||
| Myometrium | + | + | + | + | ||
| Decidua | Basalis | Stroma | ++ | ++ | +++ | ++ |
| Vessel wall | + | + | ++ | + | ||
| Myometrium | + | + | + | + | ||
| Giant cell | − | − | + | − | ||
| Parietalis | Stroma | ++ | ++ | + | ++ | |
| Vessel wall | + | + | + | + | ||
| Myometrium | + | + | + | + | ||
| Placenta | Villous core | + | + | + | + | |
| Fetal vessel wall | + | + | + | + | ||
| Basement membrane | − | − | + | + | ||
| Cell column | − | − | + | − | ||
+ Present, ++ Strong, +++ Very strong, − Absent.
Fig. 1.
The amount of col-IV increases during decidualisation. A–F Endometrial tissue sections, n = 3 of each phase, were stained for col-IV using clone CIV-22. A, mid proliferative (MP); C,D, mid secretory (MS); E,F, late secretory (LS) phase. Panel B, shows mid-proliferative endometrium stained with isotype control and is representative of all immunohistochemical investigations in this study. BM – basement membrane, G – gland, S – stroma, SA – spiral artery. G Ten proliferative endometrium (PE), 13 secretory endometrium (SE) and seven decidual (D) samples were used to quantify relative levels of transcripts for COL4A1, COL4A2, COL4A3, COL4A4, COL4A5 and COL4A6 by real-time RT–PCR through the reproductive cycle. Samples were run in duplicate. Expression levels were measured relative to hRPL-19 and 18s. The graphs show mean expression levels in arbitrary units (AU). Mean ± SD, ***P < 0.0001, **P < 0.001, *P < 0.005, ns – not significant, determined using Kruskal–Wallis and Dunn's multiple comparisons test.
Since these results suggest the hormonal regulation of col-IV, quantitative real-time PCR was carried out for alpha1 – alpha6(IV) chains (Fig. 1G). COL4A5 and COL4A6 are significantly up-regulated in samples of mid secretory compared to mid proliferative endometrium and further increased in decidua. On the contrary, COL4A1, COL4A2, COL4A3 and COL4A4 are similar in mid secretory and mid proliferative endometrium but are significantly up-regulated in decidua compared to endometrium. COL4A6 and COL4A3 represent the most up-regulated transcripts in decidual vs. proliferative endometrial samples (131-fold and 35-fold, respectively). These results show that all alpha(IV) mRNAs are up-regulated between secretory endometrium and decidua and immunohistochemistry shows this is reflected in increased staining in the stroma and vessel walls.
3.2. Primary trophoblast cells secrete col-IV in vitro
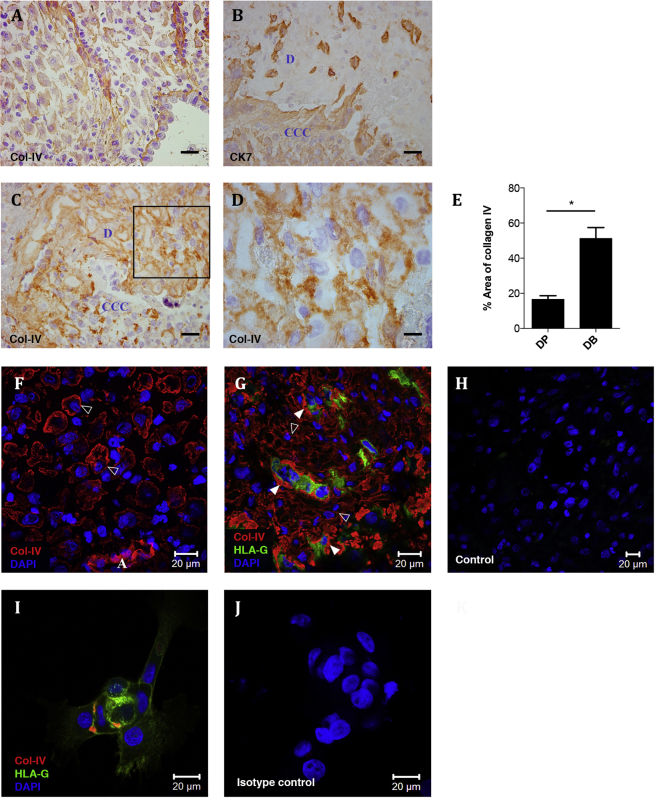
In decidua parietalis, native col-IV is present in basement membranes, vessel walls and around individual decidual stromal cells (Fig. 2A). In decidua basalis, characterised by the presence of invading trophoblast cells, even more intense staining for col-IV is noted (Fig. 2B–D). Quantification of stained tissue sections reveals a significant increase in the expression of col-IV in decidua basalis vs. parietalis (Fig. 2E). The expression pattern of native triple helical col-IV in decidua was studied in more detail using immunofluorescence with monoclonal antibody CIV-22 (Fig. 2F,G). In decidua parietalis col-IV immunostaining is pericellular around stromal cells, whilst in decidua basalis there is additional dense deposition around invading HLA-G + EVT (Fig. 2G). To confirm trophoblast do express col-IV, primary trophoblast isolated from first trimester placenta were cultured and stained for HLA-G and native col-IV (Fig. 2I). We conclude that HLA-G+ trophoblast cells secrete this collagen in vitro and contribute to the increased amount of col-IV found at the implantation site.
Fig. 2.
Col-IV is up-regulated at the implantation site and expressed by primary trophoblast cells in vitro. A–D Sections of decidua parietalis (A), n = 3, and serial sections of decidua basalis (B–D), n = 3, were stained for col-IV (A, C, D) and cytokeratin 7 (CK7). Image D) represents the box in C). Scale bars 30 μm. E Area of col-IV immunostaining in decidua basalis (DB) and decidua parietalis (DP) was quantified in ImageJ (n = 3 for each). Mean ± SD, *P < 0.001 determined using Welch's t test. F–J Confocal scanning microscopy images of decidua parietalis (F), decidua basalis (G,H) and isolated primary trophoblast cells (I,J) stained for col-IV (red), HLA-G (green) and DAPI (blue). mIgG1-FITC negative control (H) and isotype control for the anti-col-IV monoclonal antibody CIV-22, (J) show typical negative controls for immunofluorescence staining. White open arrowheads (F,G) show the expression of pericellular col-IV around individual stromal cells and white solid arrowheads show col-IV deposits around invading trophoblast cells (G). A – artery, CCC – cytotrophoblast cell column, D – decidua.
3.3. Distinctive col-IV structure in the placenta
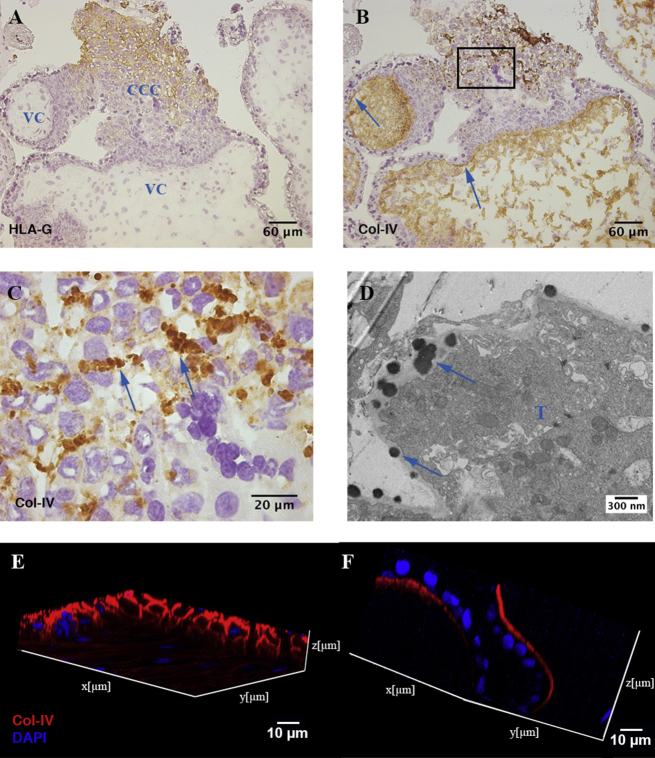
In placental villi, native col-IV is typically present in the basement membrane underlying villous trophoblast (Fig. 3B) but is also present in large amounts in the mesenchyme of the villous core (Fig. 3B) and as globules of intense staining in cytotrophoblast cell columns (CCC) (Fig. 3C). These globules are present between HLA-G+ EVT, located in the distal part of the cell columns. Transmission electron microscopy demonstrates the dense granular nature of these globules attached to a trophoblast cell within CCC (Fig. 3D). Confocal microscopy of 100 μm-thick tissue sections reveals the three-dimensional characteristics of col-IV in the villous mesenchyme compared to fetal membrane, respectively (Fig. 3E, F). In the villous mesenchyme col-IV forms a complex three-dimensional network encapsulating individual cells (Fig. 3E) while forming a thin sheet underlining the basement membrane in the amnion (Fig. 3F). There are therefore three different structural forms of col-IV in placental villi suggesting that these have different functions.
Fig. 3.
Expression and structure of col-IV in placenta. A–C Serial sections of first trimester placental villi, n = 9, were stained for HLA-G (A) and native col-IV (B, C). Arrows in B) show col-IV immunostaining in basement membranes. Image C) represents the box in B). Arrows in C) show globular col-IV in cytotrophoblast column. D Transmission electron microscopy image of globules (arrows) attached to a trophoblast cell (T) in cytotrophoblast cell columns. E, F Confocal microscopy of 100 μm-thick sections comparing z-stack of placental villous core (E) and fetal amniotic membrane (F) stained for col-IV (red) and DAPI (blue). Z-stack image of staining of serial sections with isotype-matched negative control antibodies showed no detectable staining (data not shown). VC – villous core, CCC – cytotrophoblast cell column.
3.4. The expression of collagen IV NC1 domains in the placental bed
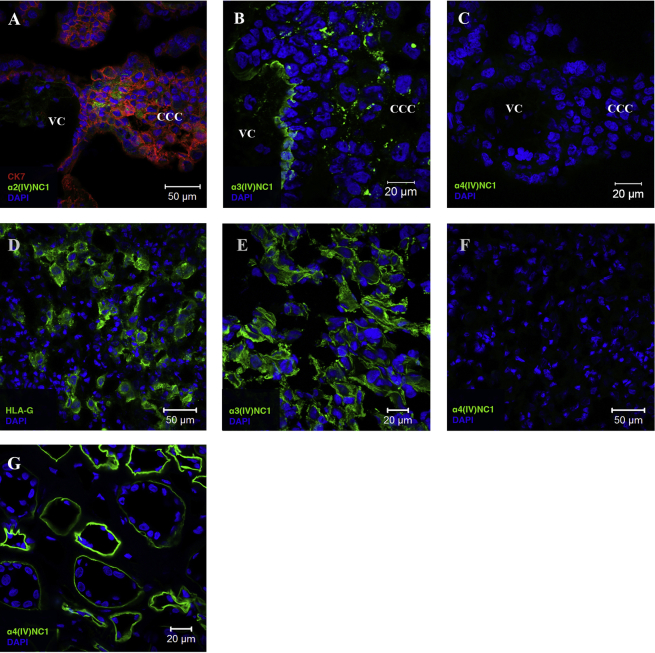
The C-terminus of col-IV chains (NC1 domains) play important roles in diminishing cell migration resulting in decreased tumour growth [17], [35], [36]. Since specific NC1 domains might have different effects on the migratory behaviour of trophoblast cells, we performed immunohistochemistry of the placental bed and decidua parietalis using antibodies specific to the different alpha(IV) NC1 domains (summarised in Table 3). In comparison to the monoclonal anti-col-IV antibody used above to detect the triple helical native conformation of col-IV, these antibodies are directed against the NC1 domains of single col-IV alpha chains. Representative pictures for presence and absence of alpha(IV) NC1 domains are depicted in Fig. 4.
Table 3.
Expression of alpha(IV)NC1 domains within the placental bed.a
| Col-IV NC1 domains |
||||||||
|---|---|---|---|---|---|---|---|---|
| Alpha1 | Alpha2 | Alpha3 | Alpha4 | Alpha5 | Alpha6 | |||
| Placenta | Villous core | + | + | + | − | + | −/+ | |
| Basement membrane | + | + | + | − | + | − | ||
| Cell column | + | + | + | − | + | − | ||
| Decidua | Basalis | Stroma | + | + | + | − | + | + |
| Vessel wall | + | + | + | − | + | + | ||
| Parietalis | Stromal cells | + | + | − | − | + | − | |
| Vessel wall | + | + | − | − | + | − | ||
+ Present, − Absent.
Fig. 4.
Expression of alpha(IV) NC1 domains in the placental bed. Adjacent sections of placenta (A–C), n = 3, decidua basalis (D–F), n = 2, and human adult kidney as positive control for alpha4(IV)NC1 (G), n = 1, were stained for cytokeratin 7 (red) and alpha2(IV)NC1 (A) (green), alpha3(IV)NC1 (B, E) (green), alpha4(IV)NC1 (C, F, G) (green) and HLA-G (D) (green). Cell nuclei were stained with DAPI (blue). VC – villous core, CCC – cytotrophoblast cell column.
The NC1 domains from alpha1(IV), alpha2(IV), alpha3(IV) and alpha5(IV) chains are present in the villous mesenchyme, basement membranes of villi and CCC of the placenta. Alpha4(IV) NC1 domain is absent from the placental bed. Some villi were devoid of alpha6(IV) NC1 immunoreactivity while others stained weakly. Alpha1-3(IV), alpha5(IV) and alpha6(IV) NC1 domains are present in decidua basalis. In contrast, only alpha1(IV), alpha2(IV) and alpha5(IV) NC1 domains are present in decidua parietalis. There is therefore a selective presence of collagen type IV NC1 domains in the placental bed.
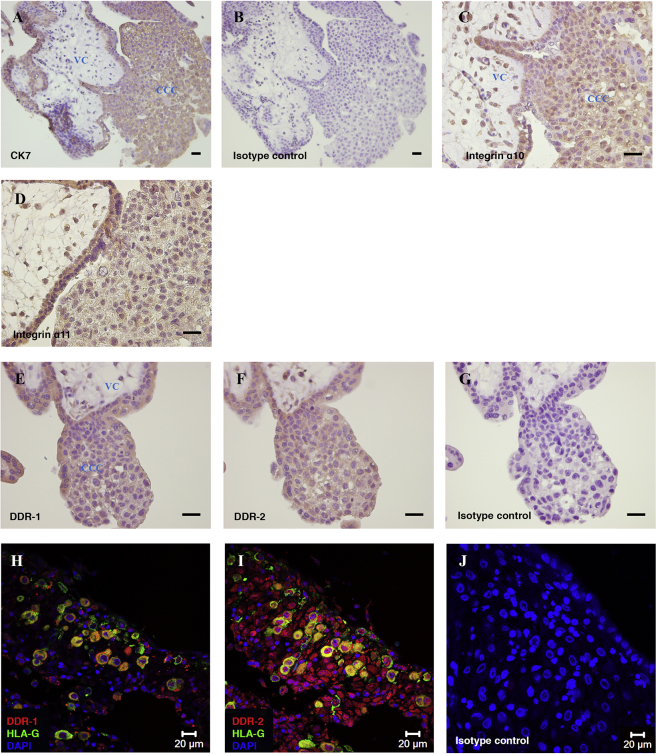
3.5. Expression of collagen receptors by trophoblast
High-affinity binding sites in collagen are recognised by receptors such as integrins which play an important role in mediating trophoblast invasion [8], [37]. Since the expression of integrins α10, α11 and collagen receptors DDR-1 and DDR-2 by trophoblast is not yet known, we examined their localisation by immunohistochemistry (Fig. 5). All these molecules are expressed by all trophoblast types. Cells within the villous core also stain positive.
Fig. 5.
Expression of collagen receptors in placenta and implantation site. Serial sections of first trimester placental villi, n = 3, were stained for cytokeratin 7 (A), isotype control (B and G), integrin α10 (C), integrin α11 (D), DDR-1 (E) and DDR-2 (F). Scale bars 30 μm. Serial sections of first trimester implantation site, n = 2, were stained for DDR-1 (red) and HLA-G (green) (H), DDR-2 (red) and HLA-G (green) (I) and isotype control (J). Cell nuclei were stained with DAPI (blue). VC – villous core, CCC – cytotrophoblast cell column.
4. Discussion
In both trophoblast invasion and cancer metastasis the extent of invasion depends on the intrinsic invasiveness of the cells and their surrounding microenvironment [38], [39], [40], [41], [42]. Implantation in humans is unique in that it is characterised by the onset of decidualisation in non-pregnant endometrium and the intrinsic invasive potential of trophoblast. Hence, successful placentation is determined by factors of both maternal and fetal origin.
Decidualisation is characterised by dramatic tissue remodelling involving differentiation of glands, stroma, immune cells and blood vessels [1]. This process is dependent on progesterone and begins during the secretory phase where the Streeter's columns surround spiral arteries. We now show col-IV appears first in this decidualising sheath and in the media of arterial walls, the focus of trophoblast cell invasion and remodelling of the arteries [5], [43]. Quantitative qPCR demonstrated selective up-regulation of mRNAs encoding alpha5(IV) and alpha6(IV) in mid secretory endometrium, suggesting they are regulated by progesterone supported by previous observation [44]. There is a further change from secretory endometrium to decidua when all six col-IV alpha chains were however up-regulated.
Col-IV is always considered as a protein specifically localised to the basal lamina [45], [46]. We found immunostaining for col-IV at unusual sites (both fetal and maternal) outside the basement membrane including the placental villous core as previously described [47], [48]. However, in comparison to the well-known two-dimensional structure of col-IV in basement membranes we report a novel three-dimensional structure in the mesenchyme of placental villi [14], [49]. The high abundance and novel ‘network’ structure of col-IV in the villous core is likely to be of functional importance. Furthermore we found col-IV immunostaining around EVT and decidual stromal cells. The unusual globular immunoreactivity around invading EVT is reminiscent of reports from cancer cells and has also been described in HLA-G + EVT in the distal part of placental cell columns [20], [50], [51], [52], [53], [54]. These globules have a granular appearance and are localised to the surface of trophoblast cells. Other reports describe these globules to be composed of col-IV and laminin [55], [56]. Since we have demonstrated secretion of col-IV by primary HLA-G+ trophoblast cells in vitro we conclude that EVT in the columns as well as invading into decidua basalis secrete this collagen. Interestingly, granules of col-IV and laminin appear significantly less around EVT in tubal pregnancy, possibly associated with uncontrolled invasion of trophoblast cells at extra-vs. intra-uterine sites [57]. Although the role of these globules is not clear, neutralising antibodies to col-IV do block trophoblast invasion in vitro [8]. Thus, maternal ECM (decidua) as well as ECM secreted by fetal trophoblasts themselves might mediate the migratory behaviour of these invasive cells [58].
Cleavage of collagen fibrils can produce short NC1 domains that act directly on target cells to regulate their migration [35], [36], [59], [60]. In our study alpha1-3(IV) and alpha5(IV) domains are present in CCC. Since EVT at the tip of CCC express integrin αvβ3, which binds to alpha2(IV) and alpha3(IV) NC1 domains, a direct interaction with trophoblast is possible [59], [61], [62]. The collagen XVIII NC1 domain (endostatin) is known to increase MMP-2 expression in trophoblast cells and the alpha(IV) NC1 domains might similarly affect MMP expression by EVT [56], [63]. In decidua, although we demonstrated the presence of all col-IV alpha chains, there was selective immunostaining of NC1 domains. The alpha4(IV) NC1 domain is absent and alpha3(IV) and alpha6(IV) NC1 domains appear only in decidua basalis. EVT express MMP-2 and MMP-9 [56], [64], [65] which both cleave alpha3(IV) NC1 domain [56], [64], [65], [66]. Like alpha6(IV), the alpha3(IV) domain, named tumstatin, exhibits anti-angiogenic functions and inhibits the proliferation and invasion of human melanoma cells in vitro [17], [35], [36], [67]. Alpha3(IV) NC1 domain is recognised by integrin α6β1 expressed by EVT [20], [68]. Thus, trophoblast cells might selectively degrade alpha(IV) chains leading to the exposure of alpha3(IV) and alpha6(IV) but not alpha4(IV) NC1 domains at the implantation site. This might function as a self-regulatory mechanism mediating a balanced trophoblast invasion.
Trophoblast ECM receptors have been studied previously but not the expression of integrins α10 and α11 and collagen receptors DDR-1 and DDR-2 [3], [20], [69]. These can bind to col-IV and are all linked with the progression of cancer [26], [27], [70], [71], [72], [73]. Interestingly, DDR-1-null mice are unable to produce offspring due to a lack of proper blastocyst implantation suggesting an important role for DDR-1 in implantation [74]. Since EVT in cell columns express integrin subunit β1 and integrins α10 and α11, as shown in this study, it is likely that trophoblast cells express the α10β1 and α11β1 heterodimers [3], [8]. Similar to the role of integrins and DDR receptors in cancer cell migration, the differential expression and activation of collagen receptors in combination with the presence of col-IV around trophoblast cells is likely to play a role in trophoblast invasion.
In conclusion, this study provides new knowledge on maternal and fetal factors that might affect trophoblast cell invasion. We describe a novel arrangement for col-IV in the form of a three-dimensional network in the villous mesenchyme. The secretion of globular forms by migrating trophoblast cells suggests that col-IV does not only represent a structural protein providing tissue integrity. The complex interplay of col-IV secreted by trophoblast cells, NC1 domains and their receptors might be involved in influencing the invasive behaviour of trophoblast cells at the implantation site. Further investigations are needed to reveal the role of these factors in mediating trophoblast cell invasion.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by funding from the Wellcome Trust [090108/Z/09/Z], [085992/Z/08/Z] and the British Heart Foundation [PG/09/077/27964]. C.M. Oefner was in receipt of a German National Academic Foundation PhD studentship. We thank Jeremy Skepper for technical assistance. The authors also thank the Centre for Trophoblast Research for generous support.
References
- 1.Deligdisch L. Hormonal pathology of the endometrium. Mod Pathol. 2000;13:285–294. doi: 10.1038/modpathol.3880050. [DOI] [PubMed] [Google Scholar]
- 2.Rodgers W.H., Matrisian L.M., Giudice L.C., Dsupin B., Cannon P., Svitek C., et al. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994;94:946–953. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrows T.D., King A., Loke Y.W. Expression of integrins by human trophoblast and differential adhesion to laminin or fibronectin. Hum Reprod. 1993;8:475–484. doi: 10.1093/oxfordjournals.humrep.a138075. [DOI] [PubMed] [Google Scholar]
- 4.Aplin J.D. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99(Pt 4):681–692. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- 5.Pijnenborg R., Bland J.M., Robertson W.B., Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- 6.Pijnenborg R., Anthony J., Davey D.A., Rees A., Tiltman A., Vercruysse L., et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 7.Ball E., Bulmer J.N., Ayis S., Lyall F., Robson S.C. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J Pathol. 2006;208:535–542. doi: 10.1002/path.1927. [DOI] [PubMed] [Google Scholar]
- 8.Damsky C.H., Librach C., Lim K.H., Fitzgerald M.L., McMaster M.T., Janatpour M., et al. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 9.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzbauer J. Basement membranes: putting up the barriers. Curr Biol CB. 1999;9:R242–R244. doi: 10.1016/s0960-9822(99)80153-5. [DOI] [PubMed] [Google Scholar]
- 11.Cutting G.R., Kazazian H.H., Antonarakis S.E., Killen P.D., Yamada Y., Francomano C.A. Macrorestriction mapping of COL4A1 and COL4A2 collagen genes on human chromosome 13q34. Genomics. 1988;3:256–263. doi: 10.1016/0888-7543(88)90086-9. [DOI] [PubMed] [Google Scholar]
- 12.Momota R., Sugimoto M., Oohashi T., Kigasawa K., Yoshioka H., Ninomiya Y. Two genes, COL4A3 and COL4A4 coding for the human alpha3(IV) and alpha4(IV) collagen chains are arranged head-to-head on chromosome 2q36. FEBS Lett. 1998;424:11–16. doi: 10.1016/s0014-5793(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto M., Oohashi T., Ninomiya Y. The genes COL4A5 and COL4A6, coding for basement membrane collagen chains alpha 5(IV) and alpha 6(IV), are located head-to-head in close proximity on human chromosome Xq22 and COL4A6 is transcribed from two alternative promoters. Proc Natl Acad Sci U S A. 1994;91:11679–11683. doi: 10.1073/pnas.91.24.11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timpl R., Wiedemann H., Van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Fed Eur Biochem Soc J. 1981;120:203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- 15.Gelse K., Pöschl E., Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Sudhakar A., Boosani C.S. Inhibition of tumor angiogenesis by tumstatin: insights into signaling mechanisms and implications in cancer regression. Pharm Res. 2008;25:2731–2739. doi: 10.1007/s11095-008-9634-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Brassart-Pasco S., Sénéchal K., Thevenard J., Ramont L., Devy J., Di Stefano L., et al. Tetrastatin, the NC1 domain of the α4(IV) collagen chain: a novel potent anti-tumor matrikine. PloS One. 2012;7:e29587. doi: 10.1371/journal.pone.0029587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortega N., Werb Z. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci. 2002;115:4201–4214. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leitinger B., Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Damsky C.H., Fitzgerald M.L., Fisher S.J. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebbink R.J., van den Berg M.C., de Ruiter T., Raynal N., van Roon J.A., Lenting P.J., et al. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J Immunol. 2008;180:1662–1669. doi: 10.4049/jimmunol.180.3.1662. [DOI] [PubMed] [Google Scholar]
- 22.Apps R., Sharkey A., Gardner L., Male V., Trotter M., Miller N., et al. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta. 2011;32:33–43. doi: 10.1016/j.placenta.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Founds S.A., Conley Y.P., Lyons-Weiler J.F., Jeyabalan A., Hogge W.A., Conrad K.P. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Founds S.A., Fallert-Junecko B., Reinhart T.A., Conley Y.P., Parks W.T. LAIR2 localizes specifically to sites of extravillous trophoblast invasion. Placenta. 2010;31:880–885. doi: 10.1016/j.placenta.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Wenke A.-K., Kjellman C., Lundgren-Akerlund E., Uhlmann C., Haass N.K., Herlyn M., et al. Expression of integrin alpha 10 is induced in malignant melanoma. Cell Oncol Off J Int Soc Cell Oncol. 2007;29:373–386. doi: 10.1155/2007/601497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu C.-Q., Popova S.N., Brown E.R.S., Barsyte-Lovejoy D., Navab R., Shih W., et al. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc Natl Acad Sci U S A. 2007;104:11754–11759. doi: 10.1073/pnas.0703040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K., Corsa C.A., Ponik S.M., Prior J.L., Piwnica-Worms D., Eliceiri K.W., et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H.-G., Hwang S.-Y., Aaronson S.A., Mandinova A., Lee S.W. DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J Biol Chem. 2011;286:17672–17681. doi: 10.1074/jbc.M111.236612. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Male V., Trundley A., Gardner L., Northfield J., Chang C., Apps R., et al. Natural killer cells in human pregnancy. Methods Mol Biol. 2010;612:447–463. doi: 10.1007/978-1-60761-362-6_30. [DOI] [PubMed] [Google Scholar]
- 30.Odermatt B.F., Lang A.B., Rüttner J.R., Winterhalter K.H., Trüeb B. Monoclonal antibodies to human type IV collagen: useful reagents to demonstrate the heterotrimeric nature of the molecule. Proc Natl Acad Sci U S A. 1984;81:7343–7347. doi: 10.1073/pnas.81.23.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sado Y., Kagawa M., Kishiro Y., Sugihara K., Naito I., Seyer J.M., et al. Establishment by the rat lymph node method of epitope-defined monoclonal antibodies recognizing the six different alpha chains of human type IV collagen. Histochem Cell Biol. 1995;104:267–275. doi: 10.1007/BF01464322. [DOI] [PubMed] [Google Scholar]
- 32.Kagawa M., Kishiro Y., Naito I., Nemoto T., Nakanishi H., Ninomiya Y., et al. Epitope-defined monoclonal antibodies against type-IV collagen for diagnosis of Alport's syndrome. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc – Eur Ren Assoc. 1997;12:1238–1241. doi: 10.1093/ndt/12.6.1238. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya Y., Kagawa M., Iyama K., Naito I., Kishiro Y., Seyer J.M., et al. Differential expression of two basement membrane collagen genes, COL4A6 and COL4A5, demonstrated by immunofluorescence staining using peptide-specific monoclonal antibodies. J Cell Biol. 1995;130:1219–1229. doi: 10.1083/jcb.130.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasco S., Han J., Gillery P., Cells T., Borel J.P., Kefalides N.A., et al. A specific sequence of the noncollagenous domain of the α 3 (IV) chain of type IV collagen inhibits expression and activation of matrix metalloproteinases by tumor cells a specific sequence of the noncollagenous domain of the α 3 (IV) chain of type IV. Cancer Res. 2000;60:467–473. [PubMed] [Google Scholar]
- 36.Mundel T.M., Yliniemi A.-M., Maeshima Y., Sugimoto H., Kieran M., Kalluri R. Type IV collagen alpha6 chain-derived noncollagenous domain 1 (alpha6(IV)NC1) inhibits angiogenesis and tumor growth. Int J Cancer. 2008;122:1738–1744. doi: 10.1002/ijc.23269. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y., Damsky C.H., Fisher S.J. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J., Weinberg R.A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Vićovac L., Aplin J.D. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat. 1996;156:202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- 40.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 41.Lockwood C.J., Oner C., Uz Y.H., Kayisli U.A., Huang S.J., Buchwalder L.F., et al. Matrix metalloproteinase 9 (MMP9) expression in preeclamptic decidua and MMP9 induction by tumor necrosis factor alpha and interleukin 1 beta in human first trimester decidual cells. Biol Reprod. 2008;78:1064–1072. doi: 10.1095/biolreprod.107.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedl P., Maaser K., Klein C.E., Niggemann B., Krohne G., Zänker K.S. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- 43.Aplin J.D., Charlton A.K., Ayad S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res. 1988;253:231–240. doi: 10.1007/BF00221758. [DOI] [PubMed] [Google Scholar]
- 44.Catalano R.D., Critchley H.O., Heikinheimo O., Baird D.T., Hapangama D., Sherwin J.R.A., et al. Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in human endometrium. Mol Hum Reprod. 2007;13:641–654. doi: 10.1093/molehr/gam021. [DOI] [PubMed] [Google Scholar]
- 45.Timpl R., Dziadek M. Structure, development, and molecular pathology of basement membranes. Int Rev Exp Pathol. 1986;29:1–112. [PubMed] [Google Scholar]
- 46.Martin G.R., Timpl R., Kuhn K. Basement membrane proteins: molecular structure and function. Adv Protein Chem. 1988;39:1–50. doi: 10.1016/s0065-3233(08)60374-5. [DOI] [PubMed] [Google Scholar]
- 47.Rukosuev V.S. Immunofluorescent localization of collagen types I, III, IV, V, fibronectin, laminin, entactin, and heparan sulphate proteoglycan in human immature placenta. Experientia. 1992;48:285–287. doi: 10.1007/BF01930477. [DOI] [PubMed] [Google Scholar]
- 48.Chen C.-P., Aplin J.D. Placental extracellular matrix: gene expression, deposition by placental fibroblasts and the effect of oxygen. Placenta. 2003;24:316–325. doi: 10.1053/plac.2002.0904. [DOI] [PubMed] [Google Scholar]
- 49.Khoshnoodi J., Pedchenko V., Hudson B.G. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kondo T., Nakazawa T., Terada N., Nakazawa K., Kawasaki T., Mochizuki K., et al. Unusual thyroid carcinoma with excessive extracellular hyaline globules: a case of “hyalinizing papillary carcinoma”. Hum Pathol. 2012;43:932–938. doi: 10.1016/j.humpath.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Gatalica Z., Miettinen M., Kovatich A., McCue P.A. Hyaline globules in renal cell carcinomas and oncocytomas. Hum Pathol. 1997;28:400–403. doi: 10.1016/s0046-8177(97)90026-5. [DOI] [PubMed] [Google Scholar]
- 52.Nucci M., Esther O. 1st ed. Elsevier Inc.; Boston: 2009. Gynecologic pathology. [Google Scholar]
- 53.Vicovac L., Jones C.J., Aplin J.D. Trophoblast differentiation during formation of anchoring villi in a model of the early human placenta in vitro. Placenta. 1995;16:41–56. doi: 10.1016/0143-4004(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez P.L., Merino M.J., Nogales F.F., Charonis A.S., Stetler-Stevenson W., Liotta L. Immunohistochemical profile of basement membrane proteins and 72 kilodalton type IV collagenase in the implantation placental site. An integrated view. Lab Invest J Tech Methods Pathol. 1992;66:572–579. [PubMed] [Google Scholar]
- 55.Huppertz B., Kertschanska S., Frank H.G., Gaus G., Funayama H., Kaufmann P. Extracellular matrix components of the placental extravillous trophoblast: immunocytochemistry and ultrastructural distribution. Histochem Cell Biol. 1996;106:291–301. doi: 10.1007/BF02473239. [DOI] [PubMed] [Google Scholar]
- 56.Huppertz B., Kertschanska S., Demir A.Y., Frank H.G., Kaufmann P. Immunohistochemistry of matrix metalloproteinases (MMP), their substrates, and their inhibitors (TIMP) during trophoblast invasion in the human placenta. Cell Tissue Res. 1998;291:133–148. doi: 10.1007/s004410050987. [DOI] [PubMed] [Google Scholar]
- 57.Kemp B., Kertschanska S., Kadyrov M., Rath W., Kaufmann P., Huppertz B. Invasive depth of extravillous trophoblast correlates with cellular phenotype: a comparison of intra- and extrauterine implantation sites. Histochem Cell Biol. 2002;117:401–414. doi: 10.1007/s00418-002-0396-0. [DOI] [PubMed] [Google Scholar]
- 58.Korhonen M., Virtanen I. The distribution of laminins and fibronectins is modulated during extravillous trophoblastic cell differentiation and decidual cell response to invasion in the human placenta. J Histochem Cytochem Off J Histochem Soc. 1997;45:569–581. doi: 10.1177/002215549704500409. [DOI] [PubMed] [Google Scholar]
- 59.Kamphaus G.D., Colorado P.C., Panka D.J., Hopfer H., Ramchandran R., Torre A., et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 60.Maeshima Y., Sudhakar A., Lively J.C., Ueki K., Kharbanda S., Kahn C.R., et al. Tumstatin, an endothelial cell-specific inhibitor of protein synthesis. Science (New York, NY) 2002;295:140–143. doi: 10.1126/science.1065298. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Y., Fisher S.J., Janatpour M., Genbacev O., Dejana E., Wheelock M., et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maeshima Y., Colorado P.C., Kalluri R. Two RGD-independent alpha vbeta 3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J Biol Chem. 2000;275:23745–23750. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
- 63.Cohen M., Meisser A., Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27:783–793. doi: 10.1016/j.placenta.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Burrows T.D., King A., Loke Y.W. Trophoblast migration during human placental implantation. Hum Reprod Update. 1996;2:307–321. doi: 10.1093/humupd/2.4.307. [DOI] [PubMed] [Google Scholar]
- 65.Xu P., Wang Y., Piao Y., Bai S., Xiao Z., Jia Y., et al. Effects of matrix proteins on the expression of matrix metalloproteinase-2, -9, and -14 and tissue inhibitors of metalloproteinases in human cytotrophoblast cells during the first trimester. Biol Reprod. 2001;65:240–246. doi: 10.1095/biolreprod65.1.240. [DOI] [PubMed] [Google Scholar]
- 66.Hamano Y., Zeisberg M., Sugimoto H., Lively J.C., Maeshima Y., Yang C., et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colorado P.C., Torre A., Kamphaus G., Maeshima Y., Hopfer H., Takahashi K., et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- 68.Mundel T.M., Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74:85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aplin J.D. Expression of integrin alpha 6 beta 4 in human trophoblast and its loss from extravillous cells. Placenta. 1993;14:203–215. doi: 10.1016/s0143-4004(05)80261-9. [DOI] [PubMed] [Google Scholar]
- 70.Engel B.E., Welsh E., Emmons M.F., Santiago-Cardona P.G., Cress W.D. Expression of integrin alpha 10 is transcriptionally activated by pRb in mouse osteoblasts and is downregulated in multiple solid tumors. Cell Death Dis. 2013;4:e938. doi: 10.1038/cddis.2013.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heinzelmann-Schwarz V.A., Gardiner-Garden M., Henshall S.M., Scurry J., Scolyer R.A., Davies M.J., et al. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2004;10:4427–4436. doi: 10.1158/1078-0432.CCR-04-0073. [DOI] [PubMed] [Google Scholar]
- 72.Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 73.Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem. 2003;278:16761–16769. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- 74.Vogel W.F., Aszódi A., Alves F., Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]