Abstract
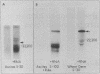
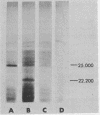
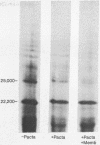
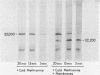
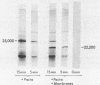
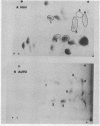
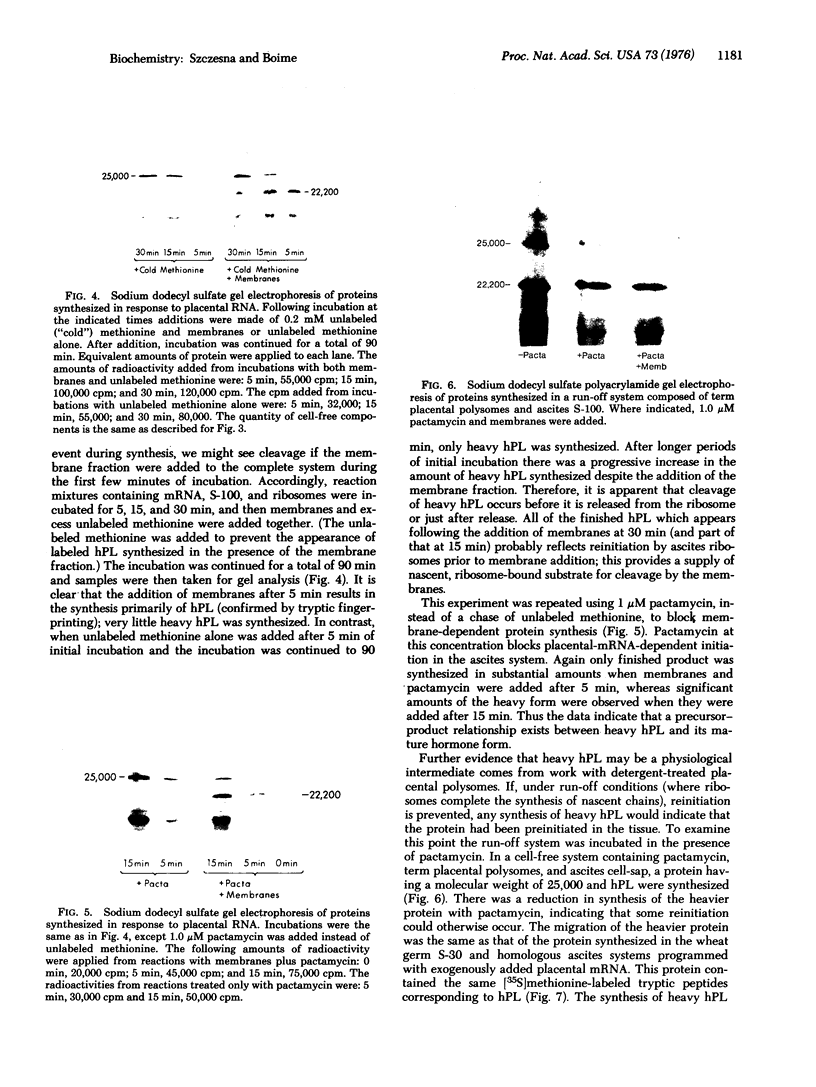
Messenger RNA derived from term placenta directs the synthesis of human placental lactogen (hPL, molecular weight 22,200) in an ascites 30,000 X g post-mitochondrial supernate (S-30). When the S-30 is fractionated into ribosome and cell-sap (S-100) fractions, and these are recombined for incubation, term placental mRNA directs the synthesis of a protein with a molecular weight of 25,000. This protein contains authentic hPL tryptic peptides. This suggested that during the separation of ribosomes and S-100 a component responsible for cleavage was lost. A 1.0 M sucrose cushion was used for the preparation of ribosomes and S-100 and membranous material accumulated at the sucrose interphase. When this membrane fraction was added back to the ribosome-S-100 system only hPl was formed. Cleavage was greatest when membranes were added within the first few minutes of incubation. In a run-off system composed of term polysomes, ascites S-100, and the inhibitor of initiation, pactamycin, the 25,000 molecular weight material, referred to as pre-hPL, was also synthesized. These data strongly suggest that (i) pre-hPL is an authentic percursor to hPL, (ii) cleavage of the precursor primarily occurs on nascent, ribosome-bound peptide chains, and (iii) pre-hPL is the primary gene product.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J. The vectorial release of nascent immunoglobulin peptides. Biochem J. 1971 Mar;122(1):5–11. doi: 10.1042/bj1220005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., Boguslawski S., Caine J. The translation of a human placental lactogen mRNA fraction in heterologous cell-free systems: the synthesis of a possible precursor. Biochem Biophys Res Commun. 1975 Jan 6;62(1):103–109. doi: 10.1016/s0006-291x(75)80411-6. [DOI] [PubMed] [Google Scholar]
- Boime I., Boguslawski S. The synthesis of human placental lactogen by ribosomes derived from human placenta. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1322–1325. doi: 10.1073/pnas.71.4.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boime I., McWilliams D., Szczesna E., Camel M. Synthesis of human placental lactogen messenger RNA as a function of gestation. J Biol Chem. 1976 Feb 10;251(3):820–825. [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Gene order of encephalomyocarditis virus as determined by studies with pactamycin. J Virol. 1972 May;9(5):823–828. doi: 10.1128/jvi.9.5.823-828.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison T. M., Brownlee G. G., Milstein C. Studies on polysome-membrane interactions in mouse myeloma cells. Eur J Biochem. 1974 Sep 16;47(3):613–620. doi: 10.1111/j.1432-1033.1974.tb03733.x. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Mulligan R. C., Potts J. T., Jr, Rich A. Pre-proparathyroid hormone: a direct translation product of parathyroid messenger RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3731–3735. doi: 10.1073/pnas.71.9.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Identification of a viral protein involved in post-translational maturation of the encephalomyocarditis virus capsid precursor. J Virol. 1975 Apr;15(4):918–928. doi: 10.1128/jvi.15.4.918-928.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Siekevitz P., Palade G. E. Synthesis and transfer of amylase in pigeon pancreatic micromosomes. J Biol Chem. 1966 Mar 10;241(5):1150–1158. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Blobel G. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. II. Location of the polypeptides in rough microsomes. J Cell Biol. 1970 Apr;45(1):146–157. doi: 10.1083/jcb.45.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Biologically and chemically pure mRNA coding for a mouse immunoglobulin L-chain prepared with the aid of antibodies and immobilized oligothymidine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2256–2260. doi: 10.1073/pnas.70.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood L. M., Handwerger S., McLaurin W. D., Lanner M. Amino-acid sequence of human placental lactogen. Nat New Biol. 1971 Sep 8;233(36):59–61. doi: 10.1038/newbio233059a0. [DOI] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]