Abstract
Objective
Despite the rarity of uterine papillary serous carcinoma (UPSC) and uterine clear cell carcinoma (UCCC), they contribute disproportionately to endometrial cancer deaths. Sufficient clinical information regarding treatment and prognosis is lacking. The aim of this study is to evaluate treatment outcomes in a rare cancer cohort based on the experience at two tertiary care cancer centers.
Methods
Clinicopathologic data were retrospectively collected on 279 patients with UPSC and UCCC treated between 1995 to 2011. Mode of surgery, use of adjuvant treatment, and dissection of paraaoritc lymph nodes were evaluated for their association with overall survival (OS) and progression-free survival (PFS).
Results
40.9% of patients presented with stage I disease, 6.8% of patients presented with stage II disease and 52.3% of patients presented with stages III and IV. Median follow-up was 31 months (range, 1 to 194 months). OS and PFS at 5 years were 63.0% and 51.9%, respectively. OS and PFS were not affected by mode of surgery (open vs. robotic approach; OS: hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.28 to 1.62; PFS: HR, 0.78; 95% CI, 0.40 to 1.56). Adjuvant treatment was associated with improved OS in stages IB-II (HR, 0.14; 95% CI, 0.02 to 0.78; p=0.026) but not in stage IA disease. There was no difference in OS or PFS based on the performance of a paraaoritc lymph node dissection.
Conclusion
Minimally invasive surgical staging appears a reasonable strategy for patients with non-bulky UPSC and UCCC and was not associated with diminished survival. Adjuvant treatment improved 5-year survival in stages IB-II disease.
Keywords: Adenocarcinoma, Clear Cell; Disease-free Survival; Endometrial Neoplasms; Lymph Node Excision; Retrospective Studies
INTRODUCTION
Uterine papillary serous carcinoma (UPSC) and uterine clear cell carcinoma (UCCC) are rare entities, accounting for 10% to 15% of all endometrial cancer cases [1,2,3,4,5]. Despite this, they contribute disproportionately to endometrial cancer mortality, with 39% to 50% of endometrial cancer-related deaths attributed to these high-risk histologic subtypes [2,3,5,6]. Data on optimal treatment is lacking secondary to small numbers and the absence of current, prospective data.
The preferred mode of surgery in these patients, has not been thoroughly evaluated. While the LAP2 trial demonstrated the feasibility and safety of laparoscopic surgical staging in endometrial cancer, only 12% of this patient cohort had UPSC or UCCC [7,8]. Aside from one recent, retrospective analysis demonstrating comparable progression-free survival (PFS) and overall survival (OS) in a cohort of high-grade endometrial cancer patients undergoing minimally invasive versus open surgical staging, no other studies have evaluated surgical staging approach in this high-risk histologic subgroup [9].
There is also limited data on appropriate adjuvant therapy in this patient population. In the largest retrospective analysis of 142 surgical stage I patients with UPSC, PFS was improved and recurrence risk was significantly reduced with the addition of adjuvant platinum/taxane chemotherapy (recurrence risk 11.2% vs. 28.3%, p=0.013) [10]. However, there may be a subset of stage IA UPSC and UCCC patients in whom recurrence risk is sufficiently low following comprehensive surgical staging and for whom observation alone may be appropriate in the postoperative setting [11,12].
Finally, while the therapeutic role of systematic pelvic lymphadenectomy in endometrial cancer has been evaluated, very few trials have assessed the role of paraaoritc lymph node dissection on PFS and OS [13,14,15,16]. In particular, survival effects have not been evaluated in patients with UPSC or UCCC. As most type II endometrial cancer patients will receive adjuvant treatment secondary to concern for the aggressive nature of these histologic subtypes, the question remains as to whether removal of nodal tissue (and potential microscopic foci of disease) leads to improved outcomes. The purpose of this analysis was therefore to broadly evaluate treatment in UPSC and UCCC at two, high volume cancer centers.
MATERIALS AND METHODS
This was an Institutional Review Board-approved retrospective chart review assessing factors associated with PFS and OS among women diagnosed with UPSC and UCCC at two tertiary academic centers, the University of Washington Medical Center and Swedish Medical Center, Seattle, WA. The study period ranged from January 1995 to December 2011. Subjects were identified by pathology and tumor registry databases at each institution. Inclusion criteria were women diagnosed with UPSC and UCCC of the uterus who underwent surgical staging. Initial staging was performed per the International Federation of Gynecology and Obstetrics (FIGO) criteria at the time of diagnosis. All patients diagnosed prior to 2009 were reassigned staging in accordance with FIGO 2009 criteria. Patients were excluded from analysis if they did not undergo pelvic lymphadenectomy unless there was evidence of stage IV disease at the time of surgery. paraaoritc lymphadenectomy was performed except in specific instances at the discretion of the surgeon. Pathology was reviewed by a gynecologic pathologist at each center to confirm UPSC and UCCC diagnosis. Abstraction of data from patient medical records for demographic information, clinicopathologic variables and clinical outcomes was performed.
The primary objective of the study was to evaluate the effect of traditional open surgical staging versus robotic surgical staging on OS and PFS. Secondary objectives of the study were to: (1) evaluate the effect of none versus any adjuvant therapy (radiation, chemotherapy, or both) on OS and PFS among patients with stage IA and stages IB-II disease; and (2) to evaluate the effect of paraaoritc lymphadenectomy on OS and PFS in stages I to II and III. The study was initially designed to evaluate the effect of specific treatment modalities, including radiation therapy (vaginal brachytherapy and/or pelvic radiation therapy), chemotherapy and combined chemoradiation therapy, on OS and PFS among early stage patients. Analysis was limited; however, based on small numbers in each group, precluding our ability to draw conclusions based on individual treatment modality. For this reason, all treatment groups were also combined into one adjuvant therapy group and compared to those patients who underwent observation alone. OS, PFS, and 95% confidence intervals (CIs) by stage were calculated from the date of diagnosis until the date of death or date of first disease progression, respectively, or date of last status if the patient was alive. Patients without disease progression or death at last follow-up visit were considered censored. A multivariate survival analysis was performed to examine the association between type of surgery (open vs. robotic) and OS and PFS among all stages, estimating hazard ratios (HRs) and 95% CIs and assessing for potential confounders of age, stage, histology, and adjuvant treatment. We adjusted for specific confounders based on those that changed the univariate crude HR by 10% or greater. We performed a subanalysis of surgical type by OS and PFS among stages I to II only, adjusting for age, histology, and adjuvant treatment. Multivariate survival analyses were performed to evaluate the association between adjuvant therapy (none vs. chemotherapy, radiation therapy or chemotherapy and radiation therapy combined, as well as none versus any, as described above) and OS and PFS among stage IA, IB, and II patients adjusting for age and histology. We assessed the relationship between histologic subtype and OS and PFS adjusting for age, stage and adjuvant treatment. Multivariate survival analysis was also performed to assess the association between paraaoritc lymph node dissection (none vs. any), adjusting for age, histology, and adjuvant treatment. Survival curves were generated according to the Kaplan-Meier method and compared using the log-rank test. Statistical analyses were performed using Stata ver. 11.0 (StataCorp., College Station, TX, USA).
RESULTS
Two hundred seventy-nine patients were identified with UPSC or UCCC during the study period. Median follow-up time for the study cohort was 31 months (range, 1 to 194 months) (Table 1). The median age was 66 years. One hundred thirty-three patients (47.7%) were stages I to II and 146 patients (52.3%) were stages III to IV. Histology was pure papillary serous in 139 patients (49.8%) and pure clear cell in 77 patients (27.6%). The remainder of patients had mixed tumors. Two hundred twenty-nine patients (82.1%) underwent open surgical staging and 49 patients (17.6%) underwent robotic surgical staging. The most notable change in treatment pattern over the study period was the introduction of surgical staging using a robotic-assisted laparoscopic technique. The earliest robotic procedures in our cohort were performed in 2007 with robotic staging becoming more routine practice in 2008 to 2009. A majority of patients (66.3%) underwent omentectomy or omental sampling and were optimally debulked (92.1%). All patients underwent pelvic lymphadenectomy with the exception of three patients who underwent an isolated paraaoritc lymphadenectomy and 20 stage IV patients who did not have lymph node sampling performed. Median number of lymph nodes retrieved was 13 (range, 1 to 46). Paraaoritc lymph node sampling was performed in 78.9% of patients with stages I and II disease and in 79.3% with stage III disease.
Table 1.
Patient and tumor characteristics among women with UPSC and UCCC treated at UWMC and SMC in Seattle, WA (n=279)
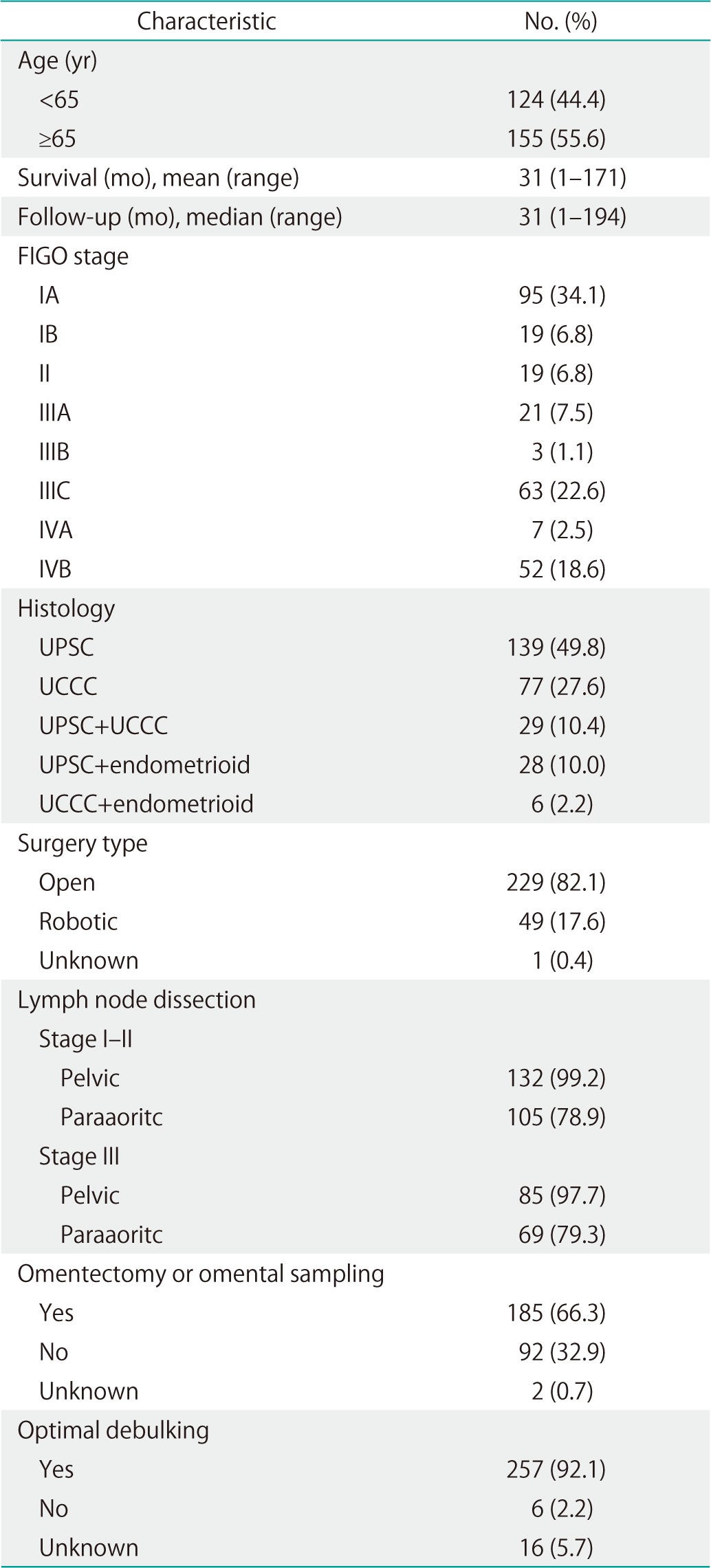
Values are presented as number (%) unless otherwise indicated.
UCCC, uterine clear cell carcinoma; FIGO, The International Federation of Gynecology and Obstetrics; SMC, Swedish Medical Center; UPSC, uterine papillary serous carcinoma; UWMC, University of Washington Medical Center.
The majority of patients received adjuvant therapy following surgical staging, including radiation therapy (pelvic, vaginal brachytherapy, or both), chemotherapy or combined chemotherapy and radiation (Table 2). Of the stage IA patients, 67 (70.5%) received adjuvant therapy, including 4 of 7 patients with disease confined to a polyp. Of the stages IB and II patients, 16 (88.9%) and 14 (87.5%) received adjuvant treatment, respectively. Among stage III and IV patients, 75 (92.6%) and 51 (91.1%) received adjuvant therapy.
Table 2.
Adjuvant treatment by stage in patients with UPCS and UCCC treated at UWMC and SMC in Seattle, WA
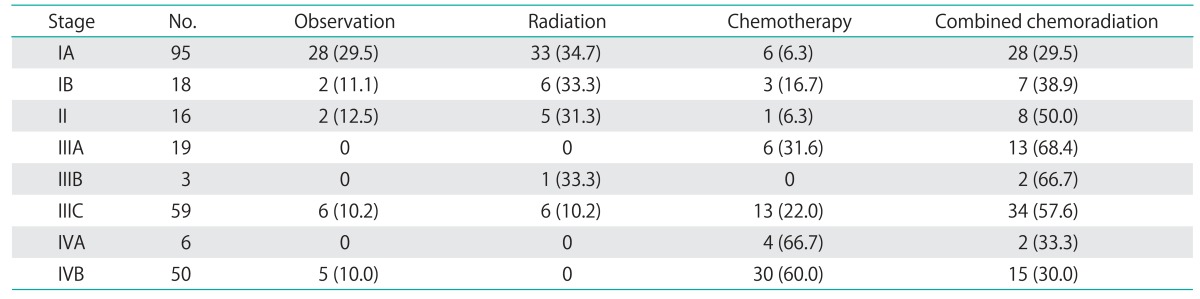
Values are presented as number (%). Adjuvant therapy was unknown in 1 stage IB patient, 3 stage II patients, 2 stage IIIA patients, 4 stage IIIC patients, 1 stage IVA patient, and 2 stage IVB patients. These patients were not included in the treatment analysis.
UCCC, uterine clear cell carcinoma; SMC, Swedish Medical Center; UPSC, uterine papillary serous carcinoma; UWMC, University of Washington Medical Center.
OS and PFS at 5 years were 63.0% (95% CI, 55.1 to 69.8) and 51.9% (95% CI, 44.5 to 58.6), respectively. OS 5 year survival by stage was as follows: stage I, 79.6%; stage II, 68.0%; stage III, 54.6%; and stage IV, 40.9% (Table 3).
Table 3.
Five-year overall and progression-free survival by substage in patients with UPSC and UCCC
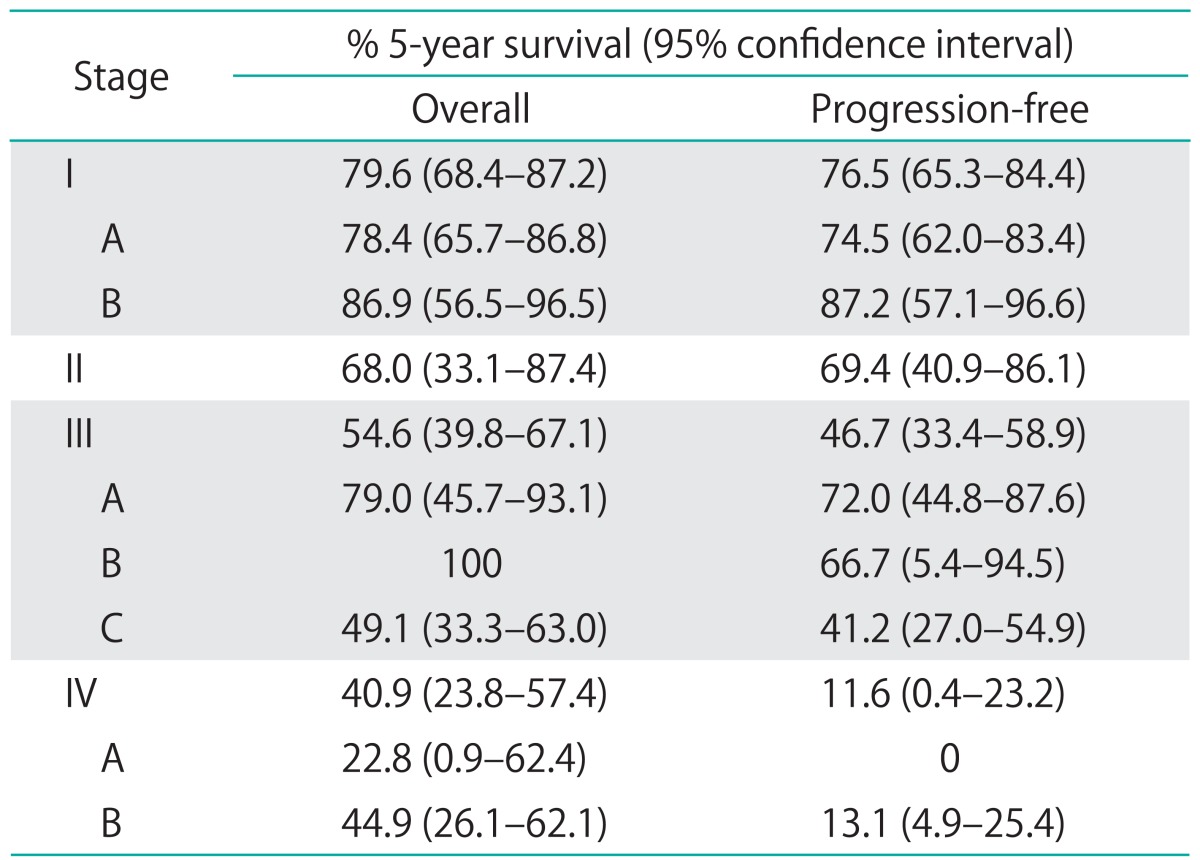
UCCC, uterine clear cell carcinoma; UPSC, uterine papillary serous carcinoma.
Evaluation of OS and PFS based on surgical approach, open versus robotic, found no significant difference in survival, for all stages combined and for women with early stage disease (Table 4). There was also no significant difference in either OS or PFS based on histologic subtype. With regards to adjuvant treatment in early stage disease, evaluation found that 5-year OS was significantly improved in stage IB to II patients who received adjuvant treatment, including radiation, chemotherapy or both (unadjusted HR, 0.14; 95% CI, 0.02 to 0.78; p=0.03) (Fig. 1). Adjuvant therapy among stage IB to II patients was not associated with a significant improvement in 5-year PFS (HR, 0.41; 95% CI, 0.05 to 3.47), although these findings were limited by small numbers. In stage IA disease, no difference in 5-year OS or PFS was observed with the addition of adjuvant treatment (OS: HR, 1.19; 95% CI, 0.38 to 3.71; PFS: HR, 3.35; 95% CI, 0.75 to 15.00).
Table 4.
Multivariate analyses of overall and progression-free survival by risk factor in UPSC and UCCC
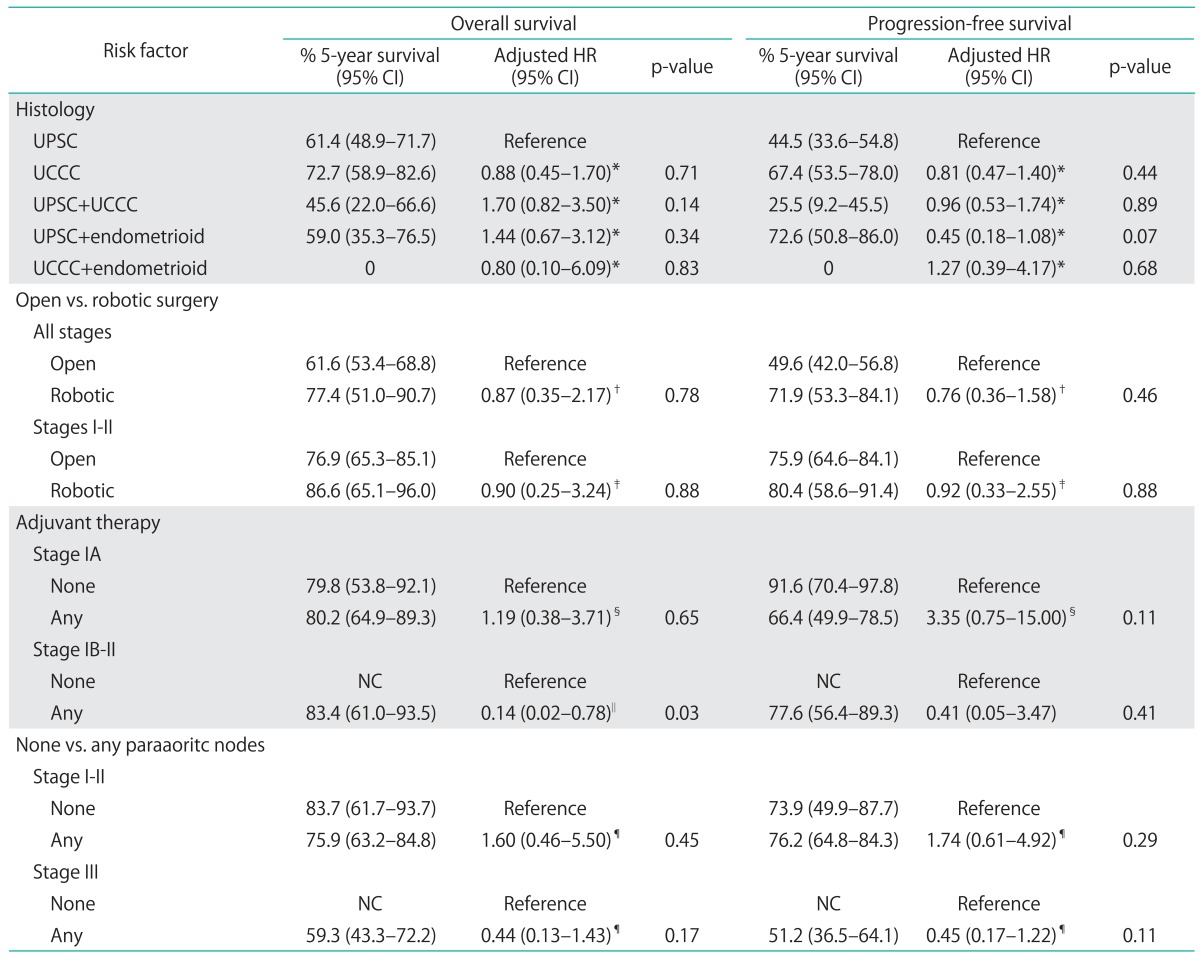
UCCC, uterine clear cell carcinoma; CI, confidence interval; HR, hazard ratio; NC, not calculable; UPSC, uterine papillary serous carcinoma.
*Adjusted for age, stage, adjuvant treatment. †Adjusted for age, stage, histology, adjuvant treatment. ‡Adjusted for age, histology, adjuvant treatment. §Adjusted for age, histology. ∥Too few cases to adjust. ¶Adjusted for age, histology, surgery, adjuvant treatment.
Fig. 1.
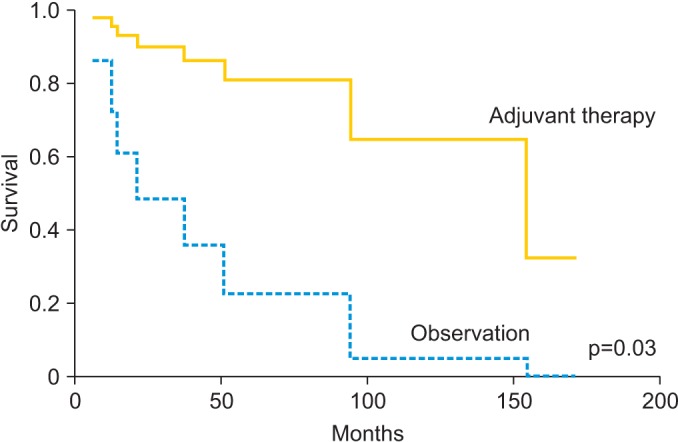
Overall survival in stages IB-II patients with uterine papillary serous carcinoma and clear cell carcinoma with and without adjuvant therapy.
The performance of a paraaoritc lymph node dissection did not improve OS or PFS, but our results may have been biased. The stage III patients who underwent paraaoritc lymph node dissection were significantly more likely to receive combined chemoradiation therapy compared to those who did not have lymph node dissection performed (data not shown).
DISCUSSION
We found that minimally-invasive, robotic surgical staging appears an appropriate strategy in type II endometrial cancers without reduction in OS or PFS, similar to what has been observed in a type I population. Our study confirms a survival benefit with any adjuvant treatment in stage IB to II disease, including radiation therapy, chemotherapy or both, although these results were based on few cases. While we did not find a therapeutic effect of paraaoritc lymphadenectomy in our high-risk patient cohort, a role for comprehensive surgical staging may be beneficial, in particular in further guiding adjuvant therapy and discussing prognosis.
While there have been several studies evaluating the role of minimally invasive surgical staging in endometrial cancer, including the LAP2 trial, there have been very few evaluating patients of high risk histology (i.e., with UPSC or UCCC) [7,8,17,18,19]. Fader et al. [9] evaluated both a traditional and minimally invasive surgical staging approach in patients with UPSC, UCCC and grade 3 endometrioid adenocarcinoma and noted similar, favorable results for laparoscopic/robotic surgical staging. Our study confirms that a minimally invasive surgical approach appears appropriate in non-bulky disease among patients with a high-risk histologic subtype.
A significant OS advantage was observed in our stage IB to II cohort of patients with the addition of any adjuvant therapy (chemotherapy, radiation therapy, or both) following surgical staging, although the findings reported are not adjusted for confounders because of small sample size. Other retrospective studies support the use of adjuvant therapy in this patient population although specific recommendations as to appropriate regimens have yet to be defined [10,20,21,22,23]. Survival outcomes in stage IA patients in our cohort were not affected by adjuvant therapy. In our cohort of 95 surgically staged IA patients, the 5-year OS was 80.2% for adjuvant therapy and 79.8% for observation. While our study was not powered sufficiently to detect a small difference in survival, the literature does suggest that there may be a subset of type II endometrial cancer patients in whom observation alone may be appropriate following complete surgical staging [12,24,25]. For example, Kelly et al. [12] demonstrated no tumor recurrences in patients with stage IA disease without residual cancer identified in the hysterectomy specimen who underwent observation alone. In contrast, in patients with stage IA disease with residual tumor identified in the hysterectomy specimen, the addition of platinum-based chemotherapy significantly improved disease-free and OS [12]. Havrilesky et al. [24] also identified a low risk of recurrence in surgically staged IA UPSC patients undergoing observation alone, with only one vaginal recurrence noted in a cohort of 22 patients, which was subsequently salvaged with radiotherapy. While there may be a subpopulation of stage IA patients appropriate for observation alone following surgical staging, further studies are warranted to identify reliable clinical characteristics in this subpopulation to predict for improved prognosis and feasibility of omitting adjuvant treatment.
The role of systematic lymphadenectomy, both pelvic and paraaoritc, as a treatment modality for endometrial cancer has been questioned [13,14,16,26]. Typically, it is part of complete surgical staging in patients with type II tumors given their propensity for metastatic spread, even in the absence of risk factors such as myometrial and lymphovascular space invasion [20,21,22,27]. No association was noted between the performance of a paraaoritc lymphadenectomy and OS or PFS in our patient cohort, despite that a larger proportion of stage III patients undergoing paraaoritc lymphadenectomy also received combined chemoradiation therapy. This suggests that stage III patients in whom paraaoritc lymph node dissection was omitted may have had other favorable tumor characteristics biasing our results. Several trials have evaluated the role of lymphadenectomy in endometrial cancer survival with differing results [13,14,15]. The A Study in the Treatment of Endometrial Cancer (ASTEC) trial, a large, prospective, randomized controlled trial, evaluated the role of pelvic lymphadenectomy in patients with suspected preoperative disease confined to the uterine corpus and found no improvement in OS or recurrence-free survival with pelvic lymphadenectomy [13]. In contrast, a large retrospective trial, the Survival Effect of Para-Aortic Lymphadenectomy (SEPAL) study, evaluated pelvic lymphadenectomy versus combined pelvic and paraaoritc lymphadenectomy and found a statistically significant improvement in OS in patients at intermediate and high risk of recurrence (including type II histologic tumor subtypes) that had both pelvic and paraaoritc lymphadenectomy. This finding, however, may have been affected by a greater proportion of patients in the pelvic plus paraaoritc lymphadenectomy group receiving adjuvant chemotherapy [15]. The therapeutic benefit of paraaoritc lymphadenectomy thus remains unclear in the endometrial cancer population. The value of comprehensive surgical staging in type II endometrial cancers may be its ability to predict recurrence risk and prognosis in patients with occult, advanced stage disease [20,21,23]. In patients with early stage UPSC and UCCC, it appears imperative that complete staging information be obtained prior to making recommendations for adjuvant treatment versus observation alone.
This study had several limitations. Despite being one of the largest series in the literature, our analyses were affected by small sample size that limited our ability to detect differences between treatment groups, possibly leading to type II errors. As a result of the small sample size, stratifying by treatment subgroups (i.e., radiation therapy alone, chemotherapy alone, and combined chemoradiation therapy) was not feasible as there were too few subjects in each group to compare. Additionally, we were unable to assess specific mode of radiation therapy (pelvic, vaginal brachytherapy, or both) and this limits our ability to draw conclusions regarding appropriate therapeutic regimens for use in this patient subset. Our study was retrospective and non-randomized regarding the specific treatments evaluated and may have resulted in selection bias in the observed associations. While the gold standard for treatment evaluation is the randomized controlled trial, such a study may be difficult to undertake in a cohort of type II endometrial cancer patients because of the rarity of the histologic subtypes that comprise this group. There is a need for further trials to assess appropriate treatment in this patient cohort and to confirm findings in our study. In the absence of large, prospective randomized trials, there is a role for a nationalized tumor database that would facilitate collection and storage of clinical information on patients with these rare tumor subtypes. A larger body of data would improve results from retrospective studies, adding to evidence guiding treatment. Collaborative efforts between centers have been performed in order to boost patient numbers and statistical power and this remains an attractive measure by which to examine outcomes in this patient group.
Footnotes
This work was presented as a poster at the Society of Gynecologic Oncology 2014 National Meeting in Tampa, FL, USA.
No potential conflict of interest relevant to this article was reported.
References
- 1.Cirisano FD, Jr, Robboy SJ, Dodge RK, Bentley RC, Krigman HR, Synan IS, et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecol Oncol. 1999;74:385–394. doi: 10.1006/gyno.1999.5505. [DOI] [PubMed] [Google Scholar]
- 2.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Nicklin JL, Copeland LJ. Endometrial papillary serous carcinoma: patterns of spread and treatment. Clin Obstet Gynecol. 1996;39:686–695. doi: 10.1097/00003081-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Webb GA, Lagios MD. Clear cell carcinoma of the endometrium. Am J Obstet Gynecol. 1987;156:1486–1491. doi: 10.1016/0002-9378(87)90021-4. [DOI] [PubMed] [Google Scholar]
- 5.Abeler VM, Vergote IB, Kjorstad KE, Trope CG. Clear cell carcinoma of the endometrium: prognosis and metastatic pattern. Cancer. 1996;78:1740–1747. doi: 10.1002/(sici)1097-0142(19961015)78:8<1740::aid-cncr14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KT, Rotmensch J, Yamada SD, Mundt AJ. Outcome and patterns of failure in pathologic stages I-IV clear-cell carcinoma of the endometrium: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2003;55:1272–1276. doi: 10.1016/s0360-3016(02)04404-8. [DOI] [PubMed] [Google Scholar]
- 7.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fader AN, Seamon LG, Escobar PF, Frasure HE, Havrilesky LA, Zanotti KM, et al. Minimally invasive surgery versus laparotomy in women with high grade endometrial cancer: a multi-site study performed at high volume cancer centers. Gynecol Oncol. 2012;126:180–185. doi: 10.1016/j.ygyno.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Fader AN, Drake RD, O'Malley DM, Gibbons HE, Huh WK, Havrilesky LJ, et al. Platinum/taxane-based chemotherapy with or without radiation therapy favorably impacts survival outcomes in stage I uterine papillary serous carcinoma. Cancer. 2009;115:2119–2127. doi: 10.1002/cncr.24247. [DOI] [PubMed] [Google Scholar]
- 11.Kelly MG, O'Malley D, Hui P, McAlpine J, Dziura J, Rutherford TJ, et al. Patients with uterine papillary serous cancers may benefit from adjuvant platinum-based chemoradiation. Gynecol Oncol. 2004;95:469–473. doi: 10.1016/j.ygyno.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Kelly MG, O'Malley DM, Hui P, McAlpine J, Yu H, Rutherford TJ, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98:353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 13.ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 15.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–1172. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]
- 16.Yaegashi N, Ito K, Niikura H. Lymphadenectomy for endometrial cancer: is paraaortic lymphadenectomy necessary? Int J Clin Oncol. 2007;12:176–180. doi: 10.1007/s10147-006-0621-2. [DOI] [PubMed] [Google Scholar]
- 17.Zullo F, Palomba S, Falbo A, Russo T, Mocciaro R, Tartaglia E, et al. Laparoscopic surgery vs laparotomy for early stage endometrial cancer: long-term data of a randomized controlled trial. Am J Obstet Gynecol. 2009;200:296.e1–296.e9. doi: 10.1016/j.ajog.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 18.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199:360.e1–360.e9. doi: 10.1016/j.ajog.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Kilgore JE, Jackson AL, Ko EM, Soper JT, Van Le L, Gehrig PA, et al. Recurrence-free and 5-year survival following robotic-assisted surgical staging for endometrial carcinoma. Gynecol Oncol. 2013;129:49–53. doi: 10.1016/j.ygyno.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994;54:264–268. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- 21.Geisler JP, Geisler HE, Melton ME, Wiemann MC. What staging surgery should be performed on patients with uterine papillary serous carcinoma? Gynecol Oncol. 1999;74:465–467. doi: 10.1006/gyno.1999.5513. [DOI] [PubMed] [Google Scholar]
- 22.Bristow RE, Asrari F, Trimble EL, Montz FJ. Extended surgical staging for uterine papillary serous carcinoma: survival outcome of locoregional (Stage I-III) disease. Gynecol Oncol. 2001;81:279–286. doi: 10.1006/gyno.2001.6159. [DOI] [PubMed] [Google Scholar]
- 23.Gehrig PA, Groben PA, Fowler WC, Jr, Walton LA, Van Le L. Noninvasive papillary serous carcinoma of the endometrium. Obstet Gynecol. 2001;97:153–157. doi: 10.1016/s0029-7844(00)01096-6. [DOI] [PubMed] [Google Scholar]
- 24.Havrilesky LJ, Secord AA, Bae-Jump V, Ayeni T, Calingaert B, Clarke-Pearson DL, et al. Outcomes in surgical stage I uterine papillary serous carcinoma. Gynecol Oncol. 2007;105:677–682. doi: 10.1016/j.ygyno.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 25.Boruta DM, 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115:142–153. doi: 10.1016/j.ygyno.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Frederick PJ, Straughn JM., Jr The role of comprehensive surgical staging in patients with endometrial cancer. Cancer Control. 2009;16:23–29. doi: 10.1177/107327480901600104. [DOI] [PubMed] [Google Scholar]
- 27.Chan JK, Loizzi V, Youssef M, Osann K, Rutgers J, Vasilev SA, et al. Significance of comprehensive surgical staging in noninvasive papillary serous carcinoma of the endometrium. Gynecol Oncol. 2003;90:181–185. doi: 10.1016/s0090-8258(03)00195-1. [DOI] [PubMed] [Google Scholar]


