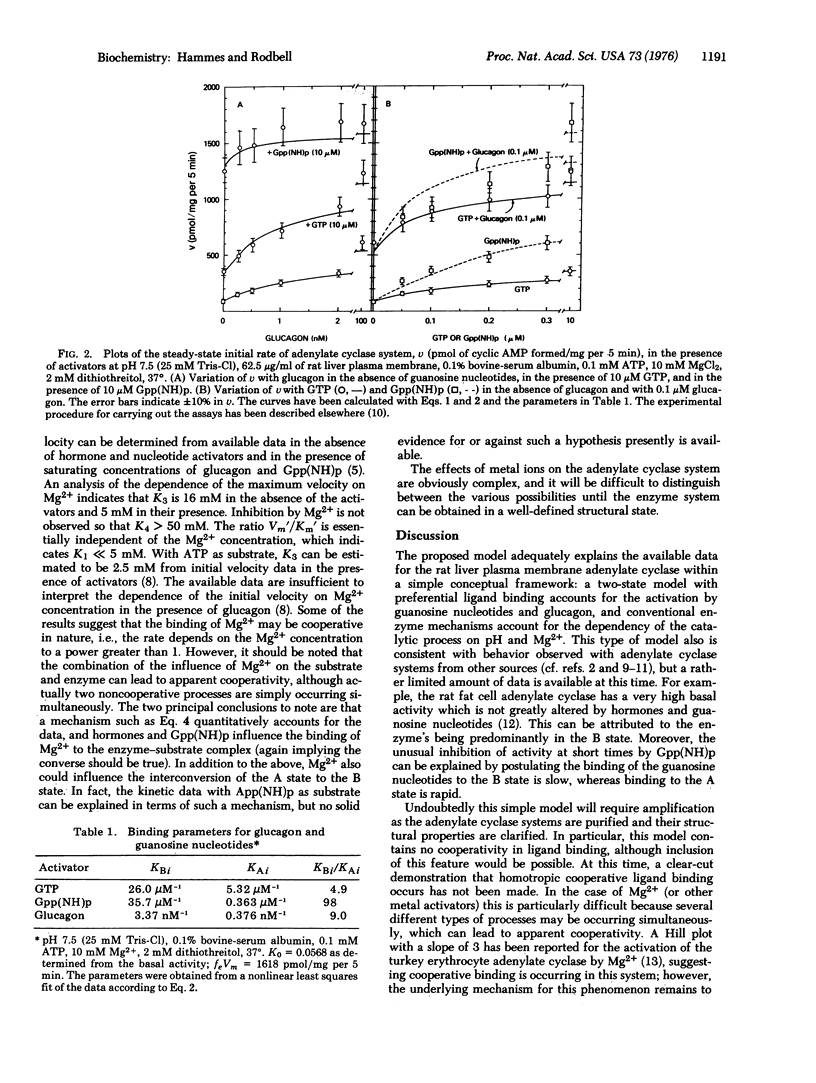
Abstract
A simple model is developed to explain the activation of rat liver plasma membrane adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] by guanosine nucleotides and glucagon and the dependence of the cATALYTIC RATE ON Mg2+, H+, and substrate concentrations. The basic model proposes that the adenylate cyclase system can exist in two states, A and B; that activating ligands bind preferentially to the B state; and that only the B state is active. Kinetic data are quantitatively fit to this model, and the binding constants for the interaction of the A and B states with glucagon, GTP, and guanyl-5'-ylimidodiphosphate are obtinaed. The substrates ATP and adenyl-5'-ylimidodiphosphate appear to show little preference between the A and B states, and simple Michaelis-Menten kinetics are sufficient to describe the dependence of the catalytic rate on substrate concentration under optimal conditions. The dependence of the rate on pH can be explained by postulating that one ionizable group in its acid form and one ionizable group in its basic form must be present at the active site in order for catalysis to occur. The activation and inhibition of the activity by Mg2+ can be explained by a similar mechanism with Mg2+ binding to activating and inhibiting sites. Glucagon and guanosine nucleotides appear to influence the dependence of the rate on Mg2+ and glucagon. The Mg2+ also may display some preference for the B state. A comparison of this model with others that have been proposed is given. The proposed model appears to provide a simple conceptual frame-work that is applicable to many adenylate cyclase systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Drummond G. I., Duncan L. Adenyl cyclase in cardiac tissue. J Biol Chem. 1970 Mar 10;245(5):976–983. [PubMed] [Google Scholar]
- Garbers D. L., Johnson R. A. Metal and metal-ATP interactions with brain and cardiac adenylate cyclases. J Biol Chem. 1975 Nov 10;250(21):8449–8456. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Lin M. C., Salomon Y., Rendell M., Rodbell M. The hepatic adenylate cyclase system. II. Substrate binding and utilization and the effects of magnesium ion and pH. J Biol Chem. 1975 Jun 10;250(11):4246–4252. [PubMed] [Google Scholar]
- Londos C., Rodbell M. Multiple inhibitory and activating effects of nucleotides and magnesium on adrenal adenylate cyclase. J Biol Chem. 1975 May 10;250(9):3459–3465. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Rendell M., Salomon Y., Lin M. C., Rodbell M., Berman M. The hepatic adenylate cyclase system. III. A mathematical model for the steady state kinetics of catalysis and nucleotide regulation. J Biol Chem. 1975 Jun 10;250(11):4253–4260. [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. IV. Effects of guanylnucleotides on binding of 125I-glucagon. J Biol Chem. 1971 Mar 25;246(6):1872–1876. [PubMed] [Google Scholar]
- Rodbell M. On the mechanism of activation of fat cell adenylate cyclase by guanine nucleotides. An explanation for the biphasic inhibitory and stimulatory effects of the nucleotides and the role of hormones. J Biol Chem. 1975 Aug 10;250(15):5826–5834. [PubMed] [Google Scholar]
- Salomon Y., Lin M. C., Londos C., Rendell M., Rodbell M. The hepatic adenylate cyclase system. I. Evidence for transition states and structural requirements for guanine nucloetide activiation. J Biol Chem. 1975 Jun 10;250(11):4239–4245. [PubMed] [Google Scholar]
- Steer M. L., Levitzki A. The control of adenylate cyclase by calcium in turkey erythrocyte ghosts. J Biol Chem. 1975 Mar 25;250(6):2080–2084. [PubMed] [Google Scholar]
- de Haën C. Adenylate cyclase. A new kinetic analysis of the effects of hormones and fluoride ion. J Biol Chem. 1974 May 10;249(9):2756–2762. [PubMed] [Google Scholar]


