Abstract
To help reduce the need for autografts, calcium sulfate-based bone graft substitutes are being developed to provide a stable platform to aid augmentation while having the ability to release a broad range of bioactive agents. Calcium sulfate (CS) has an excellent reputation as a biocompatible and osteoconductive substance, but addition of bioactive agents may further enhance these properties. Samples were produced with either directly loaded small, hydrophobic molecule (i.e., simvastatin), directly loaded hydrophilic protein (i.e., lysozyme), or 1 and 10 wt% of H6 poly(β-amino ester) (PBAE) particles containing protein. Whereas sustained release of directly loaded simvastatin was achieved, direct loading of small amounts of lysozyme resulted in highly variable release. Direct loading of a larger amount of protein generated a large burst, 65% of total loading, followed by sustained release of protein. Release of lysozyme from 1 wt% PBAE particles embedded into CS was more controllable than when directly loaded, and for 10 wt% of protein-loaded PBAE particles, a higher burst was followed by sustained release, comparable to the results for the high direct loading. Compression testing determined that incorporation of directly loaded drug or drug-loaded PBAE particles weakened CS. In particular, PBAE particles had a significant effect on the strength of the composites, with a 25% and 80% decrease in strength for 1 wt% and 10 wt% particle loadings, respectively. CS-based composites demonstrated the ability to sustainably release both macromolecules and small molecules, supporting the potential for these materials to release a range of therapeutic agents.
Keywords: Calcium sulfate, drug delivery, synthetic bone graft, simvastatin, lysozyme
Introduction
Bone augmentation through the application of onlay autogenous bone grafts is a suitable method for vertically restoring maxillofacial bone defects.1–8 Although autografts are considered the ‘gold standard’ for augmentation procedures, they have multiple disadvantages as well. Their availability from intra-oral donor sites is limited, and harvesting tissue, which requires a secondary surgery, can lead to undesirable donor site morbidity and chronic discomfort.2,9–19 Many groups are developing synthetic grafting substitutes to replace the current standard. In doing so, several performance criteria must be met. The synthetic bone graft substitute needs to be biocompatible, osteoconductive to help create a secure bond with the surrounding host tissue, and mechanically stable to ensure that vertical augmentation can be achieved without collapse.20–22 Calcium sulfate (CS) hemihydrate has an excellent reputation as a biocompatible and osteoconductive substance.16,23–27 In vivo, calcium sulfate becomes osteogenic in the presence of bone and is completely absorbed without inducing a significant inflammatory response.16,24–27 In addition, CS is mechanically similar to cancellous bone.28
Although synthetic grafting alternatives, such as CS, can be effective, their osteoconductive properties may be improved using bioactive agents, such as growth factors or other drugs capable of stimulating new bone formation.29 Studies have shown enhancement of synthetic bone grafts through the release of bioactive molecules.27,29,30 An ongoing question relates to how these molecules can be delivered to maximize their therapeutic potential.31 One possible approach is to load drug directly into a CS construct. If homogeneously distributed throughout the matrix, dissolution of CS will result in release of the drug. Based on release of fibroblast growth factor, Rosenblum et al. concluded that CS could be a suitable carrier for sustained drug delivery.27 Another way to load drug is through the aid of carrier particles. Biodegradable hydrogel networks provide specific advantages for drug delivery due to their high water content, general biocompatibility, and controlled degradation via hydrolysis or by enzymatic degradation leading to controlled drug delivery.31–33 In particular, the poly(β-amino ester) (PBAE) family of hydrogels has properties that are easily tuned to achieve great control as a drug delivery vehicle.32,34,35 Previous research has shown that PBAE particles can be homogeneously embedded into a CS matrix, which may allow further control over the release of drug from these particles as the CS matrix erodes.20
Several osteogenic drugs are available. These range from larger proteins, such as bone morphogenetic proteins (BMPs; 30–38 kDa) down to small molecules, such as the statin family of drugs (<1 kDa). BMPs control tissue induction and morphogenesis and are directly involved in the differentiation of cartilage and bone.21,36,37 Introducing BMPs into grafting devices can stimulate rapid new bone formation, allowing for a reduction in healing time to achieve sufficient new bone volume for proper anchoring of an inserted implant.30,38 Like all statins, simvastatin (419 Da) is commonly known as an inhibitor of 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase and often prescribed to control cholesterol levels.39,40 Importantly, however, simvastatin promotes bone formation in vitro and in vivo through the stimulation of osteoblastic activity and inhibition of osteoclastic activity.39,40 Due to its pharmacokinetic characteristics of being actively cleared in the liver, controlling the local release of relatively higher amounts of simvastatin from a synthetic bone graft can maintain a therapeutic level of drug suitable for accelerated bone regeneration.40
In the present study, previously developed CS-based composites were investigated for their ability to control the release of drugs.20 Because candidate bioactive agents, ranging from growth factor to intracellular signaling molecule, will differ in their structure, stability, and mode of action, a larger hydrophilic protein and a smaller hydrophobic molecule were evaluated to demonstrate that a range of osteogenic drugs could be released from the composites, either loaded in hydrogel particles or directly loaded into the CS matrix. Having the ability to deliver a wide range of osteogenic agents would allow for the devices to be tuned based on patient needs. Lysozyme was chosen as a model protein to determine the potential to adjust protein release, and simvastatin was used as a representative small molecule drug.
Materials and Methods
Hydrogel synthesis
H6 PBAE macromer was synthesized according to previously described methods.20,32 To review, polyethylene glycol (PEG) 400 diacrylate (Polysciences, Inc., Warrington, PA) and isobutylamine (Sigma-Aldrich, St. Louis, MO) were combined in a 1.2:1 molar ratio and placed in a flask partially submerged in a silicon oil bath pre-heated to 85°C. The reaction mixture was continuously stirred for 48 hr and then removed from the heat. The resulting macromer was stored at 4°C until used.
Biodegradable hydrogels (HG) were made by photopolymerization. Two grams of cooled macromer and 1 wt% of 2,2-dimethoxy-2-phenyl-acetophenone (DMPA; Sigma Aldrich, St. Louis, MO) initiator were dissolved in 1.25 mL of dimethyl sulfoxide (DMSO). H6 loaded with lysozyme was made the same way except that 100 mg of lysozyme (14 kDa; Sigma-Aldrich; solubility: ~300 mg/mL in H2O) was added along with the initiator. After vortexing for 60 sec, the homogeneous mixture was pipetted between glass plates separated by a 1.5 mm Teflon spacer. The macromer was exposed to UV radiation at room temperature under a Lesco UV flood source with an intensity of 14 mW/cm2 for 5 min. After polymerization, the gel was washed with ethanol for 30 minutes to remove any unreacted initiator and macromer and then lyophilized overnight.
Protein release from H6 PBAE gels
To assess release kinetics from the gel alone, circular (diameter: 9 mm) samples of protein-loaded H6 PBAE were weighed, submerged in 4 mL of PBS, and incubated at 37°C on a plate shaker. For the first hr, supernatant was collected for measurement and replenished every 10 min. During the second and third hr, supernatant was collected and replenished every 20 min. After 3 hr, the time intervals were increased to every hr until the samples completely degraded. Collected supernatant was measured using the MicroBCA Protein Assay (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s protocol.
Formation of drug-loaded calcium sulfate composites
The composites consisted of calcium sulfate (β-hemihydrate form; Sigma-Aldrich) as the structural matrix and different amounts of lysozyme-loaded HG particles. Hydrogel particles were hand-ground from polymerized gel slabs using a mortar and pestle at room temperature. In order to coat the gel particles and prevent them from sticking to one another, as well as to allow grinding to be efficient, small amounts of CS powder were added during grinding. Particle sizes of 53–150 µm and 150–250 µm were obtained from grinding and sieving. After grinding, gel particles were washed with 100% ethanol for 1 min to remove residual CS, filtered, and air-dried.
CS samples were fabricated as previously described.20 Briefly, control (drug- and HG-free) CS samples were made by thoroughly mixing 1 g of CS powder and 800 µL of deionized (DI) water in 3 mL syringes fitted with blunt-tipped needles. The slurry was loaded into a custom Teflon mold having a diameter of 4.7 mm and a height of 6.5 mm and allowed to set at 43°C for 24 hr. Similar to the CS control samples, 1 wt% composite samples were created by mixing 0.01 g HG particles with 0.98 g CS and 850 µL DI water. Samples with 10 wt% protein-loaded particles were made by mixing 0.1 g HG, 0.8 g CS, and 900 µL DI water. As for the controls, the composite mixtures were also allowed to set at 43°C for 24 hr.CS samples directly loaded with lysozyme were also made for comparing with protein release from CS-HG composites. Two different loadings were selected to match the amounts in hydrogel composite samples. Briefly, 500 µg or 5 mg of lysozyme were mixed with 1 g CS powder and 800 µL DI water to create the low and high loadings, respectively. The slurries were loaded into a mold and allowed to set at 43°C for 24 hr.
Simvastatin-loaded samples were created by adding 20 mg of simvastatin (Haouri Pharma-Chem, Inc., Edison, NJ; 419 Da; solubility: 12.2 µg/mL in H2O) to 1 g of CS powder prior to mixing with 850 µL of DI water. The slurry was injected into a custom-fabricated Teflon mold and placed in a 43°C oven for 24 hr to set the CS.
To visually demonstrate surface exposure of HG particles at the surface of CS, composites were loaded with 10 wt% HG particles (150–250 µm) that had been stained with blue food coloring. Samples were imaged using a Canon DS126071 camera fitted with a 60 mm macro lens prior to exposure to PBS and after 24 hr of dissolution in 4 mL PBS.
Drug release from CS samples
Protein release from CS composites was conducted with samples for each treatment (blank, 1%HG 53–150 µm, 10%HG 53–150 µm, 1%HG 150–250 µm, 10%HG 150–250 µm, direct low, and direct high) initially weighed, submerged in 4 mL of PBS, and incubated at 37°C on a plate shaker. Every 4 days, supernatant was collected for measurement and replaced with 4 mL of fresh PBS until all samples had completely degraded. Collected supernatant was measured using the MicroBCA Protein Assay slightly modified from the manufacturer’s protocol due to CS precipitation observed when the working reagent was added. Briefly, 200 µL of supernatant and 200 µL of working reagent were combined and incubated at 37°C for 2 hr. Vials were centrifuged at 10,000 G for 2 min to separate any CS precipitate that formed during incubation, and absorbance of the cleared reagent was measured at 562 nm. Lysozyme standards were also assayed using this process to allow for consistent results.
Simvastatin release was conducted by pre-weighing both blank and simvastatin-loaded CS samples, submerging them in 4 mL of PBS, and incubating at 37°C on a plate shaker. Every 4 days, supernatant was collected for measurement and then replenished with 4mL of fresh PBS. The collected supernatant was treated with 100% ethanol in a 50:50 volume ratio of supernatant to solvent. After filtration (0.45 µm), absorbances were measured at 240 nm and compared to a series of standards.
Mechanical properties
Compression testing was performed to determine whether incorporation of directly loaded drug (simvastatin and lysozyme) or protein-loaded H6-PBAE particles into CS would affect the mechanical properties. Testing was conducted using a Bose ELF 3300 system. Contact surfaces were lightly sanded, if necessary, using 600 grit SiC paper to provide smooth, parallel surfaces in contact with the compression platens. Control (blank, drug-free), directly loaded simvastatin, low and high dose of directly loaded lysozyme, 1 wt%HG, and 10 wt%HG samples were tested at a rate of 0.5 N/sec until failure. Compressive modulus (M) and ultimate compressive strength (UCS) were calculated.
Statistics
Statistical analysis of the mechanical compression results was performed using two-way ANOVA. As appropriate, the analysis was followed with a Tukey-Kramer multiple comparisons post hoc test. Differences between groups were considered to be significant with p-values < 0.05.
Results
Protein release from H6 PBAE gels
Figure 1 displays the kinetics of lysozyme release from H6 PBAE discs. During the first hr, the gels swelled steadily with little protein (~4.5%) being released. For the next two hr, the rate of protein release continued to increase from 13% per hr to a steady state of 22.8% per hr, which was sustained for the remainder of the experiment. After four hr, the gels became too difficult to handle, and consequently the degree of swelling could not be determined.
FIGURE 1.
Lysozyme release from PBAE gels along with the swelling profile of H6 PBAE. Data are mean ± standard deviation (n=3).
Surface exposure of HG particles on CS surface
Figure 2 shows images of CS composites in which blue HG particles have been embedded. In the freshly made sample, particles appeared to be homogeneously distributed at the surface. After incubation in PBS, CS dissolved from the surface to expose HG particles that degraded, leaving behind pockets in the CS.
FIGURE 2.
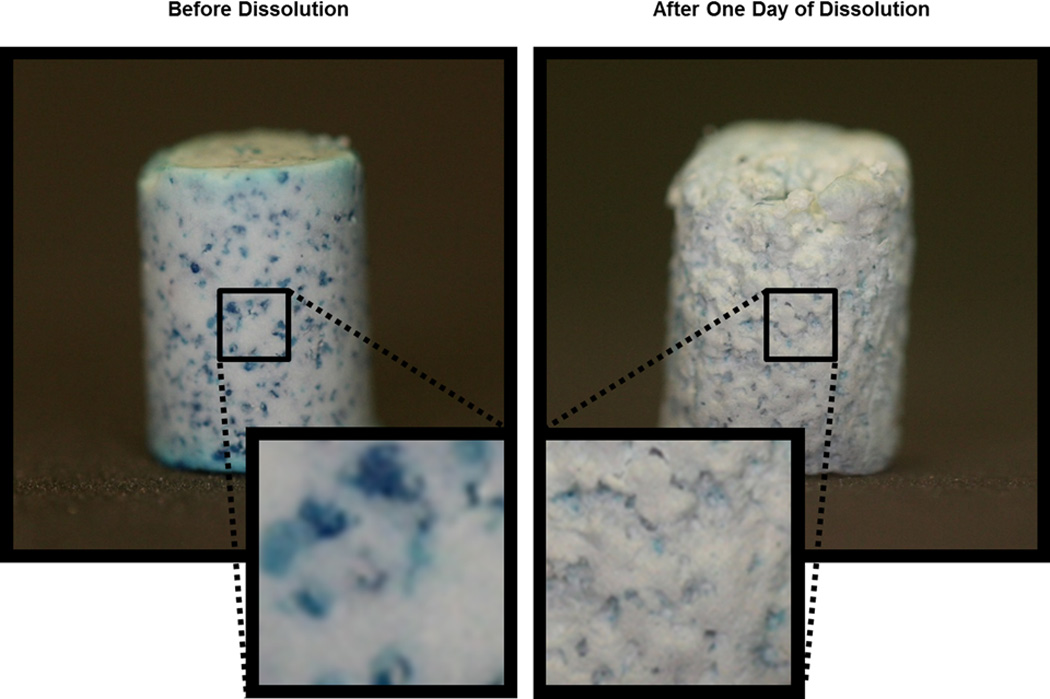
Images depicting HG surface exposure on CS composites. HG particles were dyed blue for contrast. (Left) As-prepared sample. (Right) Sample after dissolution in PBS for 24 hr.
Protein release from composites
Displayed in Figure 3 are the results for the release of lysozyme directly loaded into CS. For the lower loading, no protein was released during the first 4 days (Figure 3(a)). From 4–16 days, release was fairly steady at a rate of 4.5%/day, and at day 16, the rate decreased to about 1.4 %/day. During the final 4 days, the rest of protein was released at a rate of 8.7%/day. However, large variations in release were observed; fluctuations as high as ±30%/day were seen. The instantaneous release pattern was erratic and unpredictable. Figures 3(b) show results for samples with the high direct loading of protein. A large burst (~65%) of protein was observed, after which release was fairly steady at a rate of 1.15%/day with some fluctuations. The high direct loading also showed large deviations (±15–20%/day) among the samples.
FIGURE 3.
Cumulative release of lysozyme directly loaded into CS. (a) Low and (b) high protein loading. Data are mean ± standard deviations (n=5).
Figure 4 shows the release kinetics for CS composites containing embedded PBAE particles loaded with lysozyme. A steady release of lysozyme from 1 wt% HG composite loadings (both particle sizes) was observed, where a 30–40% burst of protein was measured during the first 4 days, and 70–80% of the protein was released after 14 days (Figure 4(a)). The rate of protein release from 1 wt% HG samples slowly decayed over the duration of the experiment. At the beginning, the average rate of release was as much as 7–10%/day at 4 days, while at later time points, the average rate decreased to 5–7%/day at 12 days and 4%/day at 24 days. On the other hand, for composites loaded with 10 wt% HG, release started with a much larger burst of protein (55–70%) at an average rate of about 17%/day during the first 4 days followed by 80–90% being released during the first 12 days of the 24 day study (Figure 4(b)). After about 8 days, release remained fairly steady at an average rate between 0.5% and 3%/day.
FIGURE 4.
Cumulative release of lysozyme from CS composites having two different HG particle sizes (53–150 µm and 150–250 µm). (a) 1 wt% HG and (b) 10 wt% HG. Data are mean ± standard deviations (n=5).
Simvastatin release from calcium sulfate
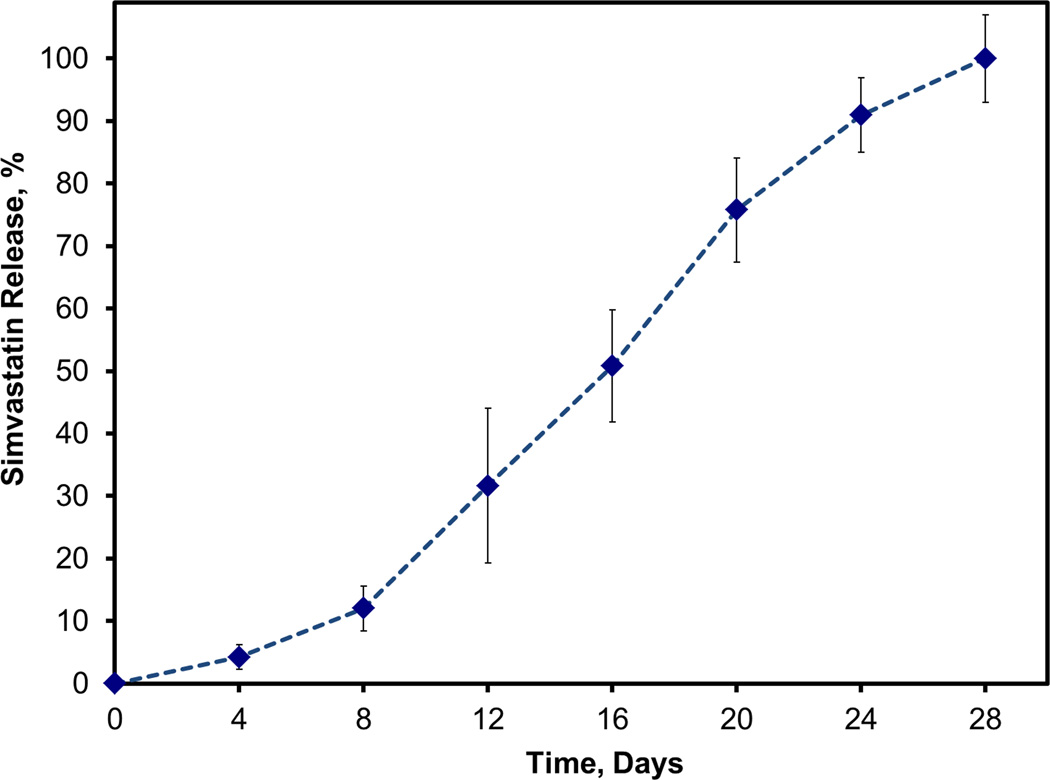
Figure 5 illustrates the release kinetics for CS composites directly loaded with simvastatin. There appeared to be an initial delay of 8 days in the release of simvastatin during which only about 12% of the drug was released. Following this lag period, the average rate shifted upward to about 5.5% of simvastatin per day and remained steady between days 8 and 20. After 20 days, release slowed to 3%/day and remained close to this rate for the final 8 days of the 28 day study.
FIGURE 5.
Cumulative release of simvastatin directly loaded into CS. Data are mean ± standard deviation (n=5).
Mechanical properties
For the ultimate compressive strength, Figure 6(a), CS blanks were significantly stronger than samples with the high dose of directly loaded lysozyme (p<0.01), 1 wt% HG composites (p<0.001), and 10 wt% HG composites (p<0.001). The strength of 10 wt% HG (1.0 ± 0.3 MPa) compared to CS blanks (5.5 ± 0.6 MPa) was about 80% weaker. Compared to all drug loaded samples, the strength of 10 wt% HG was also significantly lower than all other samples (p<0.001). In addition, the decrease in strength for the high direct loading of lysozyme (4.3 ± 0.5 MPa) and 1 wt% HG composites (4.1 ± 0.4 MPa) was only about 20–25% compared to the CS blanks.
FIGURE 6.
Mechanical properties of simvastatin- and protein-loaded CS. (a) Compressive strength; (b) Compressive elastic modulus. Data are mean ± standard deviation (n=10). Symbols (*) and (#) indicates significant difference: p<0.01and p<0.05, respectively.
The elastic modulus, Figure 6(b), for CS blanks (406 ± 126 MPa) was significantly higher than that for directly loaded simvastatin samples (267 ± 68 MPa) and 10 wt% HG composites (189 ± 89 MPa) (p<0.01). There were no significant differences between the controls and low direct lysozyme, high direct lysozyme, and 1 wt% HG composite samples. Elastic modulus for 10 wt% HG composites was also significantly less than that for low direct lysozyme (430 ± 154 MPa; p<0.001), high direct lysozyme (349 ± 110 MPa; p<0.05), and 1 wt% HG composites (383 ± 126 MPa; p<0.01).
Discussion
The aim of the present study was to demonstrate that previously developed CS composites20 are capable of controlling the release of different bioactive molecules. These molecules, which have a broad range of physical and chemical properties such as size, function, and solubility, were released from CS by means of either direct loading of drug into the CS matrix or with the aid of PBAE hydrogel particles.
Protein release from H6 PBAE gels
H6 PBAE particles were investigated as a drug delivery vehicle embedded in a CS matrix. For this study, gel particles were loaded with the protein lysozyme. Lysozyme, an extensively studied and characterized protein, has become a reliable substitute for larger osteogenic molecules, such as bone morphogenetic proteins.41,42 A study of protein release directly from the gel was conducted first. After a slow initial release of lysozyme, a higher steady state was sustained for the remainder of the experiment. The overall trend suggested that lysozyme was trapped within the gel allowing only a slow diffusion of protein from the matrix during swelling followed by protein being released as the gel degraded. This was desired for allowing release of protein from the composites to occur only when the gel particles are exposed and released themselves.
Hawkins et al. demonstrated sustained release of lysozyme from A11 poly(β-amino ester).43 The method for loading lysozyme in the A11 PBAE gels was similar to that used with H6 PBAE gels in this study. Although the two polymers have different compositions, they also have some similarities as members of the PBAE family of hydrogels.32,34,35,43,44 Among this family of materials, a variety of possible combinations of diacrylates and amines can produce similar range of properties.32,34,35,43,44 The similarities between these two PBAEs demonstrate the adaptability of this gel family to accommodate different formulations or drug types if needed.
Protein release from CS
To determine the effectiveness of controlling release of protein from CS, a series of release experiments compared the release of lysozyme from PBAE particles embedded into CS to the release of protein directly loaded into the CS matrix. To allow for an accurate comparison, the lysozyme amount for 1 wt% HG was matched with the low direct loading, and the loading for 10 wt% HG was the same as that for high direct loading. Two different release trends were seen for direct loading. During the 28 day study for the lower direct loading of lysozyme, the amount of protein released fluctuated with no noticeable consistencies. In addition to this variability, there was a delay in the release of protein during the first 4 days.
When comparing the low direct loading to the 1 wt% HG samples, release of lysozyme from the composites was more consistent. Previous research demonstrated good gel particle distribution in the composites.20 The slow decay in the release of protein from the 1 wt% HG composites was similar to that observed for a pilot study with the antioxidant curcumin.20 An important difference from the curcumin release study was that the drug was previously added during PBAE macromer synthesis, which allowed it to covalently bind with the diacrylate.45 This process helped prevent premature release or diffusion of drug from the gel matrix prior to hydrolysis, providing greater control over curcumin release.45 Instead of being covalently incorporated during macromer synthesis, the macromer was polymerized around the protein in the present studies. Because lysozyme is a much larger molecule, the protein could be entrapped in the polymer mesh structure. This trapping of protein allows release from the gel matrix only after particles near the surface of the dissolving CS have been freed and begun to degrade. As discussed in section for protein release from H6 PBAE gels, protein was prevented from leaving the gel matrix until the gel showed signs of hydrolysis. This illustrates that protein was not prematurely released prior to exposure of HG particles during composite erosion. Based on visualization of HG particles embedded into CS, gel particles that were at or near the surface degraded following erosion of CS. Further dissolution of CS exposed more particles below the surface to allow polymer degradation. This observation demonstrates how the sustained release of protein from embedded HG particles was governed by CS dissolution and HG degradation. These macroscopic observations confirm microcomputed tomography (microCT) analysis from a previous study that showed a homogeneous distribution of HG particles throughout the CS matrix.20 The slow decay in the release of protein from the 1 wt% HG composites can be attributed to the slow decrease in surface area of CS over the duration of the release study, which leads to a smaller amount of gel particles exposed over time and thus a slowly decreasing mass of protein being released.
The two different higher loadings (high direct and 10 wt% HG) resulted in similar release kinetics. The advantage of the 10 wt% composites, however, is the smaller variability among samples and therefore better control of protein release. During the first several days of the release, a large surface area of CS was exposed, allowing for a much larger volume of gel particles to be released. This could lead to an accumulation of gel particles releasing protein early on and thus contribute to the burst witnessed from the 10 wt% HG composites. As for the high direct loading of lysozyme in CS, greater early protein release could result from a segregation effect due to solubilized protein accumulating at the surfaces in excess water as the CS slurry sets. Furthermore, as water was consumed by reaction from the hemihydrate to dehydrate forms of CS, some solubilized protein may be precipitated and trapped within the setting matrix. With the addition of carrier particles, such as protein-loaded PBAE, the variations can be reduced or eliminated altogether.
Simvastatin release from CS
To demonstrate the release of small molecule osteogenic agents, simvastatin was directly loaded into CS. Ginebra et al. described how drug may be trapped between crystals in a ceramic matrix: dissolved in the liquid within pores, adsorbed to the crystal surface, or in solid form as drug crystals.46 Because simvastatin has low solubility in water, the drug was likely to be suspended in the CS slurry and not dissolved. With this in mind, as water was consumed during setting or excess water was driven to the exterior of the samples, simvastatin remained in the CS matrix. As a result, the release of drug was governed by the rate at which the material dissolved. Based on the ability to control release by directly loading simvastatin in CS, incorporating the drug in hydrogel particles was unnecessary and would introduce unneeded material in the system.
Nyan et al. also investigated simvastatin directly loaded into CS. They conducted an in vivo study in critically-sized rat calvarial defects.29 A dramatic increase in bone volume was observed after 8 weeks, which was significantly greater than for any other group.29 However, substantial soft tissue inflammation was also observed during the first several weeks, and the authors concluded that this was most likely caused by a burst release of simvastatin following early resorption of CS.29 Although dissolution of the implants was not assessed, the present results support the hypothesis that rapid erosion of CS would release a burst of drug.
Mechanical properties
With the incorporation of drug either directly loaded or in PBAE particles embedded in CS, compromise of mechanical stability can become a concern. The present drug-loaded composites are being developed as bone graft substitutes with tunable drug delivery capabilities. With this in mind, it is important to note that much of the strength of the grafting device must be preserved to allow for a stable implant capable of providing a suitable ‘healing chamber’ for tissue regeneration as well as allow for controlled release of drug as the implant dissolves. Because a previous study demonstrated that the addition of additives as high as 10 wt% HG did not substantially affect dissolution of the composites, attention was focused on preservation of the mechanical strength of the composites.20 In the present study, the comparison of compressive properties for CS with directly loaded simvastatin to those for blank CS showed that the drug did not have a significant effect on the overall strength. However, incorporation of simvastatin reduced the modulus about 30% compared to the CS control samples. Although a small molecule drug, simvastatin may be creating small disruptions in the CS crystal interactions that could lead to greater shearing along crystal surfaces resulting in more deformation prior to failure. Yin et al. reported that a small amount (10%) of directly loaded simvastatin did not significantly compromise the compressive strength of calcium phosphate.47 In the current study, 20 mg, or 20 wt%, was loaded into CS prior to sample production. Although the materials were different, this finding demonstrates that the incorporation of a small amount of simvastatin does not appear to affect the supporting material, thus preserving its overall strength.
The effect of loading a much larger drug molecule compared to simvastatin on mechanical stability of CS was also tested. Protein, i.e., lysozyme, was either directly loaded into the CS matrix, or it was polymerized into H6-PBAE hydrogel particles that were then incorporated into the CS. Misch et al. tested the mechanical properties of human trabecular bone from the mandible.48 The ultimate compressive strength and elastic modulus were determined to be 3.9 ± 2.7 MPa 96.2 ± 40.6 MPa, respectively. Based on these numbers, although CS implants containing either directly loaded lysozyme or 1 wt% HG particles exhibited a 20–25% decrease in strength compared to CS blanks, they had properties comparable to those of tissue at the intended implantation site. Increasing the HG particle loading to 10 wt%, however, has a greater adverse effect on the mechanical stability of CS. The significant decrease in strength and modulus for 10 wt% HG particle loading demonstrates a possible limit to the amount of drug-loaded particles that can be added. In a previous study, Orellana et al. showed that the addition of 10 wt% of A11 PBAE particles decreased the strength and modulus of CS by 50% and 70%, respectively20, which suggested a comparable upper threshold of particle loading. The particle loading effect could be attributed to the altered microstructure due to stress concentrators created by the gel particles or even minor bubbles/pores.20 Based on the present findings, a loading of 1 wt% HG may be the best option to preserve as much of the mechanical stability of CS while allowing for the controlled release of drug as the material erodes.
Conclusion
Controlled release of bioactive molecules has the potential to greatly enhance the ability of calcium sulfate-based composites to become more effective bone graft substitutes. In the present study, release kinetics were investigated for two different agents, a small, hydrophobic drug and a much larger, hydrophilic protein, incorporated into CS. Controlled release of both molecules could be achieved but by different means. Whereas direct loading was appropriate for simvastatin, control of lysozyme release required incorporation of the protein in degradable polymeric particles prior to embedding in CS. Adding a drug, either directly into CS or via PBAE particles, however, can negatively affect mechanical properties. In particular, greater amounts of gel particles significantly reduced compressive strength and modulus. Therefore, the balance between drug content and mechanical properties must be determined for different applications. Based on the current findings, CS-based synthetic bone graft substitutes demonstrated the ability to sustainably release both large and small bioactive molecules, opening up potential opportunities to deliver a broad range of therapeutic agents.
Acknowledgments
This research supported by the National Institutes of Health (DE019645) and Kentucky NASA EPSCoR (NNX08BA13A).
References
- 1.Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. The International Journal of Oral & Maxillofacial Implants. 2009;24:237. [PubMed] [Google Scholar]
- 2.Chiapasco M, Zaniboni M, Rimondini L. Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: a 2–4-year prospective study on humans. Clinical Oral Implants Research. 2007;18(4):432–440. doi: 10.1111/j.1600-0501.2007.01351.x. [DOI] [PubMed] [Google Scholar]
- 3.Guarnieri R, Pecora G, Fini M, Aldini NN, Giardino R, Orsini G, Piattelli A. Medical Grade Calcium Sulfate Hemihydrate in Healing of Human Extraction Sockets: Clinical and Histological Observations at 3 Months. Journal of Periodontology. 2004;75(6):902–908. doi: 10.1902/jop.2004.75.6.902. [DOI] [PubMed] [Google Scholar]
- 4.Hitti RA, Kerns DG. Guided bone regeneration in the oral cavity: a review. Open Pathology Journal. 2011;5:33–45. [Google Scholar]
- 5.Simion M, Dahlin C, Rocchietta I, Stavropoulos A, Sanchez R, Karring T. Vertical ridge augmentation with guided bone regeneration in association with dental implants: an experimental study in dogs. Clinical Oral Implants Research. 2007;18(1):86–94. doi: 10.1111/j.1600-0501.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 6.Simion M, Fontana F, Rasperini G, Maiorana C. Vertical ridge augmentation by expanded-polytetrafluoroethylene membrane and a combination of intraoral autogenous bone graft and deproteinized anorganic bovine bone (Bio Oss) Clinical Oral Implants Research. 2007;18(5):620–629. doi: 10.1111/j.1600-0501.2007.01389.x. [DOI] [PubMed] [Google Scholar]
- 7.Tamimi F, Torres J, Gbureck U, Lopez-Cabarcos E, Bassett DC, Alkhraisat MH, Barralet JE. Craniofacial vertical bone augmentation: A comparison between 3D printed monolithic monetite blocks and autologous onlay grafts in the rabbit. Biomaterials. 2009;30(31):6318–6326. doi: 10.1016/j.biomaterials.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Zhang Z, Zhao J, Zhang X, Sun X, Xia L, Chang Q, Ye D, Jiang X. Vertical alveolar ridge augmentation with β-tricalcium phosphate and autologous osteoblasts in canine mandible. Biomaterials. 2009;30(13):2489–2498. doi: 10.1016/j.biomaterials.2008.12.067. [DOI] [PubMed] [Google Scholar]
- 9.Chiapasco M. Bone augmentation procedures in implant dentistry. Quintessence Pub. Co.; 2009. [PubMed] [Google Scholar]
- 10.Clavero J, Lundgren S. Ramus or Chin Grafts for Maxillary Sinus Inlay and Local Onlay Augmentation: Comparison of Donor Site Morbidity and Complications. Clinical Implant Dentistry and Related Research. 2003;5(3):154–160. doi: 10.1111/j.1708-8208.2003.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 11.Gao C, Huo S, Li X, You X, Zhang Y, Gao J. Characteristics of calcium sulfate/gelatin composite biomaterials for bone repair. J. Biomater. Sci., Polym. Ed. 2007;18:799–824. doi: 10.1163/156856207781367710. [DOI] [PubMed] [Google Scholar]
- 12.Hitti RA, Kerns DG. Guided Bone Regeneration in the Oral Cavity: A Review. The Open Pathology Journal. 2011;5:33–45. [Google Scholar]
- 13.Jamali A, Hilpert A, Debes J, Afshar P, Rahban S, Holmes R. Hydroxyapatite/Calcium Carbonate (HA/CC) vs. Plaster of Paris: A Histomorphometric and Radiographic Study in a Rabbit Tibial Defect Model. Calcified Tissue International. 2002;71(2):172–178. doi: 10.1007/s00223-001-1087-x. [DOI] [PubMed] [Google Scholar]
- 14.Kenley RA, Yim K, Abrams J, Ron E, Turek T, Marden LJ, Hollinger JO. Biotechnology and Bone Graft Substitutes. Pharmaceutical Research. 1993;10(10):1393–1401. doi: 10.1023/a:1018902720816. [DOI] [PubMed] [Google Scholar]
- 15.Kenny SM, Buggy M. Bone cements and fillers: A review. Journal of Materials Science: Materials in Medicine. 2003;14(11):923–938. doi: 10.1023/a:1026394530192. [DOI] [PubMed] [Google Scholar]
- 16.Lewis KN, Thomas MV, Puleo DA. Mechanical and degradation behavior of polymer-calcium sulfate composites. Journal of Materials Science: Materials in Medicine. 2006;17:531–537. doi: 10.1007/s10856-006-8936-0. [DOI] [PubMed] [Google Scholar]
- 17.Tay BK, Patel VV, Bradford DS. Calcium sulfate- and calcium phosphate-based bone substitutes. Mimicry of the mineral phase of bone. The Orthopedic clinics of North America. 1999;30(4):615–623. doi: 10.1016/s0030-5898(05)70114-0. [DOI] [PubMed] [Google Scholar]
- 18.Triplett RG, Schow SR. Autologous bone grafts and endosseous implants: Complementary techniques. Journal of Oral and Maxillofacial Surgery. 1996;54(4):486–494. doi: 10.1016/s0278-2391(96)90126-3. [DOI] [PubMed] [Google Scholar]
- 19.Guarnieri R, Grassi R, Ripari M, Pecora G. Maxillary sinus augmentation using granular calcium sulfate (surgiplaster sinus): radiographic and histologic study at 2 years. International Journal of Periodontics and Restorative Dentistry. 2006;26:79–85. [PubMed] [Google Scholar]
- 20.Orellana BR, Thomas MV, Dziubla TD, Shah NM, Hilt JZ, Puleo DA. Bioerodible calcium sulfate/poly(β-amino ester) hydrogel composites. Journal of the Mechanical Behavior of Biomedical Materials. 2013;26(0):43–53. doi: 10.1016/j.jmbbm.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groeneveld E, Burger E. Bone morphogenetic proteins in human bone regeneration. European Journal of Endocrinology. 2000;142(1):9–21. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 22.Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ Journal of Surgery. 2001;71(6):354–361. [PubMed] [Google Scholar]
- 23.Pecora G, Andreana S, Margarone JE, 3rd, Covani U, Sottosanti JS. Bone regeneration with a calcium sulfate barrier. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:424–429. doi: 10.1016/s1079-2104(97)90043-3. [DOI] [PubMed] [Google Scholar]
- 24.Thomas MV, Puleo DA. Calcium sulfate: properties and clinical applications. Journal of Biomedical Materials Research Part B. 2009;88B:597–610. doi: 10.1002/jbm.b.31269. [DOI] [PubMed] [Google Scholar]
- 25.Thomas MV, Puleo DA, Al-Sabbagh M. Calcium sulfate: a review. Journal of Long-Term Effects of Medical Implants. 2005;15:599–607. doi: 10.1615/jlongtermeffmedimplants.v15.i6.30. [DOI] [PubMed] [Google Scholar]
- 26.Guarnieri R, Pecora G, Fini M, Aldini NN, Giardino R, Orsini G, Piattelli A. Medical grade calcium sulfate hemihydrate in healing of human extraction sockets: clinical and histological observations at 3 months. Journal of Periodontology. 2004;75:902–908. doi: 10.1902/jop.2004.75.6.902. [DOI] [PubMed] [Google Scholar]
- 27.Rosenblum SF, Frenkel S, Ricci JR, Alexander H. Diffusion of fibroblast growth factor from a plaster of paris carrier. Journal of Applied Biomaterials. 1993;4(1):67–72. doi: 10.1002/jab.770040109. [DOI] [PubMed] [Google Scholar]
- 28.Hak DJ. The Use of Osteoconductive Bone Graft Substitutes in Orthopaedic Trauma. Journal of the American Academy of Orthopaedic Surgeons. 2007;15(9):525–536. doi: 10.5435/00124635-200709000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Nyan M, Sato D, Oda M, Machida T, Kobayashi H, Nakamura T, Kasugai S. Bone Formation With the Combination of Simvastatin and Calcium Sulfate in Critical-Sized Rat Calvarial Defect. Journal of Pharmacological Sciences. 2007;104(4):384–386. doi: 10.1254/jphs.sc0070184. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki Y, Oida S, Akimoto Y, Shioda S. Response of the mouse femoral muscle to an implant of a composite of bone morphogenetic protein and plaster of Paris. Clinical Orthopaedics and related research. 1988;234:240–249. [PubMed] [Google Scholar]
- 31.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. Journal of Controlled Release. 2002;83(1):53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins AM, Puleo DA, Hilt JZ. Effect of macromer synthesis time on the properties of the resulting poly(β-amino ester) degradable hydrogel. Journal of Applied Polymer Science. 2011;122(2):1420–1426. [Google Scholar]
- 33.Kamath KR, Park K. Biodegradable hydrogels in drug delivery. Advanced Drug Delivery Reviews. 1993;11(1–2):59–84. [Google Scholar]
- 34.Brey DM, Ifkovits JL, Mozia RI, Katz JS, Burdick JA. Controlling poly(Î2-amino ester) network properties through macromer branching. Acta Biomaterialia. 2008;4(2):207–217. doi: 10.1016/j.actbio.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson DG, Tweedie CA, Hossain N, Navarro SM, Brey DM, Van Vliet KJ, Langer R, Burdick JA. A Combinatorial Library of Photocrosslinkable and Degradable Materials. Advanced Materials. 2006;18(19):2614–2618. [Google Scholar]
- 36.Ripamonti U, Herbst N-N, Ramoshebi LN. Bone morphogenetic proteins in craniofacial and periodontal tissue engineering: Experimental studies in the non-human primate Papio ursinus. Cytokine & Growth Factor Reviews. 2005;16(3):357–368. doi: 10.1016/j.cytogfr.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Wozney JM. Overview of bone morphogenetic proteins. Spine. 2002;27(16S):S2–S8. doi: 10.1097/00007632-200208151-00002. [DOI] [PubMed] [Google Scholar]
- 38.Cho D-C, Kim K-T, Jeon Y, Sung J-K. A synergistic bone sparing effect of curcumin and alendronate in ovariectomized rat. Acta Neurochirurgica. 2012;154(12):2215–2223. doi: 10.1007/s00701-012-1516-9. [DOI] [PubMed] [Google Scholar]
- 39.Park J-B. The use of simvastatin in bone regeneration. Med. Oral Patol. Oral Cir. Bucal. 2009;14(9):485–489. [PubMed] [Google Scholar]
- 40.Wu Z, Liu C, Zang G, Sun H. The effect of simvastatin on remodelling of the alveolar bone following tooth extraction. International Journal of Oral and Maxillofacial Surgery. 2008;37(2):170–176. doi: 10.1016/j.ijom.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Puleo DA, Kissling RA, Sheu MS. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials. 2002;23(9):2079–2087. doi: 10.1016/s0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan C, Katare YK, Muthukumaran T, Panda AK. Effect of additives on encapsulation efficiency, stability and bioactivity of entrapped lysozyme from biodegradable polymer particles. Journal of Microencapsulation. 2005;22(2):127–138. doi: 10.1080/02652040400026400. [DOI] [PubMed] [Google Scholar]
- 43.Hawkins AM, Milbrandt TA, Puleo DA, Zach Hilt J. Composite hydrogel scaffolds with controlled pore opening via biodegradable hydrogel porogen degradation. Journal of Biomedical Materials Research Part A. 2013 doi: 10.1002/jbm.a.34697. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 44.Hawkins AM, Milbrandt TA, Puleo DA, Hilt JZ. Synthesis and analysis of degradation, mechanical and toxicity properties of poly(β-amino ester) degradable hydrogels. Acta Biomaterialia. 2011;7(5):1956–1964. doi: 10.1016/j.actbio.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Wattamwar PP, Biswal D, Cochran DB, Lyvers AC, Eitel RE, Anderson KW, Hilt JZ, Dziubla TD. Synthesis and characterization of poly(antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomaterialia. 2012;8(7):2529–2537. doi: 10.1016/j.actbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 46.Ginebra M-P, Canal C, Espanol M, Pastorino D, Montufar EB. Calcium phosphate cements as drug delivery materials. Advanced Drug Delivery Reviews. 2012;64(12):1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Yin H, Li Y-G, Si M, Li J-M. Simvastatin-loaded macroporous calcium phosphate cement: Preparation, in vitro characterization, and evaluation of in vivo performance. Journal of Biomedical Materials Research Part A. 2012;100A(11):2991–3000. doi: 10.1002/jbm.a.34228. [DOI] [PubMed] [Google Scholar]
- 48.Misch CE, Qu Z, Bidez MW. Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. Journal of Oral and Maxillofacial Surgery. 1999;57(6):700–706. doi: 10.1016/s0278-2391(99)90437-8. [DOI] [PubMed] [Google Scholar]