Abstract
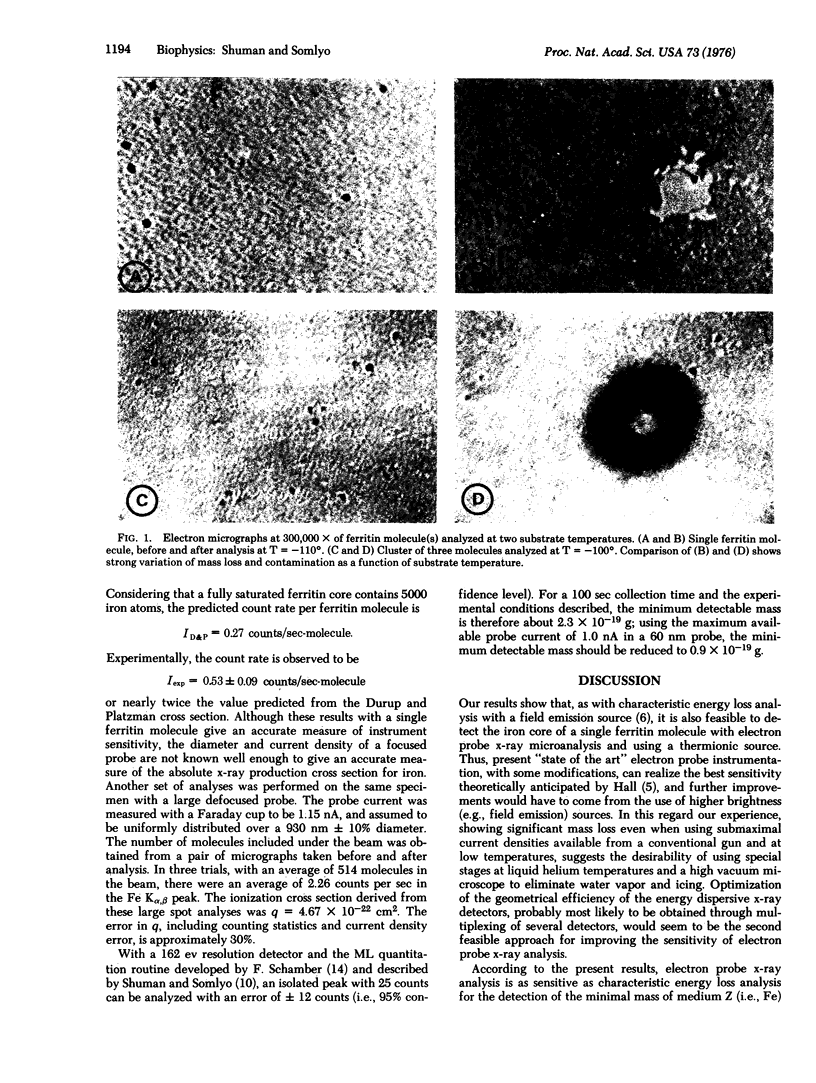
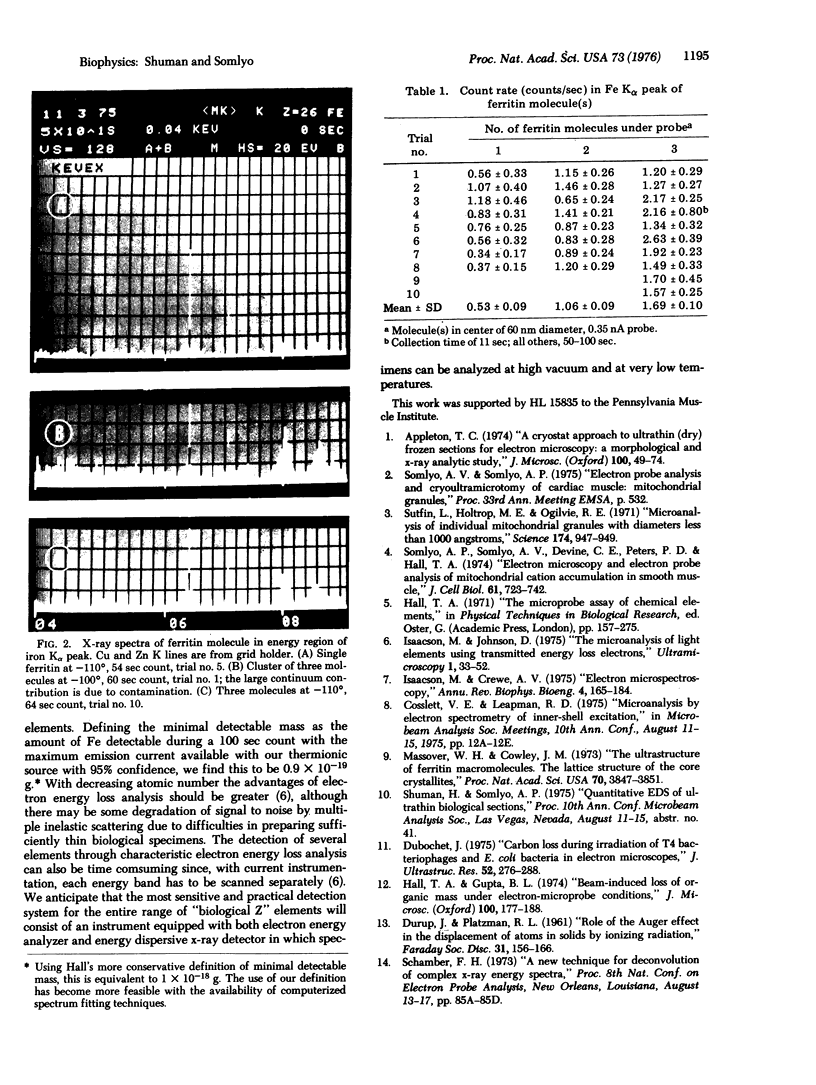
Single molecules and groups of two or three ferritin molecules were subjected to electron probe x-ray microanalysis in a transmission electron microscope equipped with a liquid nitrogen cooled stage. Significant Fe Kalpha peaks were generated during 100-sec counts when single ferritin molecules were excited with a probe current of 0.35 nA/60 nm spot, less than the maximal current available in a thermionic gun. There was a linear relationship between the number of ferritin molecules analyzed and count rates. The experimental results are compared to the theoretically calculated Fe Kalpha yields and to the results of Isaacson and Johnson [(1975) Ultramicroscopy I, 33-52] with electron energy loss analysis. We conclude that current state of the art electron probe x-ray analysis can realize the theoretically predicted sensitivity of the method, and estimate 0.9 X 10(-19) g of Fe as the minimal mass detectable with maximal (thermionic) probe current during a 100-sec count and with 95% confidence.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton T. C. A cryostat approach to ultrathin "dry" frozen sections for electron microscopy: a morphological and x-ray analytical study. J Microsc. 1974 Jan;100(1):49–74. doi: 10.1111/j.1365-2818.1974.tb03913.x. [DOI] [PubMed] [Google Scholar]
- Dubochet J. Carbon loss during irradiation of T4 bacteriophages and E. coli bacteria in electron microscopes. J Ultrastruct Res. 1975 Aug;52(2):276–288. doi: 10.1016/s0022-5320(75)80118-3. [DOI] [PubMed] [Google Scholar]
- Hall T. A., Gupta B. L. Bean-induced loss of organic mass under electronmicroprobe conditions. J Microsc. 1974 Mar;100(2):177–188. doi: 10.1111/j.1365-2818.1974.tb03927.x. [DOI] [PubMed] [Google Scholar]
- Isaacson M. S., Crewe A. V. Electron microspectroscopy. Annu Rev Biophys Bioeng. 1975;4(00):165–184. doi: 10.1146/annurev.bb.04.060175.001121. [DOI] [PubMed] [Google Scholar]
- Isaacson M. The microanalysis of light elements using transmitted energy loss electrons. Ultramicroscopy. 1975 Jul;1(1):33–52. doi: 10.1016/s0304-3991(75)80006-4. [DOI] [PubMed] [Google Scholar]
- Massover W. H., Cowley J. M. The ultrastructure of ferritin macromolecules. The lattice structure of the core crystallites. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3847–3851. doi: 10.1073/pnas.70.12.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Devine C. E., Peters P. D., Hall T. A. Electron microscopy and electron probe analysis of mitochondrial cation accumulation in smooth muscle. J Cell Biol. 1974 Jun;61(3):723–742. doi: 10.1083/jcb.61.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutfin L. V., Holtrop M. E., Ogilvie R. E. Microanalysis of individual mitochondrial granules with diameters less than 1000 angstroms. Science. 1971 Nov 26;174(4012):947–949. doi: 10.1126/science.174.4012.947. [DOI] [PubMed] [Google Scholar]