Abstract
Objective
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been recently reported with favorable oncological outcomes as treatment of advanced epithelial ovarian cancer (EOC). The aim of this study was to demonstrate the feasibility of CRS+HIPEC with cisplatin and paclitaxel for the treatment of advanced EOC.
Methods
This is a prospective observational study of 54 patients, from April 2007 to October 2013, with primary or recurrent peritoneal carcinomatosis due to EOC. The mean age was 54.51±9.34. Thirty patients (59%) had primary EOC, and 24 patients (41%) had recurrent disease.
Results
Mean peritoneal cancer index was 10.11 (range, 0 to 28), complete cytoreduction (CC0) was achieved for 47 patients (87%), CC1 for seven patients (13%). Patients with suboptimal cytoreduction (CC2 and CC3) were not included in the study. The mean stay in intensive care unit was 4.73±5.51 days and the mean hospitalization time was 24.0±10.03 days. We did not observe any intraoperative death. Seven patients (13%) required additional operations. Three patients (5.6%) died within 30 days from the procedure. Severe complications were seen in 19 patients (35.2%). During the follow-up period, disease recurred in 33 patients (61.1%); the median disease-free survival time was 12.46 months and the median overall survival time was 32.91 months.
Conclusion
CRS+HIPEC with cisplatin and paclitaxel for advanced EOC is feasible with acceptable morbidity and mortality. Additional follow-up and further studies are needed to determine the effects of HIPEC on long term survival.
Keywords: Cisplatin, Disease-free Survival, Ovarian Neoplasms, Paclitaxel, Prospective studies
INTRODUCTION
Epithelial ovarian cancer (EOC) is the fifth most frequent cancer among females and approximately two thirds of the patients present with advanced disease (International Federation of Gynecology and Obstetrics [FIGO] stage III or IV) at diagnosis [1]. The current standard treatment for these patients consists in maximum cytoreductive surgery (CRS) and platinum-based chemotherapy [2]. Although platinum drugs are the most active agents in ovarian cancer, taxanes have emerged as an important group of drugs, particularly when given in conjunction with platinum. Though the response rate for first line carboplatin and paclitaxel (PTX) is 70% to 80%, this approach still yields poor results and overall 5-year survival rate is less than 30%. Several studies have sought to improve survival by addition of a third agent (topotecan, gemcitabine, doxorubicin) without any survival advantage. Currently the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (Baden-Baden, Germany, 2004) proposed carboplatin and PTX as standard of care and as the favored standard treatment protocol for comparison in clinical trials [3]. In ovarian cancer, the peritoneal cavity is the main site of disease diffusion and the administration of chemotherapy into the peritoneal cavity increases the drug's dose delivered to the tumor site without compromising plasma drugs levels. Based on this idea, three studies [4,5,6] compared intravenous versus intraperitoneal (IP) administration of cisplatin (CDDP) and PTX and showed an increase of median survival in the IP treatment group. Based on these results, the US National Cancer Institute prompted a clinical announcement in 2006 stating that IP chemotherapy should be considered for optimally debulked patients. Despite the advantage of this approach, IP chemotherapy has not become routine practice because of major toxicity, lack of experience in placing and managing indwelling catheters, and the difficulty to diffuse the drugs in all peritoneal cavity due to adhesions and anatomic niches. In recent years, maximum cytoreductive effort combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been a promising treatment for non-ovarian carcinomatosis (e.g., carcinoma of the appendix, or colon). The procedure exploits the advantages of IP therapy and the synergistic enhancement of drug cytotoxicity induced by heat. Various protocols have been described using CDDP, doxorubicin, mitomycin C, oxaliplatin, interferon α, and mitoxantrone [7]. These drugs have been chosen because of their favorable IP pharmacokinetics and their high local efficacy. Taxane have already been tested only as single drugs in HIPEC procedures [8,9]. Our group studied the pharmacokinetics of a combination of CDDP and PTX during HIPEC after CRS in 10 patients in a previous feasibility study approved by the ethics committee (of the Papa Giovanni XXIII Hospital): the data showed that HIPEC with concomitant CDDP and PTX following CRS is associated with a highly favorable pharmacokinetic profile, despite its short treatment duration. Based on these encouraging results we decided to test the CDDP and PTX association as HIPEC drugs in advanced ovarian cancer in a larger series.
The primary aim of this study was to analyze the morbidity of HIPEC using a CDDP and PTX after CRS in patients with peritoneal carcinomatosis for EOC; secondary aims were overall survival (OS) and disease-free survival (DFS).
MATERIALS AND METHODS
1. Population
This is an open-label, prospective, phase II study performed at the Unit of General, Emergency and Transplant Surgery at Sant'Orsola-Malpighi Hospital, University of Bologna, Italy, the Unit of General Surgery and Gynaecologic Surgery of Papa Giovanni XXIII Hospital, Bergamo and the Unit of Gynaecology of the Jena University Hospital, Germany. Patients with primary, advanced (FIGO stage IIIC to IV), or recurrent EOC were eligible for the present study to be treated with CRS+HIPEC with CDDP (100 mg/m2) and PTX (175 mg/m2). The study was approved by local Ethical Committee. Inclusion criteria: age younger than 75, Eastern Cooperative Oncology Group performance status 0 to 2 [10], resectable disease evaluated by computed tomography (CT) scan and/or positron emission tomography (PET) and diagnostic laparoscopy to identify the possibility to achieve optimal cytoreduction with residual disease less than 1 cm, no significant co-morbidities precluding the combined treatments (CRS and HIPEC), informed written consent. Exclusion criteria: other malignant pathologies, extra-abdominal metastasis, complete intestinal obstruction, active infections. The treatment plan was to perform the maximum surgical effort, aimed to remove all visible disease using peritonectomy procedures and multiorgan resection depending on the abdominal disease involvement. If residual disease <1 cm was achieved, the patients were submitted to HIPEC. Patients with residual disease larger than 1 cm were excluded from the study.
CRS and HIPEC were performed at one of the following five different timings: T1 at the time of primary treatment if optimal cytoreduction was achieved, T2 at the time of interval debulking, T3 as a consolidation therapy following complete pathological response after initial therapy as confirmed by a second-look laparotomy, T4 at the time of first recurrence, and T5 as salvage therapy [11].
2. Study parameters
Histological type and grade were assessed according to the World Health Organization classification, and surgical stage according to the FIGO criteria [12]. Patients with recurring disease were classified according to their platinum free interval as platinum sensitive (≥6 months) or insensitive (<6 months).
During the laparotomy, the extension of peritoneal carcinomatosis was recorded according to peritoneal cancer index (PCI) [13]. The number of peritonectomy procedures according to the affected anatomical area (right and left subphrenic, Glisson's capsule, right and left paracolic gutters, lesser omentectomy, pelvic) was assessed in every patient (0, no peritonectomy; 7, all peritonectomies). Completeness of cytoreduction was assessed by measuring the size of the residual peritoneal implants following surgery and assigning a complete cytoreduction (CC) score: CC0, no residual disease; CC1, residual nodules measuring less than 2.5 mm; CC2, residual nodules measuring between 2.5 mm and 2.5 cm; or CC3, residual nodules greater than 2.5 cm [14].
3. Hyperthermic intraperitoneal chemotherapy
Following surgery HIPEC was performed in patients with CC0 and CC1 as an open procedure with the Coliseum technique or as a closed technique [15] using CDDP (100 mg/m2) and PTX (175 mg/m2). IP chemotherapy was performed for 60 or 90 minutes, with a peritoneal and outflow thermal plateau of 41.5℃. Perfusate (4 to 6 L) was circulated using an extracorporeal circulation device at a flow rate of 700 mL/minute with an intra-abdominal target temperature of 42.5℃. The surgical procedure length was calculated from the induction of anesthesia to the closure of the abdominal wall.
4. Postoperative outcomes and follow-up
During the immediate postoperative period, patients were assisted in an intensive care unit. To analyze postoperative morbidity, all surgical and nonsurgical complications that occurred within 30 days from the procedure were considered and all complications were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) scale ver. 3.0 (August 9, 2006) [16]. After hospital discharge, patients were referred to the medical oncologic staff to plan systemic chemotherapy and were followed after chemotherapy with clinical examination and serum levels of CA-125 every 4 months for the first 2 years and every 6 months for 3 years. Every further evaluation (CT/PET) was indicated depending on the patients' clinical presentation and the increase of CA-125 levels. Recurrence and progression of disease were evaluated using the RECIST criteria [17]. The time and type of recurrence, the nature of treatment and date of death were recorded. The median follow-up was calculated from the date of HIPEC to the date of last visit or date of death.
5. Statistical analysis
Patient data, including epidemiological, surgical, pathological, and survival figures, were compiled into a database (IBM SPSS ver. 20, IBM Co., Armonk, NY, USA). Survival rates were calculated using the Kaplan-Meier method, and were compared using the log-rank test (p<0.05 was considered statistically significant). Associations were calculated with the chi-square; correlations were analyzed with the binomial logistic regression method and Cox regression model, p<0.05 was considered statistically significant.
RESULTS
Fifty-four patients were included between April 2007 and October 2013: 29 at Unit of General, Emergency and Transplant Surgery of St. Orsola-Malpighi Hospital, University of Bologna; 23 at Unit of General Surgery I of Papa Giovanni XXIII Hospital, Bergamo; and 2 at the Unit of Gynaecology of the Jena University Hospital. HIPEC was performed at T1 in one patient (1.9%), at T2 in 29 patients (56.7%), at T4 in 14 patients (25.9%), and at T5 in 10 patients (18.5%). Population and tumor characteristics are shown in Table 1. At laparotomy the mean PCI was 10.11 (range, 0 to 28), in 38 cases (70.4%) the PCI was ≤15 and in 16 cases (29.6%) the PCI was >15. CC0 was achieved for 47 patients (87%), CC1 for seven patients (13%), we did not record CC2 and CC3 patients, since suboptimal cytoreducted patients were not candidates for this study. HIPEC was performed as an open procedure with the Coliseum technique in 42 cases (77.8%) and closed technique in 12 (22.2%); the mean time of IP chemotherapy was 89.4±4.4 minutes and the peritoneal and outflow mean temperature was 41.5℃ (range, 40℃ to 42℃); the mean operation time was 8.85±1.68 hours. Mean ICU stay was 4.73±5.51 days, and mean hospital stay was 24.0±10.03 days.
Table 1.
Patient characteristics (n=54)
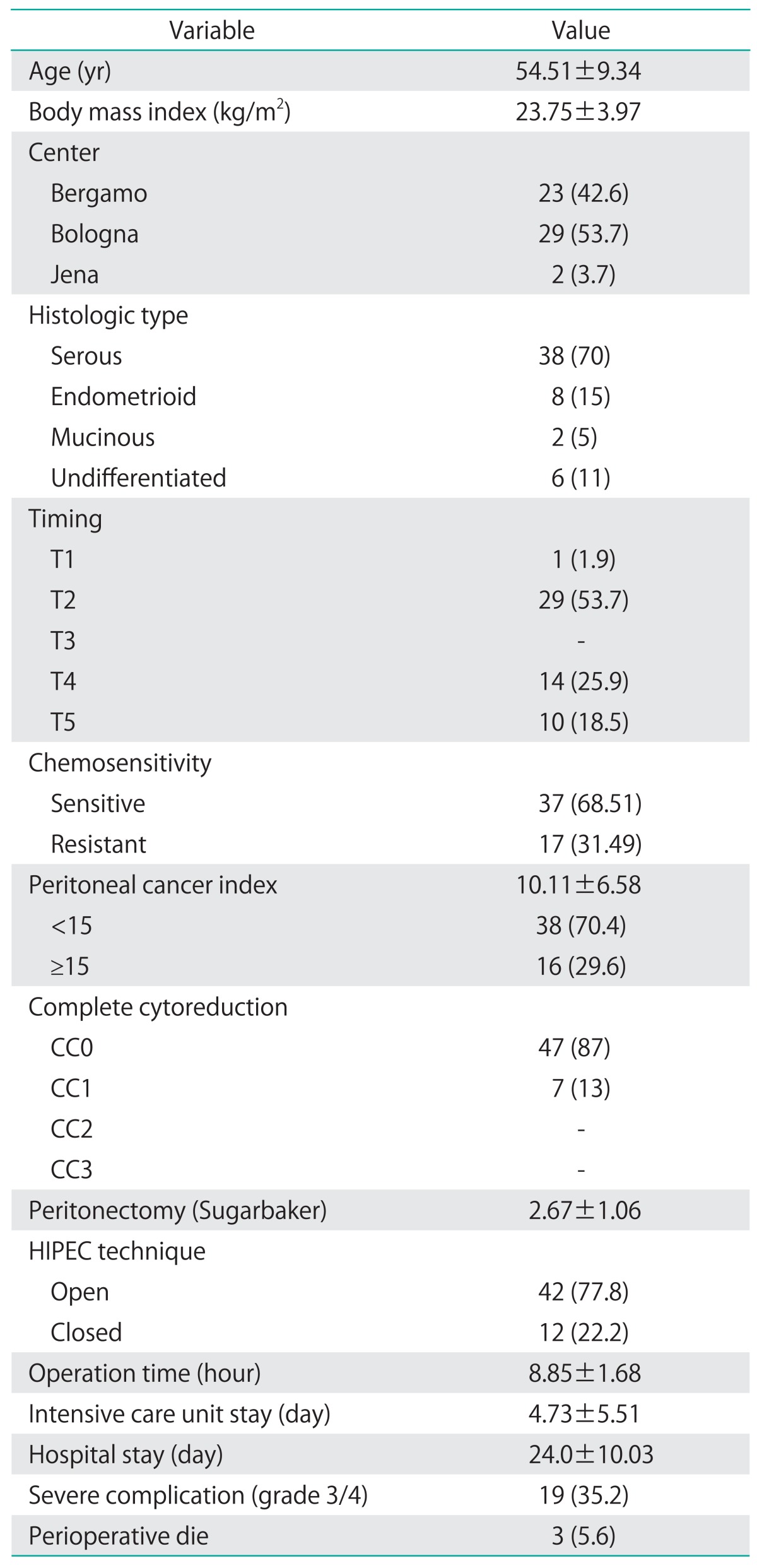
Values are presented as mean±SD or number (%).
HIPEC, hyperthermic intraperitoneal chemotherapy.
1. Postoperative complications
We did not observe any intraoperative death. Reoperation was required for seven patients (13%) due to intestinal perforation (3), gastrointestinal (GI) fistula (2), abdominal abscess (1), and colonic obstruction (1). Three patients (5.6%) died within 30 days from the procedure: one died of peritonitis following colonic perforation, one died of septicemia due to a pelvic abscess and one for pulmonary embolism. PCI, upfront treatment, number of peritonectomy procedures, HIPEC technique, age, body mass index (BMI), and operation time were not associated with perioperative death; CC1 was associated with increased perioperative death (p=0.004); multivariate analysis showed no correlations. Minor complications (grade 1 to 2) were observed in 32 patients (60.2%). Most common complications affected urinary system, infection, and cytopenia. Severe complications (grade 3 to 4 CTCAE 3.0) were seen in 19 patients (35.2%). Grade 3 complications (13 patients) include wound dehiscence (3 patients), GI fistula (2 patients), wound infection (1 patient), deep vein thrombosis (1 patient), anemia (1 patient), acute renal failure (1 patient, from neurogenic bladder), leukocytopenia (2 patients), and thrombocytopenia (2 patients). Grade 4 complications (6 patients) included leukocytopenia (1 patient), thrombocytopenia (1 patient), bowel obstruction (1 patient), and septic shock due to perforation (3 patients).
By univariate and multivariate analysis of HIPEC as an upfront treatment, with or without neoadjuvant chemotherapy (NACT), was associated with more severe postoperative complications compared to HIPEC used to treat recurring disease (p=0.002 and p=0.004, respectively), all the other variables (age, BMI, PCI, number of peritonectomy procedures, operative time, chemosensitivity, and CC score) were not significant (NS).
2. Follow-up
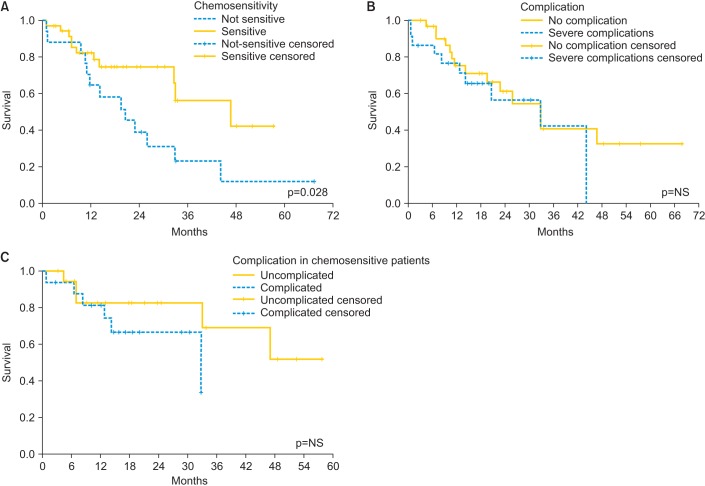
Patients were followed for a median of 20.0 months (range, 0.7 to 67.9 months). Three patients were lost at follow-up, all of them after relapse. Thirty-three patients had disease relapse (61.1%) and 19 (35.2%) died of disease during the follow-up period. DFS and OS, calculated with the Kaplan-Meier method, are reported in Fig. 1 with a median of 12.5 and 32.9 months, respectively. Relapse was not associated with age, BMI, timing, chemosensitivity, number of peritonectomies, CC, or PCI in the multivariate analysis. The log-rank test and the Cox regression model showed a significant improvement in OS in chemosensitive patients (p=0.028 and p=0.026, respectively) (Fig. 2A). Major complications did not affect the OS.
Fig. 1.
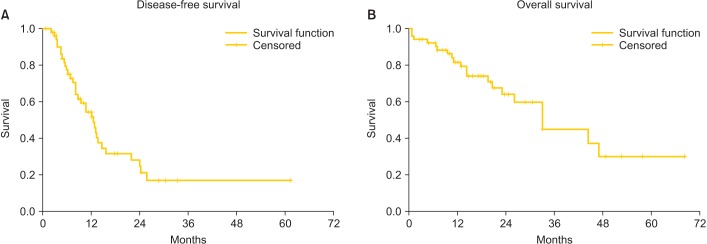
Disease-free (A) and overall survival (B) in 54 patients with primary advanced or recurrent ovarian cancer treated with cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel.
Fig. 2.
(A) Effect of chemosensitivity on overall survival. (B) Effect of severe complication on OS. (C) Effect of severe complication on OS in the subgroup of chemosensitive patients. NS, not significant.
DISCUSSION
The rationale for the CRS+HIPEC treatment of advanced EOC is to focus on the cytotoxicity of chemotherapic drugs on the peritoneal surface after debulking surgery in order to treat even the microscopic lesions and to limit the systemic toxicity of the drugs.
PTX has been widely used since 1992 becoming a standard therapy for patients with ovarian cancer [18]. A high molecular weight and hepatic metabolism are features that make PTX an attractive agent for IP administration. The average maximal concentration ratio and the area under the timeconcentration curve (AUC) ratio between the peritoneal cavity and the peripheral blood are approximately 800 to 1,000 and 550 to 2,000, respectively [19,20,21], so high cytotoxic drug levels in the peritoneal cavity can be achieved and sustained for several days [21]. CDDP is a well-known drug largely used in IP therapeutic regimens since it has a low lipophility and a high molecular weight with advantageous plasma/peritoneal AUC ratio (20±6). This drug has been shown to have reduced tendency to diffuse through the plasma-peritoneal barrier, even after extensive removal of the peritoneum, achieving very high IP concentrations without incurring significant systemic toxicity [21]. In a feasibility study at our institution (data unpublished), the pharmacokinetics of CDDP and PTX during HIPEC were analyzed, demonstrating a good pharmacokinetic profile with a perfusated concentration 1,216 and 405 times higher than plasma for PTX and CDDP, respectively.
This positive pharmacokinetic profile encouraged us to analyze in a larger cohort of patients the clinical feature of this drug combination: to our knowledge, this is the first study that reported the results of HIPEC performed with CDDP and PTX in association; our results, in terms of outcome and morbidity, showed that HIPEC with this drugs after CRS could be a valid option of treatment for advanced EOC. CRS and HIPEC are associated to severe morbidity rate ranging from 12% to 52% [22], this percentage decreases in high volume specialized centers. In our experience the total morbidity was 35%, similar to those reported in literature with other chemotherapeutic agents. In these procedures, the morbidity is due to surgery and toxicity of chemotherapeutic agents: surgical complications are associated with performance status, extent of carcinomatosis, duration of surgery, number of peritonectomies, number of anastomoses, and extension of cytoreduction, since peritoneal carcinomatosis requires extensive CRS with a variable number of peritonectomies and a long lasting procedures (4 to 10 hours) [22]. The loco-regional administration of the perfusate could hamper wound healing and induce local immunodeficiency with an increase of the postoperative complications but how the chemotherapy impacts on these events remains to be elucidated. In our study the morbidity rate related to surgery was 18% and follow-up operations were necessary in 13% of cases, confirming that the association carboplatin-taxane did not increase surgical morbidity. Systemic chemotherapy-related complications as hematological toxicity and renal insufficiency range from 0% to 28% and 0% to 7%, respectively [22]. In our study we observed hematological complications in 11% of the patients and one renal failure (2%). This confirms that the toxicity of the carboplatin-taxane cocktail is analogous to other chemotherapeutic agents, as single drug regimens or as combination of two different agents [22,23,24]. Analyzing the patients on the basis of their chemoresistance or chemosensitivity, we observed a significant increase of OS in the chemosensitive group. In the upfront treatment group we observed an increased comorbidity: with this heterogeneous and small sample of patients we can not find a reasonable explanation; despite it the OS was not significantly different in patients with major complications (Fig. 2B); and also in the subgroup analysis of chemosensitive patients (Fig. 2C). This information may indicate that using NACT could help us to select patients which may benefit from HIPEC with higher chance of longer OS. This aspect needs to be studied more deeply with a well designed study of a homogenous population. Recent literature shows a positive effect of NACT [25] but data about the association of NACT and HIPEC are missing.
Median disease free survival in patients treated with CRS and HIPEC for advanced ovarian cancer ranges from 10 to 26 months and median OS ranges from 24 to 106 months [26]: these data are extremely variable because in the reported studies, HIPEC was performed at different time points and populations. With a mean follow-up of 20 months we observed a median DFS of 12.46 months and a median OS of 32.9 months. Survival data were not different between patients with primary or recurrent disease, with a median DFS of 13 and 12 months and a median OS of 22 and 44 months, respectively (p=NS in both cases) (Fig. 3).
Fig. 3.
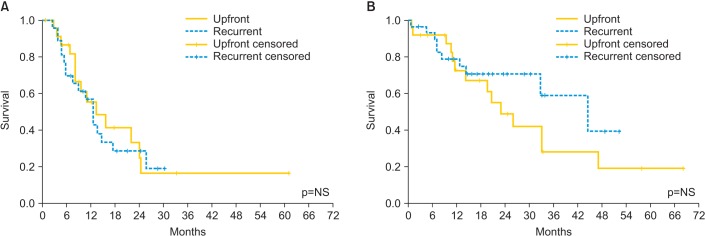
(A) Disease-free survival and (B) overall survival in 54 patients with ovarian cancer treated with cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel between primary and recurrent diseases. NS, not significant.
The aim of this paper is to demonstrate the feasibility of CRS+HIPEC using CDDP and PTX in terms of morbidity and mortality related to procedure. Present data can confirm the safety of this drug regimen. Due to short follow-up window, we cannot provide satisfactory data to show the long term survival of patients; in addition our data are too heterogeneous and the number of patients is too small to perform any comparison or subgroups analysis: there are patients treated in different time points, with different response to chemotherapy and different chemosensitivity. Nevertheless, the data shown are not inferior to those reported in literature suggesting new studies with prospective series focused in each time point.
CRS+HIPEC with CDDP (100 mg/m2) and PTX (175 mg/m2) as treatment for advanced EOC is feasible and comparable with the other drugs regimens with similar mortality and morbidity rate. In the subgroup analysis, morbidity was higher in upfront treatment patients but could give the possibility to select chemosensitive patients with an increased survival. These data are to be validated in larger studies of patients with a homogenous population, even in order to demonstrate the DFS and OS with a longer follow-up time.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 3.Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21:750–755. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- 4.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 5.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 7.Chua TC, Robertson G, Liauw W, Farrell R, Yan TD, Morris DL. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol. 2009;135:1637–1645. doi: 10.1007/s00432-009-0667-4. [DOI] [PubMed] [Google Scholar]
- 8.de Bree E, Romanos J, Michalakis J, Relakis K, Georgoulias V, Melissas J, et al. Intraoperative hyperthermic intraperitoneal chemotherapy with docetaxel as second-line treatment for peritoneal carcinomatosis of gynaecological origin. Anticancer Res. 2003;23:3019–3027. [PubMed] [Google Scholar]
- 9.Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, et al. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol. 2007;106:193–200. doi: 10.1016/j.ygyno.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 10.North Bristol NHS Trust. WHO Performance status [Internet] Bristol: North Bristol NHS Trust; 2013. [cited 2014 Oct 17]. Available from: http://www.nbt.nhs.uk/sites/default/files/filedepot/incoming/WHO_Performance_Status.doc. [Google Scholar]
- 11.Helm CW, Bristow RE, Kusamura S, Baratti D, Deraco M. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol. 2008;98:283–290. doi: 10.1002/jso.21083. [DOI] [PubMed] [Google Scholar]
- 12.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg. 1999;384:576–587. doi: 10.1007/s004230050246. [DOI] [PubMed] [Google Scholar]
- 14.Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996;15:49–58. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 15.Sarnaik AA, Sussman JJ, Ahmad SA, McIntyre BC, Lowy AM. Technology for the delivery of hyperthermic intraoperative intraperitoneal chemotherapy: a survey of techniques. In: Gonzaalez-Moreno S, editor. Advances in peritoneal surface oncology. Berlin: Springer; 2007. pp. 75–82. [DOI] [PubMed] [Google Scholar]
- 16.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [Internet] Bethesda, MD: Cancer Therapy Evaluation Program; c2006. [cited 2014 Oct 17]. Available from: http://www.eortc.be/services/doc/ctc/ctcaev3.pdf. [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 19.Markman M, Rowinsky E, Hakes T, Reichman B, Jones W, Lewis JL, Jr, et al. Phase I trial of intraperitoneal taxol: a Gynecoloic Oncology Group study. J Clin Oncol. 1992;10:1485–1491. doi: 10.1200/JCO.1992.10.9.1485. [DOI] [PubMed] [Google Scholar]
- 20.Hofstra LS, Bos AM, de Vries EG, van der Zee AG, Willemsen AT, Rosing H, et al. Kinetic modeling and efficacy of intraperitoneal paclitaxel combined with intravenous cyclophosphamide and carboplatin as first-line treatment in ovarian cancer. Gynecol Oncol. 2002;85:517–523. doi: 10.1006/gyno.2002.6665. [DOI] [PubMed] [Google Scholar]
- 21.Rossi CR, Mocellin S, Pilati P, Foletto M, Quintieri L, Palatini P, et al. Pharmacokinetics of intraperitoneal cisplatin and doxorubicin. Surg Oncol Clin N Am. 2003;12:781–794. doi: 10.1016/s1055-3207(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 22.Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009;249:900–907. doi: 10.1097/SLA.0b013e3181a45d86. [DOI] [PubMed] [Google Scholar]
- 23.Pomel C, Ferron G, Lorimier G, Rey A, Lhomme C, Classe JM, et al. Hyperthermic intra-peritoneal chemotherapy using oxaliplatin as consolidation therapy for advanced epithelial ovarian carcinoma: results of a phase II prospective multicentre trial. CHIPOVAC study. Eur J Surg Oncol. 2010;36:589–593. doi: 10.1016/j.ejso.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Konigsrainer I, Horvath P, Struller F, Grischke EM, Wallwiener D, Konigsrainer A, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in recurrent epithelial ovarian cancer with peritoneal metastases: a single centre experience. Langenbecks Arch Surg. 2014;399:589–594. doi: 10.1007/s00423-014-1207-5. [DOI] [PubMed] [Google Scholar]
- 25.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 26.Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–6994. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]