Abstract
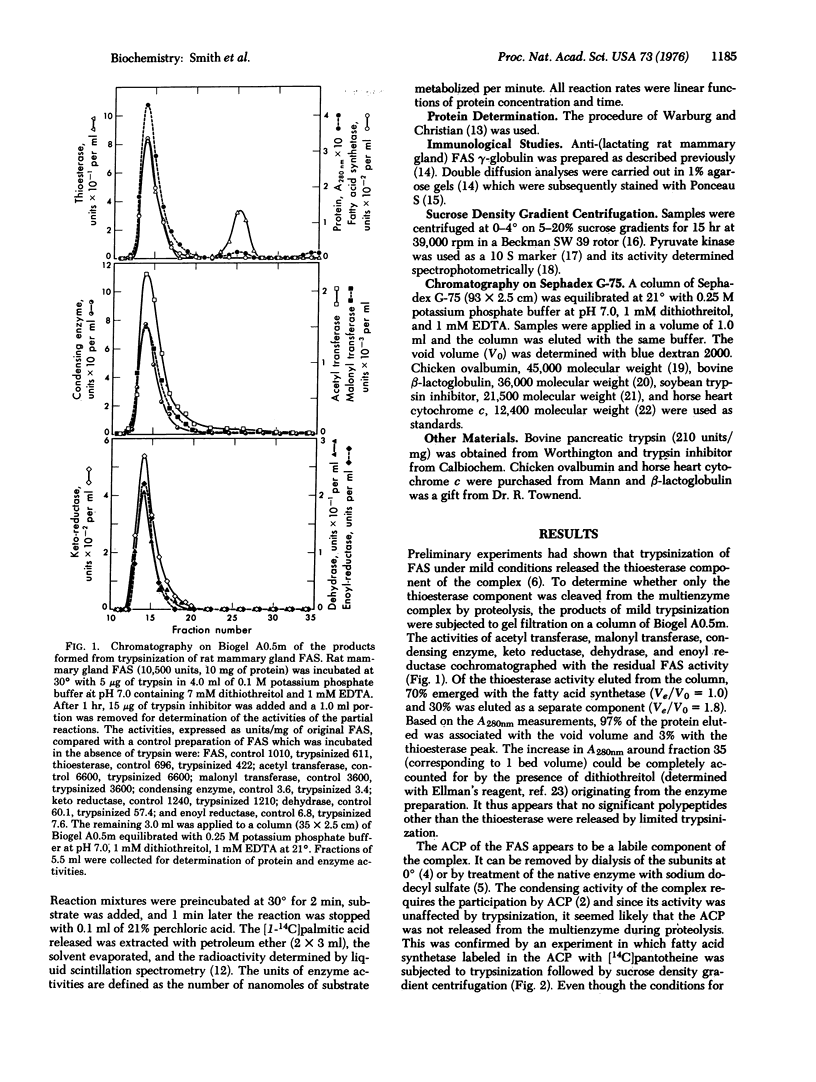
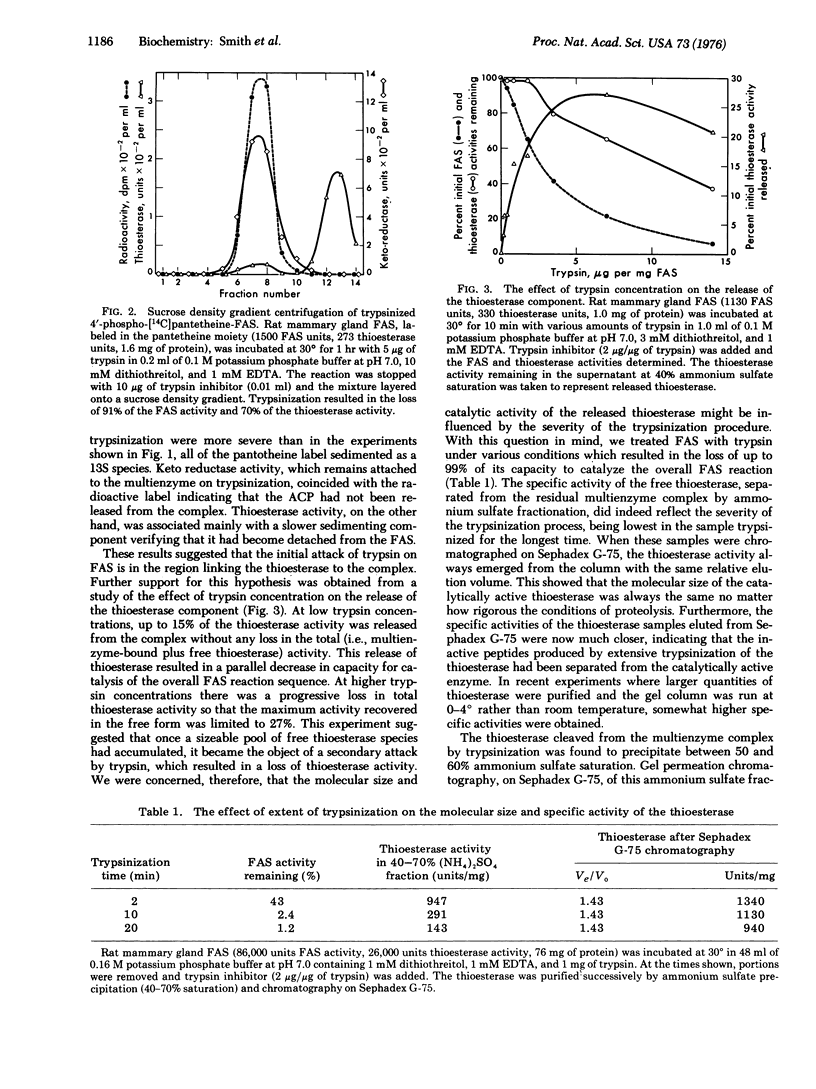
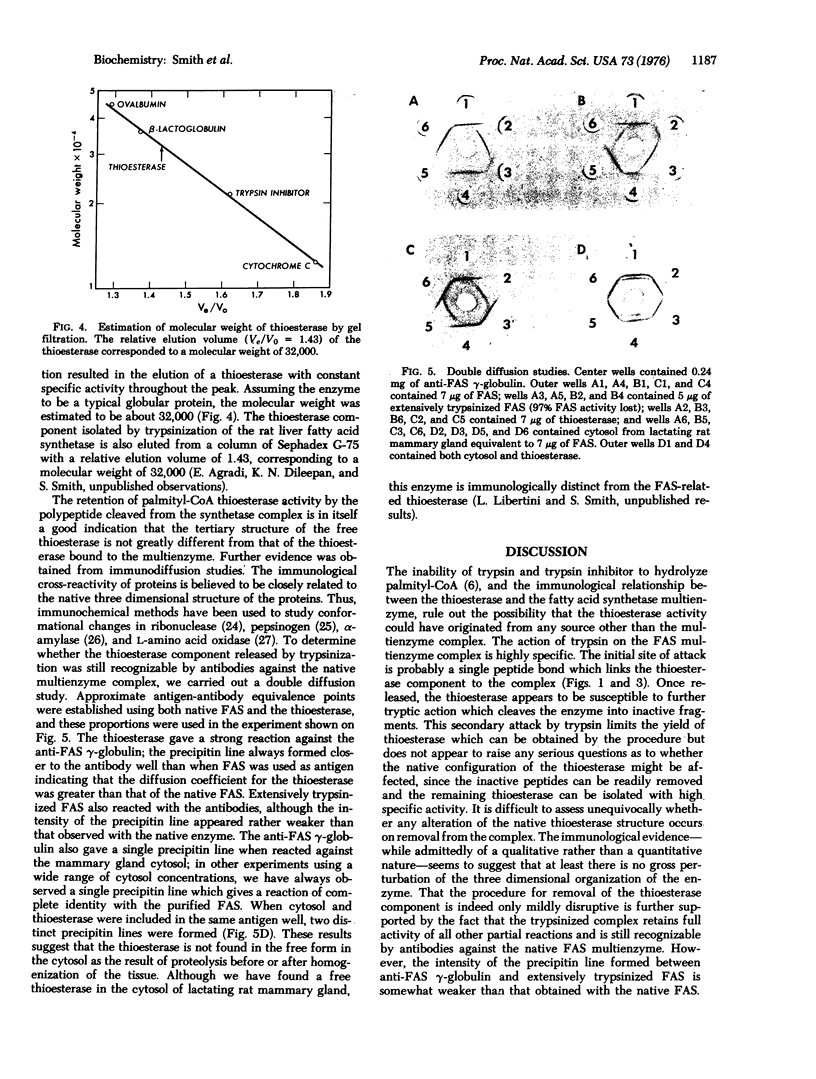
Limited trypsinization of the fatty acid synthetase multienzyme complex from rat mammary gland results in the release of a protein, molecular weight 32,000, with thioesterase activity. The other components of the multienzyme complex--the acyl carrier protein, acetyl and malonyl transferases, condensing enzyme, keto reductase, dehydrase and enoyl reductase--are not affected and remain associated with the complex. The thioesterase can be isolated by ammonium sulfate precipitation and gel filtration. Extensive trypsinization of fatty acid synthetase multienzyme complex results in a loss of thioesterase activity, probably due to cleavage of the thioesterase component into inactive peptides. However, the molecular weight and specific activity of the thioesterase isolated after limited trypsinization is relatively unaffected by the severity of the conditions of proteolysis. Both the thioesterase and the residual trypsinized complex react with antibodies produced against the native multienzyme. The results demonstrate that mild trypsinization can be used to release the thioesterase component of the multienzyme with little perturbation of either the thioesterase or the other components of the complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agradi E., Libertini L., Smith S. Specific modification of fatty acid synthetase from lactating rat mammary gland by chymotrypsin and trypsin. Biochem Biophys Res Commun. 1976 Feb 9;68(3):894–900. doi: 10.1016/0006-291x(76)91229-8. [DOI] [PubMed] [Google Scholar]
- Al-Arif A., Blecher M. Synthesis of fatty acyl CoA and other thiol esters using N-hydroxysuccinimide esters of fatty acids. J Lipid Res. 1969 May;10(3):344–345. [PubMed] [Google Scholar]
- Alberts A. W., Strauss A. W., Hennessy S., Vagelos P. R. Regulation of synthesis of hepatic fatty acid synthetase: binding of fatty acid synthetase antibodies to polysomes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3956–3960. doi: 10.1073/pnas.72.10.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN R. K., DELANEY R., LEVINE L., VAN VUNAKIS H. Studies on the antigenic structure of ribonuclease. I. General role of hydrogen and disulfide bonds. J Biol Chem. 1959 Aug;234(8):2043–2049. [PubMed] [Google Scholar]
- Barnes E. M., Jr, Wakil S. J. Studies on the mechanism of fatty acid synthesis. XIX. Preparation and general properties of palmityl thioesterase. J Biol Chem. 1968 Jun 10;243(11):2955–2962. [PubMed] [Google Scholar]
- Barnes E. M., Jr, Wakil S. J., Swindell A. C. Purification and properties of a palmityl thioesterase II from Escherichia coli. J Biol Chem. 1970 Jun;245(12):3122–3128. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GERSTEIN J. F., LEVINE L., VANVUNAKIS H. ALTERED ANTIGENICITY OF PEPSINOGEN AND PEPSIN AS AN INDEX OF CONFORMATIONAL CHANGE: EFFECT OF UREA AND REDUCING AGENTS. Immunochemistry. 1964 Apr;1:3–14. doi: 10.1016/0019-2791(64)90050-3. [DOI] [PubMed] [Google Scholar]
- Kass L. R., Brock D. J., Bloch K. Beta-hydroxydecanoyl thioester dehydrase. I. Purification and properties. J Biol Chem. 1967 Oct 10;242(19):4418–4431. [PubMed] [Google Scholar]
- Knudsen J., Clark S., Dils R. Acyl-CoA hydrolase(s) in rabbit mammary gland which control the chain length of fatty acids synthesised. Biochem Biophys Res Commun. 1975 Aug 4;65(3):921–926. doi: 10.1016/s0006-291x(75)80473-6. [DOI] [PubMed] [Google Scholar]
- Knudsen J., Dils R. Partial purification from rabbit mammary gland of a factor which controls the chain length of fatty acids synthesised. Biochem Biophys Res Commun. 1975 Apr 7;63(3):780–785. doi: 10.1016/s0006-291x(75)80451-7. [DOI] [PubMed] [Google Scholar]
- Kumar S., Dorsey J. A., Muesing R. A., Porter J. W. Comparative studies of the pigeon liver fatty acid synthetase complex and its subunits. Kinetics of partial reactions and the number of binding sites for acetyl and malonyl groups. J Biol Chem. 1970 Sep 25;245(18):4732–4744. [PubMed] [Google Scholar]
- Lornitzo F. A., Qureshi A. A., Porter J. W. Subunits of fatty acid synthetase complexes. Enzymatic activities and properties of the half-molecular weight nonidentical subunits of pigeon liver fatty acid synthetase. J Biol Chem. 1975 Jun 25;250(12):4520–4529. [PubMed] [Google Scholar]
- MARGOLIASH E., LUSTGARTEN J. Interconversion of horse heart cytochrome C monomer and polymers. J Biol Chem. 1962 Nov;237:3397–3405. [PubMed] [Google Scholar]
- Qureshi A. A., Lornitzo F. A., Porter J. W. The isolation of acyl carrier protein from the pigeon liver fatty acid synthetase complex1 II. Biochem Biophys Res Commun. 1974 Sep 9;60(1):158–165. doi: 10.1016/0006-291x(74)90186-7. [DOI] [PubMed] [Google Scholar]
- Ringle D. A., Herndon B. L. A micromanipulator-based system for immunologic analyses with microliter and submicroliter reactant volumes. J Immunol. 1965 Nov;95(5):966–979. [PubMed] [Google Scholar]
- Roncari D. A. Mammalian acyl carrier protein. Dissociation of the acyl carrier protein subunit from dog liver fatty acid synthetase complex. J Biol Chem. 1974 Nov 10;249(21):7035–7037. [PubMed] [Google Scholar]
- Sirisinha S., Allen P. Z. Immunochemical studies on alpha-amylase. I. Effects of denaturing agents and proteolytic enzymes on the immunochemical reactivity of alpha-amylase from Aspergillus oryzae. Arch Biochem Biophys. 1965 Oct;112(1):137–148. doi: 10.1016/0003-9861(65)90021-4. [DOI] [PubMed] [Google Scholar]
- Smith S., Abraham S. Fatty acid synthetase from lactating rat mammary gland. I. Isolation and properties. J Biol Chem. 1970 Jun;245(12):3209–3217. [PubMed] [Google Scholar]
- Smith S., Abraham S. Fatty acid synthetase from lactating rat mammary gland. II. Studies on the termination sequence. J Biol Chem. 1971 Apr 25;246(8):2537–2542. [PubMed] [Google Scholar]
- Smith S. Lipogenesis in rabbit adipose tissue. J Lipid Res. 1975 Jul;16(4):324–331. [PubMed] [Google Scholar]
- Smith S. Studies on the immunological cross-reactivity and physical properties of fatty acid synthetases. Arch Biochem Biophys. 1973 Jun;156(2):751–758. doi: 10.1016/0003-9861(73)90328-7. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Arslanian M. J., Oh Y. H., Aune K. C., Vanaman T. C., Wakil S. J. Presence of two polypeptide chains comprising fatty acid synthetase. Proc Natl Acad Sci U S A. 1975 May;72(5):1940–1944. doi: 10.1073/pnas.72.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Saturated fatty acid biosynthesis and its regulation. Annu Rev Biochem. 1973;42:21–60. doi: 10.1146/annurev.bi.42.070173.000321. [DOI] [PubMed] [Google Scholar]
- WARNER R. C. Physical properties of crystalline fluorokinase. Arch Biochem Biophys. 1958 Dec;78(2):494–496. doi: 10.1016/0003-9861(58)90373-4. [DOI] [PubMed] [Google Scholar]
- WU Y. V., SCHERAGA H. A. Studies of soybean trypsin inhibitor. I. Physicochemical properties. Biochemistry. 1962 Jul;1:698–705. doi: 10.1021/bi00910a025. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. E., Brown R. K., Curti B., Massey V. Immunochemical studies of L-amino acid oxidase. Biochim Biophys Acta. 1971 Jan 19;229(1):260–270. doi: 10.1016/0005-2795(71)90341-2. [DOI] [PubMed] [Google Scholar]





