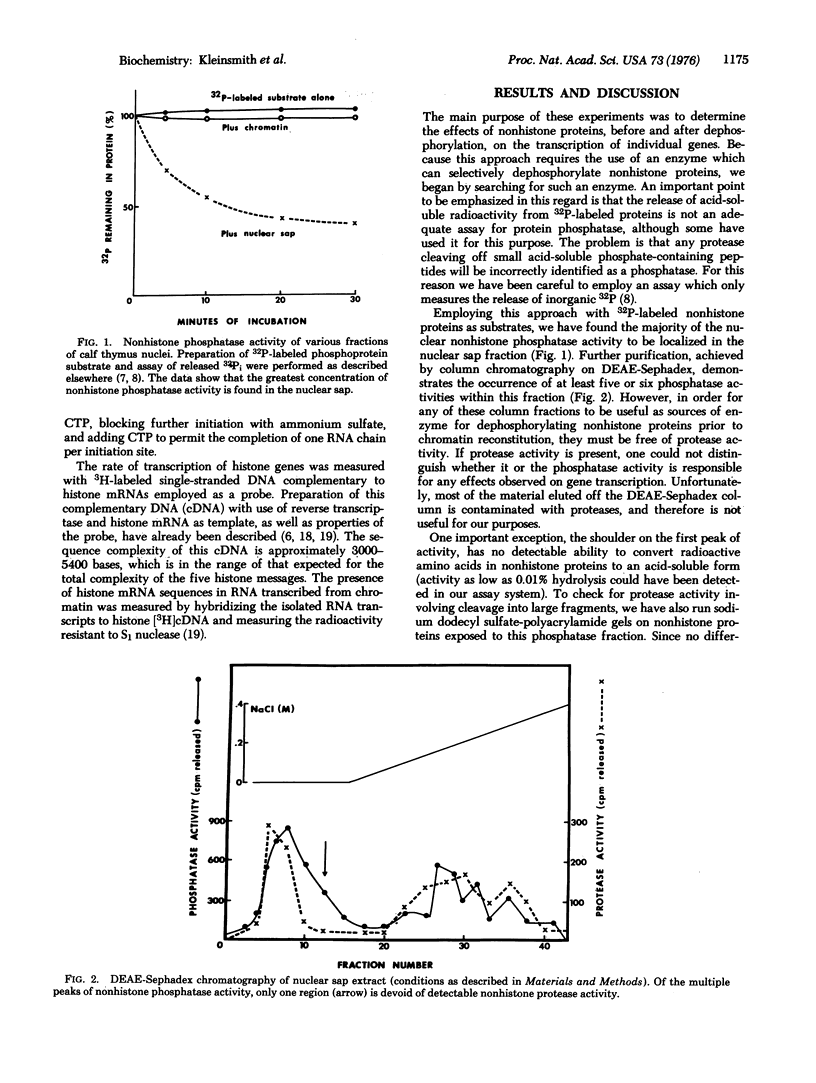
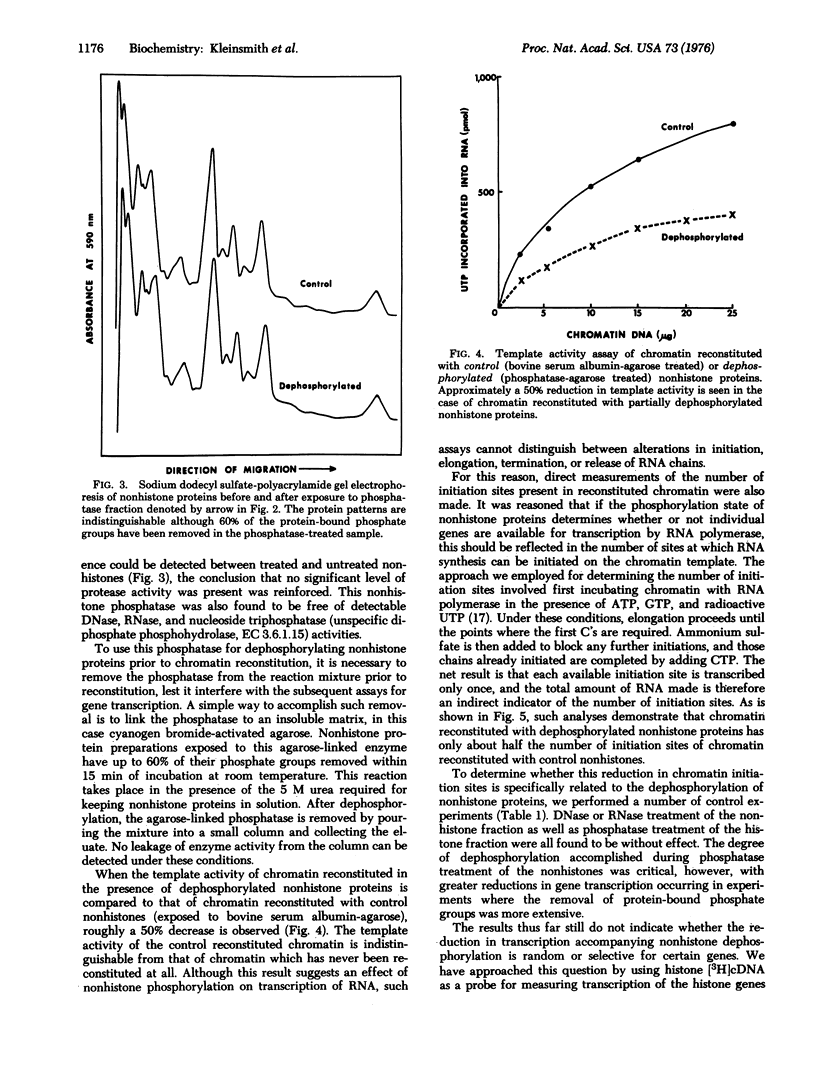
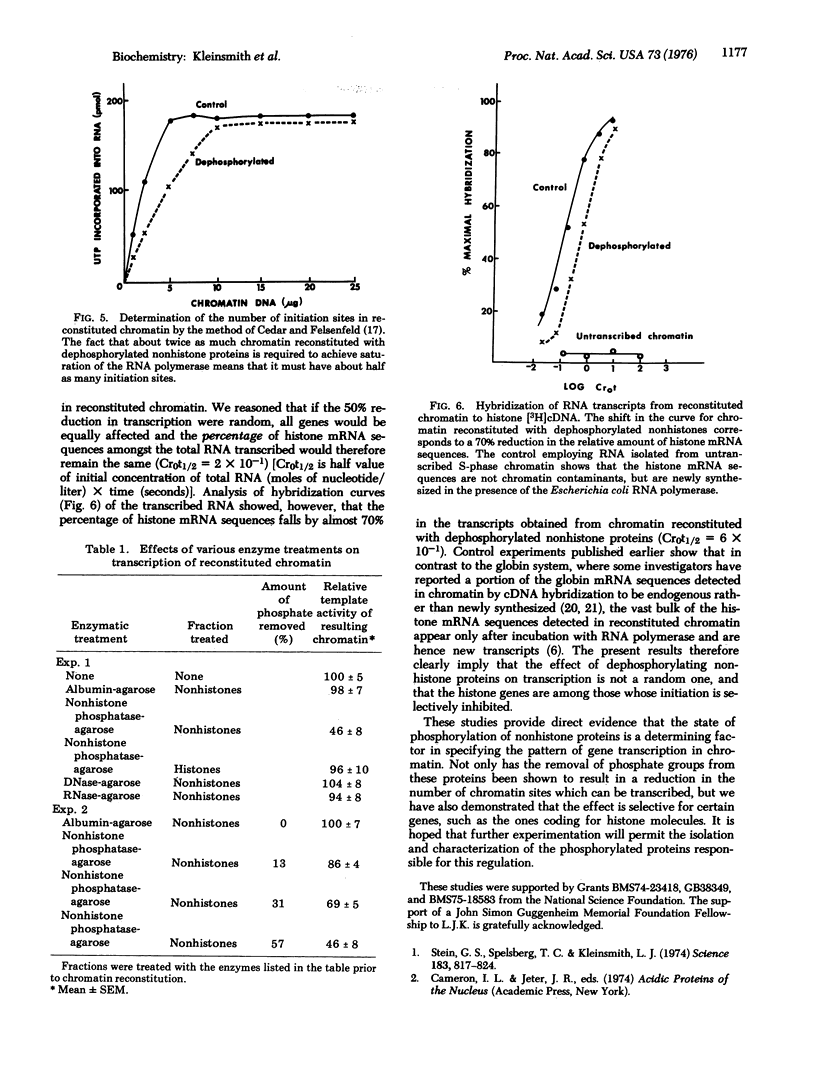
Abstract
A nonhistone phosphatase devoid of detectable protease activity has been purified from nuclear sap by chromatography on DEAE-Sephadex. After linkage to an insoluble agarose matrix, this enzyme was used to dephosphorylate nonhistone proteins obtained from S-phase HeLa cells. Chromatin reconstituted with these dephosphorylated proteins exhibited roughly a 50% reduction in the overall number of initiation sites available for transcription when compared to controls. Specific measurements of transcription of histone genes with use of a complementary DNA probe showed that genes coding for histones are preferentially inhibited after nonhistone dephosphorylation. These results provide the first direct support for the theory that phosphorylation of nonhistone proteins is involved in regulating the availability of individual genes for transcription.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekhor I., Kung G. M., Bonner J. Sequence-specific interaction of DNA and chromosomal protein. J Mol Biol. 1969 Jan;39(2):351–364. doi: 10.1016/0022-2836(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Cedar H., Felsenfeld G. Transcription of chromatin in vitro. J Mol Biol. 1973 Jun 25;77(2):237–254. doi: 10.1016/0022-2836(73)90334-3. [DOI] [PubMed] [Google Scholar]
- Chae C. B., Carter D. B. Degradation of chromosomal proteins during dissociation and reconstitution of chromatin. Biochem Biophys Res Commun. 1974 Apr 8;57(3):740–746. doi: 10.1016/0006-291x(74)90608-1. [DOI] [PubMed] [Google Scholar]
- Chae C. B., Godski R. A., Carter D. B., Efird P. H. Integrity of proteins in reconstituted chromatin. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1459–1465. doi: 10.1016/0006-291x(75)90190-4. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Role of non-histone components in determining organ specificity of rabbit chromatins. FEBS Lett. 1970 Aug 17;9(4):242–244. doi: 10.1016/0014-5793(70)80366-0. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G. Nuclear phosphoproteins. I. Isolation and characterization of a phosphoprotein fraction from calf thymus nuclei. Biochim Biophys Acta. 1969 Feb 4;175(1):123–135. [PubMed] [Google Scholar]
- Kleinsmith L. J. Phosphorylation of non-histone proteins in the regulation of chromosome structure and function. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):459–475. doi: 10.1002/jcp.1040850412. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Mans R. J., Gabbay E. J., Stein J. L., Davis J., Adawadkar P. D. Evidence for fidelity of chromatin reconstitution. Biochemistry. 1975 May 6;14(9):1859–1866. doi: 10.1021/bi00680a009. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Park W. D., Thrall C. L., Mans R. J., Stein J. L. Cell cycle stage-specific transcription of histone genes. Biochem Biophys Res Commun. 1975 Apr 21;63(4):945–949. doi: 10.1016/0006-291x(75)90660-9. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Stein G., Farber J. Role of nonhistone chromosomal proteins in the restriction of mitotic chromatin template activity. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2918–2921. doi: 10.1073/pnas.69.10.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G., Park W., Thrall C., Mans R., Stein J. Regulation of cell cycle stage-specific transcription of histone genes from chromatin by non-histone chromosomal proteins. Nature. 1975 Oct 30;257(5529):764–767. doi: 10.1038/257764a0. [DOI] [PubMed] [Google Scholar]
- Thrall C. L., Park W. D., Rashba H. W., Stein J. L., Mans R. J., Stein G. S. In vitro synthesis of DNA complementary to polyadenylated histone messenger RNA. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1443–1449. doi: 10.1016/s0006-291x(74)80445-6. [DOI] [PubMed] [Google Scholar]
- Wilson G. N., Steggles A. W., Kantor J. A., Nienhuis A. W., Anderson W. F. Cell-free transcription of mammalian chromatin. Quantitative measurement of newly synthesized globin messenger RNA sequences. J Biol Chem. 1975 Nov 25;250(22):8604–8613. [PubMed] [Google Scholar]



