Abstract
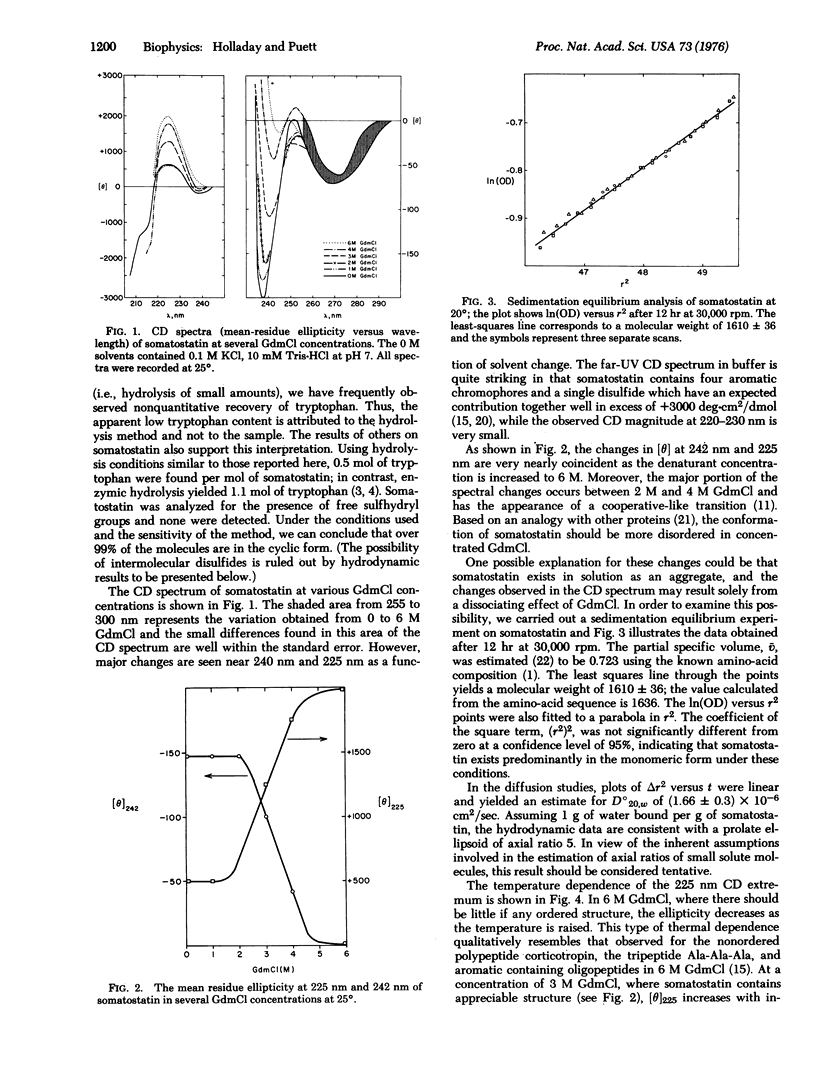
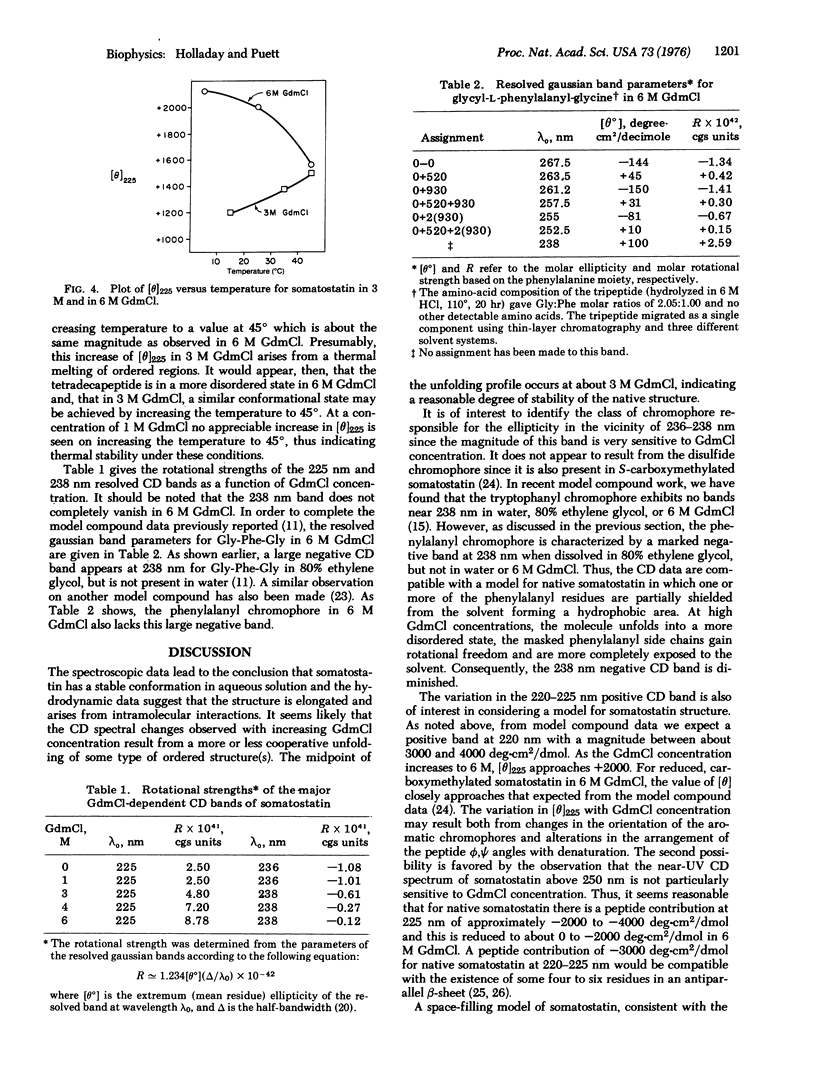
Somatostatin is a hypothalamic tetradeca peptide that inhibits the release of growth hormone insulin, and glucagon. The circular dichroism spectrum is characterized by negative extrema at 238 nm and 270 nm, and a positive extremum at 225 nm. The far ultraviolet circular dichroism spectrum is consistent with the presence of ordered secondary structure such as beta-structure, but not alpha-helix. Sedimentation equilibrium results demonstrate that somatostatin exists in its monomeric form (i.e., a molecular weight of 1610 +/- 36 was obtained) and, thus, the structure must arise from intramolecular interactions. The diffusion constant of somatostatin was estimated to be 1.66 X 10(-6) cm2/sec. These data are consistent with an ellipsoidal rather than a spherical shape. The magnitude of the ellipticity at both 225 nm and 238 nm is quite dependent on guanidinium hydrochloride concentration; the midpoint occurs at about 3 M and the transition is cooperative-like. These data strongly suggest that somatostatin has a stable conformation in aqueous solution. A model, consistent with the results of the physicochemical studies and with semi-empirical rules for secondary structure formation, is proposed for somatostatin. The proposed structure consists of a hairpin loop with several residues in an antiparallel beta-pleated sheet, is somewhat elongated, and contains a hydrophobic domain at one end and a hydrophilic domain at the other end.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Rivier J., Guillemin R. Acylated des-(Ala1-Gly2)-somatostatin analogs: prolonged inhibition of growth hormone secretion. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1202–1207. doi: 10.1016/0006-291x(74)90326-x. [DOI] [PubMed] [Google Scholar]
- Burgus R., Ling N., Butcher M., Guillemin R. Primary structure of somatostatin, a hypothalamic peptide that inhibits the secretion of pituitary growth hormone. Proc Natl Acad Sci U S A. 1973 Mar;70(3):684–688. doi: 10.1073/pnas.70.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Fasman G. D., Potter J. The optical rotatory dispersion of two beta structures. Biochem Biophys Res Commun. 1967 Apr 20;27(2):209–216. doi: 10.1016/s0006-291x(67)80063-9. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Schneider V., Karam J. H., Rivier J., Guillemin R., Forsham P. H. Effects of somatostatin on plasma glucose and glucagon levels in human diabetes mellitus. Pathophysiologic and therapeutic implications. N Engl J Med. 1974 Sep 12;291(11):544–547. doi: 10.1056/NEJM197409122911102. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Hammonds R. G., Jr, Puett D. Growth hormone conformation and conformational equilibria. Biochemistry. 1974 Apr 9;13(8):1653–1661. doi: 10.1021/bi00705a015. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Levine J. H., Nicholson W. E., Orth D. N., Salmon W. D., Jr, Puett D. Evidence for structural dissociation of two biologic actions of growth hormone. Biochim Biophys Acta. 1975 Jan 13;381(1):47–60. doi: 10.1016/0304-4165(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Puett D. Circular dichroism of corticotropin, fragment 1-24, and model compounds. An assessment of the contributions of the peptide chromophore and armoatic residues. Biopolymers. 1976 Jan;15(1):43–59. doi: 10.1002/bip.1976.360150106. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Puett D. Gonadotropin and subunit conformation. Arch Biochem Biophys. 1975 Dec;171(2):708–720. doi: 10.1016/0003-9861(75)90083-1. [DOI] [PubMed] [Google Scholar]
- Koerker D. J., Ruch W., Chideckel E., Palmer J., Goodner C. J., Ensinck J., Gale C. C. Somatostatin: hypothalamic inhibitor of the endocrine pancreas. Science. 1974 Apr 26;184(4135):482–484. doi: 10.1126/science.184.4135.482. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Pace C. N. The stability of globular proteins. CRC Crit Rev Biochem. 1975 May;3(1):1–43. doi: 10.3109/10409237509102551. [DOI] [PubMed] [Google Scholar]
- Rivier J. E. Somatostatin. Total solid phase synthesis. J Am Chem Soc. 1974 May 1;96(9):2986–2992. doi: 10.1021/ja00816a053. [DOI] [PubMed] [Google Scholar]
- Rivier J., Brazeau P., Vale W., Guillemin R. Somatostatin analogs. Relative importance of the disulfide bridge and of the Ala-Gly side chain for biological activity. J Med Chem. 1975 Feb;18(2):123–126. doi: 10.1021/jm00236a001. [DOI] [PubMed] [Google Scholar]
- Rivier J., Brown M., Vale W. D-Trp8-somatostatin: an analog of somatostatin more potent than the native molecule. Biochem Biophys Res Commun. 1975 Jul 22;65(2):746–751. doi: 10.1016/s0006-291x(75)80208-7. [DOI] [PubMed] [Google Scholar]
- Robinson J. P., Holladay L. A., Picklesimer J. B., Puett D. Tetanus toxin conformation. Mol Cell Biochem. 1974 Dec 20;5(3):147–151. doi: 10.1007/BF01731377. [DOI] [PubMed] [Google Scholar]
- Rohrbach M. S., Humphries B. A., Yost F. J., Jr, Rhodes W. G., Boatman S., Hiskey R. G., Harrison J. H. The reaction of 4,4'-bis-dimethylaminodiphenylcarbinol with the sulfhydryl group. A new reagent for sulfhydryl analysis. Anal Biochem. 1973 Mar;52(1):127–142. doi: 10.1016/0003-2697(73)90338-2. [DOI] [PubMed] [Google Scholar]
- Simmons N. S., Barel A. O., Glazier A. N. High-resolution circular dichroism of N-acetyl-L-phenylalaninamide: solvent effects. Biopolymers. 1969;7(2):275–279. doi: 10.1002/bip.1969.360070213. [DOI] [PubMed] [Google Scholar]
- Stevens L., Townend R., Timasheff S. N., Fasman G. D., Potter J. The circular dichroism of polypeptide films. Biochemistry. 1968 Oct;7(10):3717–3720. doi: 10.1021/bi00850a051. [DOI] [PubMed] [Google Scholar]