Abstract
Combination chemotherapy is standard for metastatic colorectal cancer (CRC); however, nearly all patients develop drug resistance. Understanding the mechanisms that lead to resistance to individual chemotherapeutic agents may enable identification of novel targets and more effective therapy. Irinotecan is commonly used in first- and second-line therapy for patients with metastatic CRC, with the active metabolite being SN38. Emerging evidence suggests that altered metabolism in cancer cells is fundamentally involved in the development of drug resistance. Using Oncomine and unbiased proteomic profiling, we found that ATP citrate lyase (ACLy), the first-step rate-limiting enzyme for de novo lipogenesis, was upregulated in CRC compared to its levels in normal mucosa and in chemoresistant CRC cells compared to isogenic chemo-naïve CRC cells. Overexpression of exogenous ACLy by lentivirus transduction in chemo-naïve CRC cells led to significant chemoresistance to SN38 but not to 5-fluorouracil or oxaliplatin. Knockdown of ACLy by siRNA or inhibition of its activity by a small-molecule inhibitor sensitized chemo-naïve CRC cells to SN38. Furthermore, ACLy was significantly increased in cancer cells that had acquired resistance to SN38. In contrast to chemo-naïve cells, targeting ACLy alone was not effective in re-sensitizing resistant cells to SN38, due to a compensatory activation of the AKT pathway triggered by ACLy suppression. Combined inhibition of AKT signaling and ACLy successfully re-sensitized SN38-resistant cells to SN38. We conclude that targeting ACLy may improve the therapeutic effects of irinotecan and that simultaneous targeting of ACLy and AKT may be warranted to overcome SN38 resistance.
Keywords: Chemoresistance, ATP citrate lyase, SN38, irinotecan, colorectal cancer
INTRODUCTION
Current standard combination chemotherapy regimens for patients with metastatic CRC contain 5-fluorouracil (5-FU) in combination with oxaliplatin or irinotecan. Although the response rate to systemic therapies is ~50%, drug resistance develops in nearly all patients with metastatic CRC, leading to ~50,000 deaths each year in the United States (1). Advances in targeted therapy have not dramatically improved the outcomes of patients with metastatic CRC, and the majority of those with unresectable metastatic disease still die within 2 years of the diagnosis of metastasis (2). A better understanding of the mechanisms by which cancer cells develop resistance to individual chemotherapeutic agents is urgently needed to identify novel therapeutic targets and methods that would improve survival.
The chemotherapeutic agent Irinotecan (CPT-11, [Camptosar]), which is commonly used in treating cancers such as metastatic CRC, causes S-phase-specific death of proliferating cells by inhibiting DNA topoisomerase I (3, 4). Irinotecan is converted by carboxylesterase into SN38 (Figure S1), which is 1,000-fold more potent than unconverted Irinotecan (5). Cancer cells may resist SN38-mediated cell death by various mechanisms: upregulation of drug efflux (6, 7), reduction of topoisomerase I levels (8, 9), development of mutations in topoisomerase I (10), enhancement of DNA repair (11), and activation of nuclear factor kappa B regulated pathways (12). Emerging evidence suggests that altered metabolism in cancer cells is fundamentally involved in the development of drug resistance (13–15). Many of the above mechanisms may result from a reprogramming of cellular metabolism, one of the hallmarks of cancer, as in enhanced aerobic glycolysis, glutaminolysis, and de novo lipogenesis (16–18). The targeting of key metabolic enzymes sustaining these cancerous metabolic adaptations bears great promise for improving treatment efficacy in patients with metastatic diseases (14, 19).
Our laboratory has developed an in vitro model of acquired drug resistance based on chronic exposure of HT29 CRC cells to incrementally increasing doses of SN38, oxaliplatin, or 5-FU (20, 21). The selected resistant cells maintain a stable chemoresistant phenotype and provide an opportunity to study mechanisms of single-agent resistance. Our unbiased proteomic profiling studies comparing parental cells with resistant cells showed that many metabolic enzymes involved in mitochondrial respiration, glycolysis, and lipogenesis are altered (22). ATP citrate lyase (ACLy), the first-step rate-limiting enzyme for de novo lipogenesis, is one of the proteins that are upregulated in the resistant CRC cells. Recently, ACLy has been investigated as an anti-cancer therapeutic target (23), however, the contribution of ACLy to drug resistance of cancer cells remains to be elucidated. In this study, we tested the hypothesis that ACLy activation plays a role in the development of drug resistance in CRC cells. We found that activations of the ACLy and AKT pathways play critical roles in CRC cell resistance to SN38.
MATERIALS AND METHODS
Cell Lines and in vitro Chemoresistance Model
The human CRC cell line HT29 was obtained from the American Type Culture Collection (ATCC; Manassas, VA). Oxaliplatin- and 5-FU-resistant cell lines were developed in our laboratory as previously described (20, 21). SN38-resistant cell lines were developed by using a similar protocol. Briefly, parental HT29 cells were exposed to an initial SN38 dose of 1 nM and cultured to a confluency of 80% for three passages (~6 weeks). The cells that survived the initial SN38 treatment were then exposed to 5 nM SN38 for three passages (~8 weeks) and to 10 nM for three more passages (~8 weeks). Finally, the SN38 concentration was increased to the clinically relevant plasma drug concentration of 15 nM for 3 weeks (~10 weeks). The surviving resistant cells were named HT29-SNR.
All cells were cultured in minimal essential medium (MEM) containing 5 mM glucose and supplemented with 10% fetal bovine serum, vitamins, nonessential amino acids, penicillin-streptomycin, sodium pyruvate, and L-glutamine (Life Technologies, Grand Island, NY). HT29-SNR cells were continuously cultured in 15 nM SN38 unless otherwise indicated. Cell viability was measured using a Vi-Cell XR cell viability analyzer (Beckman Coulter, Brea, CA). In vitro experiments were carried out at 70% cell confluency at least three times. All cell lines were authenticated by short tandem repeat sequencing and matched with 100% accuracy to the ATCC database.
Lentivirus Transduction
To stably overexpress ACLy in HT29 cells, lentivirus transduction particles were generated using 293 packaging cells transfected by plasmid constructs containing full-length ACLy cDNA. ACLy cDNA was subcloned into the mammalian expression vector pCDNA3.1(-) (Invitrogen, Carlsbad, CA) from bacterial cloning vector pOTB6 (MG1813; ATCC) using EcoR1 and HindIII sites and subsequently cloned into a green fluorescent protein (GFP)-expressing vector for virus production. Virus particles and polybrene (10 µl/ml) were added to cells at 70% confluency in 6-well plates for 24 hr. After cell expansion for one passage, GFP-positive cells were subjected to fluorescence-activated cell sorting (FACS) to obtain cells stably expressing ACLy.
Flow Cytometry Analysis for Cell Death
Apoptotic cells and total dead cells (sub-G1 population) were quantified by Annexin V-propidium iodide (PI) staining and flow cytometry. Apoptosis was analyzed by an Annexin V assay kit (BD Biosciences Pharmingen, San Diego, CA) according to the manufacturer’s instructions. For sub-G1 phase quantification, cells grown in 6-cm-diameter petri dishes were harvested with trypsin and washed in phosphate-buffered saline. Cells were then resuspended in 10% ethanol for fixation. Next, 100 µl of a cell suspension (105 cells) was stained with PI at room temperature for at least 15 minutes in the dark. Cells were then analyzed in a FACSCalibur flow cytometer (Becton Dickinson). Data were analyzed by FlowJo software (Tree Star, Ashland, OR).
RNA Interference for ACLy Knockdown
SmartPool siRNA oligonucleotides for ACLy and a negative control were purchased from Dharmacon (Lafayette, CO). Four independently validated siRNAs were transfected into HT29-SNR cells together at a final concentration of 40 nM by Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. First, 120 pmol of siRNA and 16 µl of Lipofectamine were mixed in 500 µl of Opti-MEM medium (10 nM final concentration for each individual siRNA in the SmartPool). After 20 min of incubation, the mixture was added to cells at 70% confluency plated in a 6-well plate (2 ml final volume). Fresh medium was added to cells after 6 hours. Twenty-four hours after transfection, cells were either collected for protein harvest or continued for SN38 treatment for additional 48 hr before analysis for apoptosis markers by Western blot.
Spectrophotometric Assay for ACLy Activity
ACLy activity was determined as described previously (24) in an assay mixture containing 100 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 20 mM K3Citrate, 1 mM dithiothreitol, 10 U of malic dehydrogenase, 300 µM coenzyme A (CoA), 280 µM NADH, and various amounts of purified ACLy (BPS Bioscience, San Diego, CA), and optimal conditions for measuring enzyme activities were identified. The reaction was initiated by adding 10 mM ATP at 37°C, and NADH oxidation, as evidenced by a decrease in absorbance at 340 nm, was monitored continuously for 10 min using a microplate reader. To measure the activity of GSK165, a small-molecule inhibitor of ACLy (25) (provided by GlaxoSmithKline, Collegeville, PA, under a material transfer agreement), 400 µg of purified ACLy was used in a final volume of 100 µl. Controls not containing ACLy were used to account for nonspecific NADH oxidation.
MTT Assay
Cell growth inhibition was quantified by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. First, HT29 cells were seeded in 96-well plates at 3000 cells in 100 µl per well and incubated overnight. The next day, a 100 µl working stock solution containing GSK165 at 2X final concentration was added to the cell suspension. After 72 hr incubation, 40 µl of the MTT reagent (3 mg/ml) was added to each well, and the mixtures were incubated for 2-4 hr. After the supernatant was removed, the formazan precipitates in the cells were dissolved in 100 µl of dimethyl sulfoxide (DMSO). Absorbance at 570 nm was determined using a MultiSkan plate reader (LabSystems, Lowell, MA). Fractional survival was plotted against the logarithm of the drug dose, and 50% inhibitory concentrations (IC50) were calculated by Prism software (GraphPad Software, La Jolla, CA).
Western Blot
Antibodies against ACLy, p-ACLy (ACLy phosphorylated at the S454 site), AKT, p-AKT S473, cleaved poly(ADP-ribose) polymerase (PARP), and cleaved caspase 3 were purchased from Cell Signaling (Danvers, MA). β-actin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Whole-cell lysate was collected from cells cultured at 70% confluency before standard Western blot analysis was performed.
Drugs and Other Reagents
Oxaliplatin, 5-fluorouracil and SN38 were obtained from The University of Texas MD Anderson Cancer Center pharmacy. Stock drugs of GSK165, phosphoinositide 3-kinase (PI3K) inhibitor Wortmannin (Sigma, St. Louis, MO), AKT specific inhibitor MK2206 (Selleckbio, Houston, TX) and fatty acid synthase inhibitor Cerulenin (Sigma) were reconstituted in DMSO at 10 mM and stored in aliquots at −20°C.
Statistical Analysis
For all in vitro experiments, statistical analyses were conducted using Student’s t test (Prism). All statistical tests were two-sided, and p values ≤ 0.05 were considered to be significant.
RESULTS
ACLy Expression in Human CRC
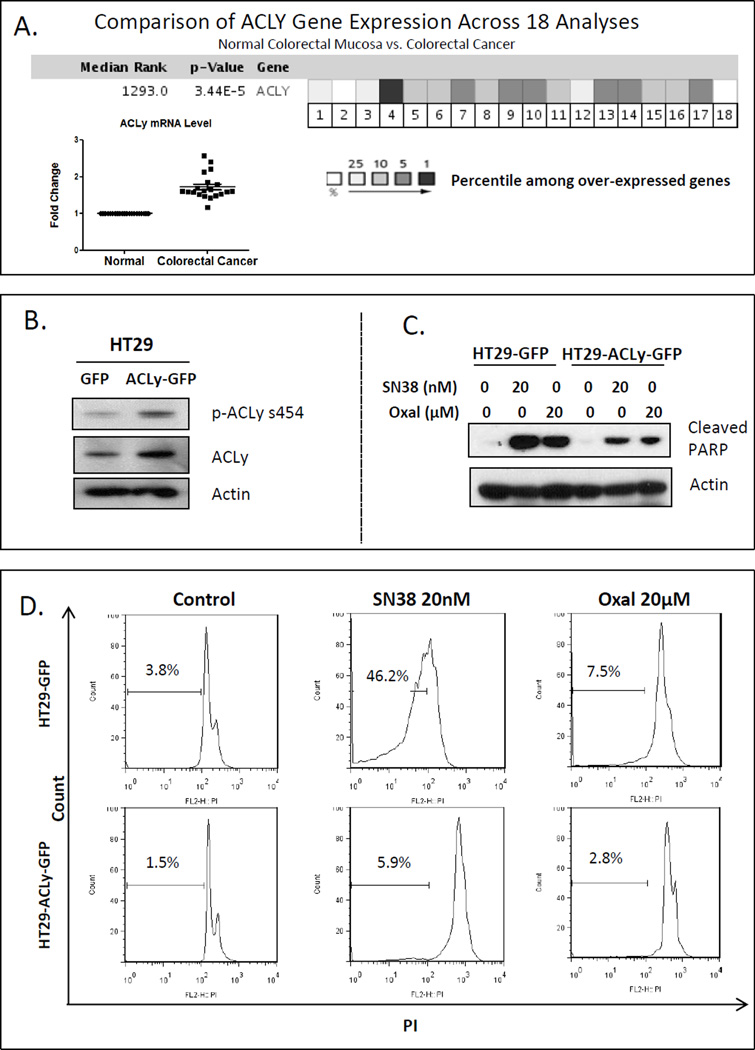
We searched the Oncomine database using criteria “ACLy, colorectal cancer, normal vs cancer”, and found that a total of 18 studies deposited gene expression data on ACLy expression to the database. As summarized in Figure 1A, the ACLy gene is among the top 1-10% overexpressed genes in colon adenocarcinoma compared with normal colon mucosa. The ACLy mRNA level is increased by 2-fold in malignant colon carcinoma tissues according to Oncomine data from 18 independent studies. In addition, our unbiased proteomic profiling suggests that drug-resistant CRC cells have elevated ACLy expression (22).
Figure 1. Overexpression of ACLy induced chemoresistance in chemo-naïve HT29 cells.
A. ACLy is among the top 1-10% overexpressed genes in malignant colon adenocarcinoma tissue compared with expression in adjacent normal colon mucosa. Its mRNA level is increased 2-fold according to Oncomine data from 18 studies. B. Stable overexpression of ACly was achieved by lentivirus transduction. C. Overexpression of ACLy partially blocked induction of cleaved PARP by SN38 and oxaliplatin (Oxal) treatment (48 hr) in HT29 cells. D. ACLy overexpression blocked cell killing by SN38 (but to less extent by oxaliplatin), as indicated by the decreased sub-G1 cell count.
Overexpression of ACLy Induces Chemoresistance in Chemo-Naïve HT29 Cells
To determine the role of ACLy in drug resistance, we stably overexpressed ACLy protein by lentivirus transduction in HT29 cells (Figure 1B), which have relatively low levels of basal ACLy and p-ACLy expression compared to other CRC cell lines (Figure S2). Overexpression of ACLy decreased the sensitivity of HT29 cells to SN38, as evidenced by decreased levels of cleaved PARP, an apoptosis marker, on Western blot (Figure 1C). Furthermore, PI (propidium iodide) staining and flow cytometry showed that ACLy overexpression inhibited cell killing by SN38. Figure 1D shows that the sub-G1 (apoptotic and necrotic) cell population decreased from 46.2% ± 2.5% (standard error of the mean [SEM] throughout) to 5.9% ± 0.5% (p < 0.05) after ACLy overexpression following SN38 treatment but to a less extent from 7.5% ± 0.5% to 2.8% ± 0.5% (p < 0.05) following oxaliplatin treatment. We did not observe any protective effect of ACLy overexpression against 5-FU treatment (data not shown). Because the most striking effect was observed with SN38, we conducted further experiments with SN38-treated cells.
Inhibition of ACLy Sensitizes Chemo-Naïve HT29 Cells to SN38 Treatment
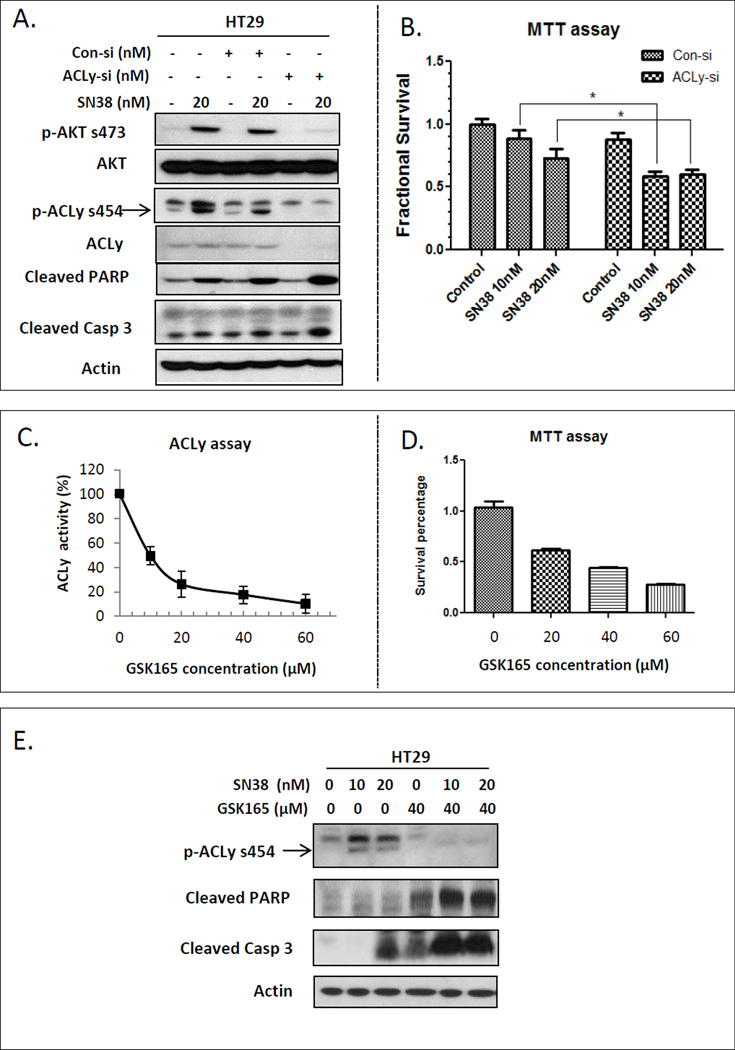
We next explored the relationship between SN38 and ACLy activity, and the effect of targeting ACLy in combination with SN38 treatment on chemo-naïve HT29 cells. Surprisingly, SN38 activated both AKT and ACLy (Figure 2A). A transient transfection of ACLy siRNA efficiently knocked down ACLy protein for 24 to 72 hr. After a 24 hr ACLy knockdown, we exposed the cells to 20 nM SN38 for an additional 48 hr. Knockdown of ACLy sensitized HT29 cells to SN38 without affecting AKT activity, as evidenced by increased PARP and caspase 3 cleavage (Figure 2A) and enhanced cell growth inhibitory effect of SN38 by MTT assay (Figure 2B).
Figure 2. Inhibition of ACLy sensitized chemo-naïve HT29 cells to SN38.
A. SN38 activated AKT and ACLy, and knockdown of ACLy by siRNA (si) enhanced apoptosis induction by SN38, as indicated by increased levels of cleaved PARP and cleaved caspase (Casp) 3 in chemo-naïve HT29 cells. Con, control. B. Combination treatment of ACLy siRNA (24 hr) and SN38 (48 hr) has significantly enhanced growth inhibitory effect compared with SN38 single agent in HT29 cells by MTT assay. C. Concentration-dependent inhibition of ACLy activity by GSK165 in a cell-free biochemical assay. D. Concentration-dependent inhibition of cell proliferation by GSK165 in HT29 cells. E. GSK165 in combination with SN38 treatment (48 hr) induced enhanced apoptosis, as indicated by increased cleavage of PARP and caspase 3 in a Western blot. * p < 0.05.
We next investigated the effect of GSK165, which is a novel ACLy inhibitor modified from 2-hydroxy-N-phenylbenzenesulfonamide pharmacophore, structurally different from the known ACLy inhibitor SB-20499 and its pro-drug SB-201076 (24, 25). As shown in Figure 2C and D, GSK165 inhibited ACLy activity in a concentration-dependent manner and had a growth-inhibitory effect on cancer cells as a single agent with an IC50 of ~30 µM. When 40 µM GSK165 was combined with SN38 treatment, GSK165 sensitized the cells to SN38, as shown by Western blot analysis of PARP and caspase 3 cleavage (Figure 2E). The chemosensitization effect of GSK165 could be inhibited by over-expression of fatty acid synthase (FASN) (Figure S3), suggesting ACLy dependent de novo lipogenesis is involved in ACLy mediated chemoresistance of HT29 cells to SN38.
ACLy Activation is Associated with Acquired Resistance to SN38
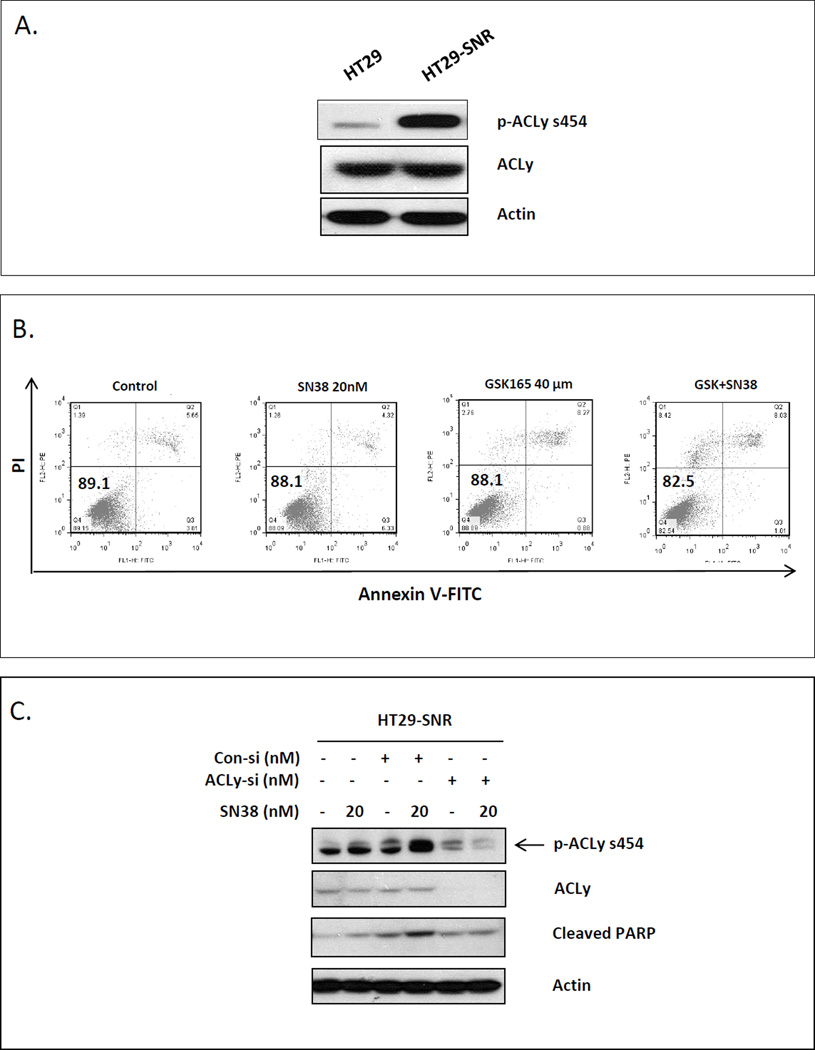
To investigate whether ACLy is also involved in the development of acquired resistance to SN38, we determined the levels of total ACLy and p-ACLy in chemo-naïve and SN38-resistant HT29 cells (HT29-SNR). We found that the level of p-ACLy, but not total ACLy, was significantly higher in HT29-SNR cells than in parental cells (Figure 3A). Increased p-ACLy activation may lead to increased de novo lipogenesis. We observed that the numbers of cytoplasmic lipid droplets by electron microscope imaging also increased significantly in drug resistant cells (Supplemental materials and Figure S4)., To further confirm the increase of lipid content in HT29-SNR cells is due to de novo lipogenesis, we examined the fatty acid synthase (FASN) level, and discovered that the HT29-SNR cells significantly upregulated FASN expression compared with the parental HT29 cells (Figure S5A). Treatment with SN38 acutely increased intracellular lipid content in the parental HT29 cells as measured by AdipoRed assay, and this increase can be blocked by a specific FASN inhibitor, cerulenin (Supplemental materials and Figure S5B, S5C). Similarly, cerulenin also significantly reduced the lipid content of HT29SNR cells (Figure S5D). These data indicate that SN38 induced intracellular lipid accumulation is due to enhanced de novo lipogenesis.
Figure 3. Knockdown of ACLy failed to re-sensitize resistant CRC cells to SN38.
A. Activation of ACLy was increased in HT29-SNR cells as determined by Western blot. Par, parental cells. B. GSK165 failed to re-sensitize HT29-SNR cells to SN38, as quantified by Annexin V-PI staining for FACS analysis. C. Knockdown of ACLy by siRNA (si) failed to enhance SN38-induced apoptosis, as determined by Western blot analysis of cleaved PARP. Con, control. SN38 treatment was 48 hr for all experiments.
These findings prompted us to test the possibility that targeting ACLy may re-sensitize HT29-SNR cells to SN38. However, treatment of cells with GSK165 or with ACLy siRNA failed to sensitize HT29-SNR cells to SN38, as demonstrated by analysis for apoptotic cells by Annexin V-coupled flow cytometry (Figure 3B) and Western blot analysis for cleaved PARP (Figure 3C). These data suggest that, in addition to the activation of ACLy, SN38 resistant cells must have acquired other survival mechanisms.
Dual Inhibition of ACLy and AKT Pathways Re-sensitized HT29-SNR Cells to SN38
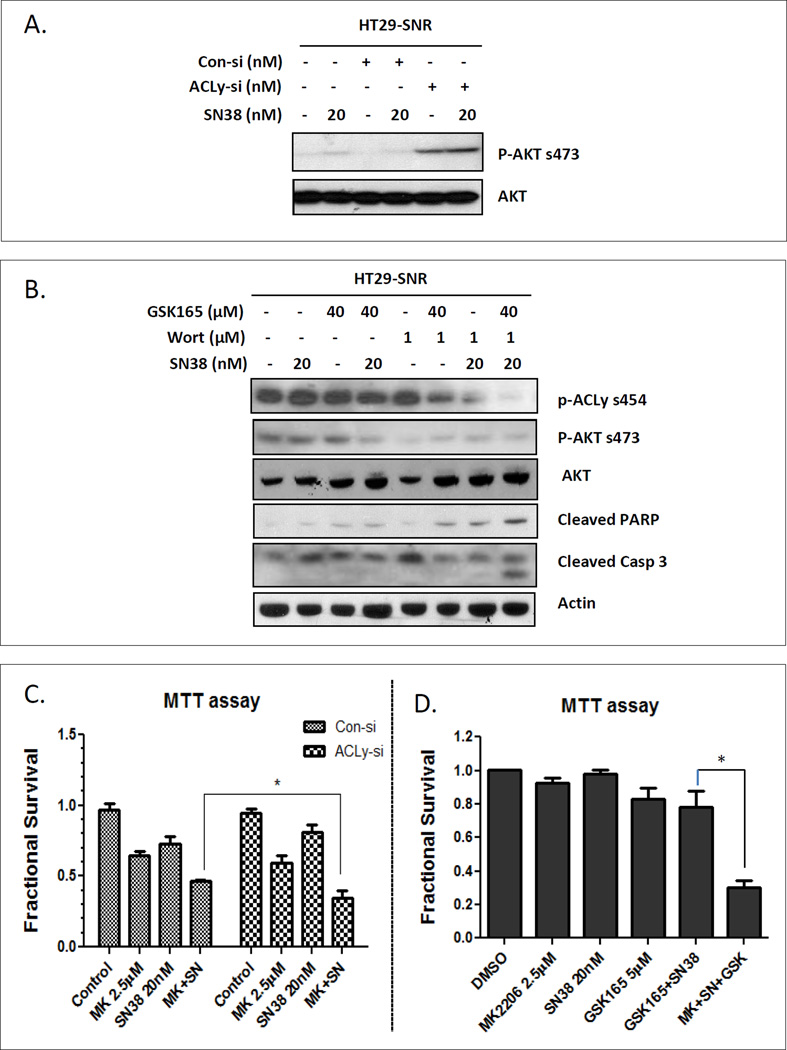
To explore the mechanisms behind the failure of re-sensitizing HT29-SNR cells to SN38 by ACLy inhibition, we measured the activity of AKT in response to ACLy knockdown. Knockdown of ACLy activated AKT in HT29-SNR cells regardless of SN38 (Figure 4A). These data suggest that the activated AKT pathway likely contributes to the failure of re-sensitizing HT29-SNR cells to SN38 by ACLy inhibition.
Figure 4. Dual targeting of the ACLy and AKT pathways re-sensitized HT29-SNR cells to SN38.
A. Increased activation of the AKT pathway was induced in HT29-SNR cells by ACLy knockdown (ACLy-si) and was sustained under SN38 treatment. B. PI3K inhibitor Wortmannin (Wort) in combination with GSK165 and SN38 inhibited p-AKT and induced cleavage of PARP and caspase 3 (Casp 3) in HT29-SNR cells. C. Pre-treatment of AKT inhibitor MK2206 for 4 hr significantly sensitized HT29-SNR cells to SN38 treatment (48 hr) under condition of ACLy knockdown. D. Triple combination of MK2206, SN38 and GSK165 has synergistic effect to inhibit HT29-SNR cell growth. * p < 0.05.
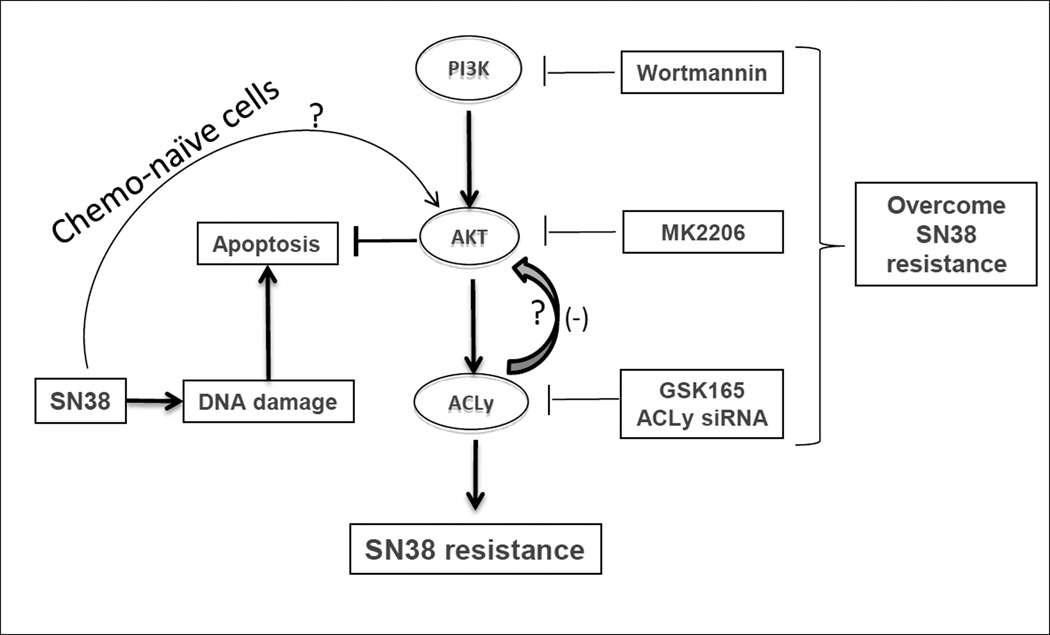
Speculating that a dual targeting of the ACLy and AKT pathways may re-sensitize HT29-SNR cells to SN38, we next tested the effect of a triple combination composed of the phosphoinositide 3-kinase (PI3K) inhibitor Wortmannin, GSK165, and SN38 on HT29-SNR cells. The triple combination suppressed activation of both the ACLy and the AKT pathways and successfully re-sensitized HT29-SNR cells to SN38-induced apoptosis, as demonstrated by increased levels of cleaved PARP and cleaved caspase 3 (Figure 4B). We next confirmed the role of AKT in chemoresistance of HT29-SNR cells using a more potent AKT inhibitor, MK2206 (26). MK2206 significantly re-sensitized HT29-SNR cells to SN38 treatment under the condition of ACly knock down by siRNA treatment (Figure 4C). We next treated HT29-SNR cells with a triple combination of MK2206 (2.5µM, sufficient to suppress AKT activation and ACLy (Figure S6), SN38 (20nM) and GSK165 (5µM, concentration reduced to show a better combination effect). Synergistic effect of growth inhibition and re-sensitization of HT29-SNR cells to SN38-induced apoptosis were observed using the triple combination (Figure 4D and S6). Figure 5 summarizes the proposed negative feedback regulation of ACLy-AKT and activation of AKT is a compensatory survival pathway under ACLy inhibition in SN38-resistant CRC cells.
Figure 5. Hypothesized ACLy-AKT pathway crosstalk in SN38-resistant CRC cells, with a negative feedback loop between ACLy and AKT.
SN38 activates AKT and ACLy in chemo-naïve cells. A negative feedback loop between ACLy and AKT is established in cells with acquired SN38 resistance. Question marks indicate mechanisms to be determined.
DISCUSSION
Approximately 50,000 patients with metastatic CRC die each year in the United States due to resistance to chemotherapy. An understanding of the mechanisms of resistance to individual drugs is critical to develop more effective treatment strategies. Irinotecan is commonly used as part of the first-line chemotherapy backbone for patients with metastatic CRC. Chemoresistance in cancer cells is known to be mediated by one or more of the following mechanisms: increased drug efflux, enhanced drug inactivation, enhanced DNA damage repair, mutated survival-related genes, deregulated growth factor signaling pathways, increased expression of anti-apoptotic genes, and/or activated intracellular survival signaling following chemotherapeutic stress(9, 10, 12, 27, 28). However, little is known about the role of metabolic changes in drug-resistant cancer cells. Our previous findings indicated that metabolic changes are critically involved in the development of cross-chemoresistance in CRC cells (29). This study revealed a novel mechanism specific to SN38 resistance that involves activation of ACLy and a potential negative feedback loop in which suppression of ACLy activates AKT signaling.
ACLy is the major enzyme for producing non-mitochondrial acetyl-CoA that is needed for de novo lipogenesis and substrate acetylation (24, 30). Upregulation of ACLy has been found in several types of cancer (31–36). The mechanism by which ACLy is involved in the survival machinery of cancer cells might be complex. Together with the other two critical lipogenic enzymes, acetyl-CoA carboxylase and fatty acid synthase, ACLy can promote cell growth and survival by increasing de novo synthesized fatty acids on which cancer cells depend (37). ACLy can participate in epigenetic events of cancer cells by regulating the levels of the key substrate of histone acetyltransferase, acetyl-CoA (30). ACLy can regulate cell survival through coordination with cellular pro-survival metabolic network by controlling cytosolic levels of citrate and oxaloacetate, i.e. decrease of cytosolic citrate levels can promote glycolsis by activating phosphofructose kinase (31, 38), and increase of oxaloacetate levels can facilitate gluconeogenesis and glycolysis.
Enhanced de novo lipogenesis is one of the major metabolic alterations used by cancer cells to sustain survival and growth (37). Almost all kinds of carbohydrate can be sources of lipid synthesis, directly or indirectly. As one of the direct substrates (not carbon source) for cytoplasmic fatty acid synthase, acetyl-CoA is mainly derived from the citrate effluxes from the mitochondria, however studies have shown that colonic epithelial cells may have alternative pathways of synthesizing fatty acids independent of ACLy (39), which is likely to be catalyzed by cytosolic acetyl-CoA synthase using acetate as its substrate (40). Our data suggest that ACLy dependent de novo lipogenesis plays a critical role in SN38 resistance. Several protein kinases have been found to activate ACLy by phosphorylation, which include the nucleotide diphosphate kinase (41), the cAMP-dependent protein kinase (38), and the AKT (42). The concurrent activations of AKT and ACLy by SN38 in the chemo-naïve cells (Figure 2A) suggest that AKT plays a major role in SN38 induced ACLy activation, which is supported by the data that inhibition of AKT almost completely abolished the phosphorylation of ACLy at its S454 (Figure S6).
Cells commonly respond to growth factor signaling (to regulate cellular metabolism) through activation of AKT, which subsequently activates ACLy (42). The reported indispensability of ACLy in AKT-induced cell transformation of several cancer types including CRC suggests that ACLy is a critical component of the AKT pathway (43). This can be partially explained by the fact that ACLy activity connects two major cellular metabolic pathways, namely the glycolysis and the fatty acid synthesis, both of which are regulated by AKT-dependent mechanisms (31, 44). The relationship between the ACLy and AKT pathways seems to be a critical axis on which CRC cells rely to develop resistance to SN38. However, after achieving the irreversible resistance to SN38, a new compensatory survival relationship between ACLy and AKT is established, i.e. suppression of ACLy activity activates AKT (Figure 5). According to this perspective, inhibition of ACLy releases the “brake” and results in compensatory AKT overactivation, which provides a survival advantage to cells to resist SN38. It has previously been shown that ACLy inhibition leads to a decrease in AKT activity in a chemo-naive lung cancer cell line (45). The activation of AKT by suppression of ACLy in the SN38 resistant cells is intriguing. The absence of this pathway in the chemo-naïve cells (Figure 2A) indicates that this feedback loop is unlikely mediated by possible preexisting mechanisms that can link ACLy functions with the AKT pathway in chemo-naïve cells. Considering that SN38 causes genotoxic stress by inducing single-strand and double-strand DNA breakage (8), ACLy can participate in signaling transduction and epigenetic alterations through protein acetylation (30, 43), and the irreversible drug resistant phenotype of HT29SNR cells, we speculate that new factors emerged from irreversible epigenetic alterations under long term SN38 exposure mediate the negative feedback loop from ACLy to AKT.
While the underlining mechanism by which suppression of ACLy activates the AKT pathway requires further investigations, the compensatory role of AKT in sustaining cell survival of SN38 resistant cells under conditions of ACLy inhibition can be explained from a metabolic point of view. The effectiveness of co-targeting ACLy and AKT in resensitizing SN38 resistant cells suggests that shared targets by ACLy and AKT play important roles in the development of SN38 resistance of CRC cells. Previously, we have reported that enhanced aerobic glycolysis is a pivotal factor in the development of cross-drug resistance of CRC cells (29). Both ACLy and AKT regulate glycolysis. ACLy can enhance glycolysis by activating phosphofructosekinase via reducing the cytosolic citrate levels (31). AKT activates glycolysis through multiple molecular mechanisms (46).
Metabolic reprogram in adaptation to chemotherapeutic stresses may be a fundamental mechanism of drug resistance, although cancer cells may achieve some common metabolic alterations, such as elevations of glycolysis and lipogenesis, via different mechanisms in response to different chemotherapeutic reagents. A better understanding of the mechanisms by which cancer cells gain resistance to commonly used chemotherapeutic reagents through metabolic adaptation bears a great potential of identifying novel targets for preventing and overcoming chemoresistance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kenneth Dunner, Jr., and Robert Langley, Ph.D., of the Electron Microscope Core Facility of MD Anderson Cancer Center for their assistance in lipid droplet morphology analysis. We thank Li Huang of the Subcloning Core of the Department of Cancer Biology for generation of lentivirus particles containing the ACLy gene. We thank the Flow Cytrometry Core Facility for the Annexin V-PI assay analysis of apoptosis. We thank Rita Hernandez of the Departments of Surgical Oncology for editorial assistance.
This research was supported, in part, by the National Institutes of Health (Cancer Center Support Grant CA016672) to L. E. Ellis, F. Tozzi was supported by NIH grant T32 CA009599. Z. Weihua was supported by grants from the American Cancer Society and Congressional Directed Medical Research Programs of the Department of Defense. L. M. Ellis was supported by the William C. Liedtke, Jr., Chair in Cancer Research.
Abbreviations
- mCRC
metastatic colorectal cancer
- ACLy
ATP citrate lyase
- PARP
poly-ADP-ribose polymerase
Footnotes
Financial disclosure:
Conflict of interest: The authors declare no conflict of interest.
Reference
- 1.Davies JM, Goldberg RM. First-line therapeutic strategies in metastatic colorectal cancer. Oncology (Williston Park) 2008;22:1470–1479. [PubMed] [Google Scholar]
- 2.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 5.Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. [PubMed] [Google Scholar]
- 6.Chen ZS, Furukawa T, Sumizawa T, Ono K, Ueda K, Seto K, et al. ATP-Dependent efflux of CPT-11 and SN-38 by the multidrug resistance protein (MRP) and its inhibition by PAK-104P. Mol Pharmacol. 1999;55:921–928. [PubMed] [Google Scholar]
- 7.Schellens JH, Maliepaard M, Scheper RJ, Scheffer GL, Jonker JW, Smit JW, et al. Transport of topoisomerase I inhibitors by the breast cancer resistance protein. Potential clinical implications. Ann N Y Acad Sci. 2000;922:188–194. doi: 10.1111/j.1749-6632.2000.tb07037.x. [DOI] [PubMed] [Google Scholar]
- 8.Giovanella BC, Stehlin JS, Wall ME, Wani MC, Nicholas AW, Liu LF, et al. DNA topoisomerase I--targeted chemotherapy of human colon cancer in xenografts. Science. 1989;246:1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- 9.Kanzawa F, Sugimoto Y, Minato K, Kasahara K, Bungo M, Nakagawa K, et al. Establishment of a camptothecin analogue (CPT-11)-resistant cell line of human non-small cell lung cancer: characterization and mechanism of resistance. Cancer Res. 1990;50:5919–5924. [PubMed] [Google Scholar]
- 10.Saleem A, Edwards TK, Rasheed Z, Rubin EH. Mechanisms of resistance to camptothecins. Ann N Y Acad Sci. 2000;922:46–55. doi: 10.1111/j.1749-6632.2000.tb07024.x. [DOI] [PubMed] [Google Scholar]
- 11.Desai SD, Li TK, Rodriguez-Bauman A, Rubin EH, Liu LF. Ubiquitin/26S proteasome-mediated degradation of topoisomerase I as a resistance mechanism to camptothecin in tumor cells. Cancer Res. 2001;61:5926–5932. [PubMed] [Google Scholar]
- 12.Huang TT, Wuerzberger-Davis SM, Seufzer BJ, Shumway SD, Kurama T, Boothman DA, et al. NF-kappaB activation by camptothecin. A linkage between nuclear DNA damage and cytoplasmic signaling events. J Biol Chem. 2000;275:9501–9509. doi: 10.1074/jbc.275.13.9501. [DOI] [PubMed] [Google Scholar]
- 13.Djiogue S, Nwabo Kamdje AH, Vecchio L, Kipanyula MJ, Farahna M, Aldebasi YH, et al. Insulin resistance and cancer: the role of insulin and insulin-like growth factors. Endocr Relat Cancer. 2012;20:R1–R17. doi: 10.1530/ERC-12-0324. [DOI] [PubMed] [Google Scholar]
- 14.Hiller K, Metallo CM. Profiling metabolic networks to study cancer metabolism. Curr Opin Biotechnol. 2013;24:60–68. doi: 10.1016/j.copbio.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Tamada M, Nagano O, Tateyama S, Ohmura M, Yae T, Ishimoto T, et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72:1438–1448. doi: 10.1158/0008-5472.CAN-11-3024. [DOI] [PubMed] [Google Scholar]
- 16.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Brit J Cancer. 2009;100:1369–1372. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Yang JM. Altered energy metabolism in cancer: A unique opportunity for therapeutic intervention. Cancer Biol Ther. 2013;14:81–89. doi: 10.4161/cbt.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, 2nd, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 2009;69:1951–1957. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12:4147–4153. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 22.Bose D, Zimmerman LJ, Pierobon M, Petricoin E, Tozzi F, Parikh A, et al. Chemoresistant colorectal cancer cells and cancer stem cells mediate growth and survival of bystander cells. Brit J Cancer. 2011;105:1759–1767. doi: 10.1038/bjc.2011.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zu XY, Zhang QH, Liu JH, Cao RX, Zhong J, Yi GH, et al. ATP citrate lyase inhibitors as novel cancer therapeutic agents. Recent Pat Anticancer Drug Discov. 2012;7:154–167. doi: 10.2174/157489212799972954. [DOI] [PubMed] [Google Scholar]
- 24.Pearce NJ, Yates JW, Berkhout TA, Jackson B, Tew D, Boyd H, et al. The role of ATP citrate-lyase in the metabolic regulation of plasma lipids. Hypolipidaemic effects of SB-204990, a lactone prodrug of the potent ATP citrate-lyase inhibitor SB-201076. Biochem J. 1998;334:113–119. doi: 10.1042/bj3340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JJ, Wang H, Tino JA, Robl JA, Herpin TF, Lawrence RM, et al. 2-hydroxy-N-arylbenzenesulfonamides as ATP-citrate lyase inhibitors. Bioorg Med Chem Lett. 2007;17:3208–3211. doi: 10.1016/j.bmcl.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 27.Gatti L, Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods Mol Med. 2005;111:127–148. doi: 10.1385/1-59259-889-7:127. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Villalona-Calero MA. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol. 2002;13:1841–1851. doi: 10.1093/annonc/mdf337. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckner ME, Fellows-Mayle W, Zhang Z, Agostino NR, Kant JA, Day BW, et al. Identification of ATP citrate lyase as a positive regulator of glycolytic function in glioblastomas. Int J Cancer. 2010;126:2282–2295. doi: 10.1002/ijc.24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008;68:8547–8554. doi: 10.1158/0008-5472.CAN-08-1235. [DOI] [PubMed] [Google Scholar]
- 33.Szutowicz A, Kwiatkowski J, Angielski S. Lipogenetic and glycolytic enzyme activities in carcinoma and nonmalignant diseases of the human breast. Brit J Cancer. 1979;39:681–687. doi: 10.1038/bjc.1979.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turyn J, Schlichtholz B, Dettlaff-Pokora A, Presler M, Goyke E, Matuszewski M, et al. Increased activity of glycerol 3-phosphate dehydrogenase and other lipogenic enzymes in human bladder cancer. Horm Metab Res. 2003;35:565–569. doi: 10.1055/s-2003-43500. [DOI] [PubMed] [Google Scholar]
- 35.Varis A, Wolf M, Monni O, Vakkari ML, Kokkola A, Moskaluk C, et al. Targets of gene amplification and overexpression at 17q in gastric cancer. Cancer Res. 2002;62:2625–2629. [PubMed] [Google Scholar]
- 36.Wang Y, Wang Y, Shen L, Pang Y, Qiao Z, Liu P. Prognostic and therapeutic implications of increased ATP citrate lyase expression in human epithelial ovarian cancer. Oncology Reports. 2012;27:1156–1162. doi: 10.3892/or.2012.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 38.Potapova IA, El-Maghrabi MR, Doronin SV, Benjamin WB. Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry. 2000;39:1169–1179. doi: 10.1021/bi992159y. [DOI] [PubMed] [Google Scholar]
- 39.Zambell KL, Fitch MD, Fleming SE. Acetate and butyrate are the major substrates for de novo lipogenesis in rat colonic epithelial cells. J Nutr. 2003;133:3509–3515. doi: 10.1093/jn/133.11.3509. [DOI] [PubMed] [Google Scholar]
- 40.Yoshii Y, Waki A, Furukawa T, Kiyono Y, Mori T, Yoshii H, et al. Tumor uptake of radiolabeled acetate reflects the expression of cytosolic acetyl-CoA synthetase: implications for the mechanism of acetate PET. Nucl Med Biol. 2009;36:771–777. doi: 10.1016/j.nucmedbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Wagner PD, Vu ND. Phosphorylation of ATP-citrate lyase by nucleoside diphosphate kinase. J Biol Chem. 1995;270:21758–21764. doi: 10.1074/jbc.270.37.21758. [DOI] [PubMed] [Google Scholar]
- 42.Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- 43.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 44.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanai J, Doro N, Sasaki AT, Kobayashi S, Cantley LC, Seth P, et al. Inhibition of lung cancer growth: ATP citrate lyase knockdown and statin treatment leads to dual blockade of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K)/AKT pathways. J Cell Physiol. 2012;227:1709–1720. doi: 10.1002/jcp.22895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robey RB, Hay N. Is Akt the "Warburg kinase"?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.