Abstract
Advancements in genetic screening have generated massive amounts of data on genetic variation; however, a lack of clear pathogenic stratification has left most variants classified as being of unknown significance. This is a critical limitation for translating genetic data into clinical practice. Genetic screening is currently recommended in the guidelines for diagnosis and treatment of cardiac channelopathies, which are major contributors to sudden cardiac death in young people. We propose to characterize the pathogenicity of genetic variants associated with cardiac channelopathies using a stratified scoring system. The development of this system was considered by using all of the tools currently available to define pathogenicity. The use of this scoring system could help clinicians to understand the limitations of genetic associations with a disease, and help them better define the role that genetics can have in their clinical routine.
Sudden Cardiac Death
Sudden cardiac death (SCD) is defined as an unexpected and non-traumatic death of an individual who had been observed healthy in previous 6 hours of the death1. In western countries, SCD underlies 20% of total mortality. Although heart failure and coronary artery disease are the most prevalent substrates2, epidemiological studies indicate that monogenic syndromes –called inherited arrhythmogenic diseases- also plays an important role in cardiac electrical instability3. Thus, the inherited arrhythmogenic diseases have been defined broadly as 2 categories of pathologies: channelopathies and cardiomyopathies. Channelopathies are caused by pathogenic variations in genes encoding ion channels, and include Long QT Syndrome (LQTS), Brugada Syndrome (BrS), Short QT Syndrome (SQTS), and Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT). Cardiomyopathies results from variations in genes encoding sarcomeric, cytoskeletal, and desmosomal proteins, and include Hypertrophic Cardiomyopathy (HCM), Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), and Dilated Cardiomyopathy (DCM).
Significant progress has been made in the identification of a genetic basis of SCD-related disorders. In particular, next-generation sequencing (NGS) of the human genome has increased dramatically the amount of genetic data available. Despite this advancement, 2 major genetic challenges in SCD-related diseases remain to be overcome4. First, a more definite conclusion regarding pathogenicity of variations must be reached. Most of the genetic data, even after bioinformatic evaluation, remains of uncertain significance. In addition, current international guidelines often focus on the prediction of disease outcomes and/or therapeutic measures only if patients carry a variant classified as pathogenic5. To our knowledge, few reports have defined pathogenicity and how to differentiate genetic causality from background noise. For example, a recent study indicated that 30% of disease-causing genetic variations cited in the literature are common polymorphisms or misinterpreted variants6. Thus, the second challenge is in translating the genetic data into clinical practice. Clinical interpretation of genetic data is becoming increasingly complex, and this is particularly true of SCD-related diseases, which are characterized by incomplete penetrance and variable expressivity and thereby complicate diagnosis and treatment. Current guidelines recommend adopting preventive measures depending on both clinical context and family history, but heritability remains of lower priority5.
If the genetic variations associated with SCD are to be of value in the clinic, their pathogenicity must be defined. A recent report describes a methodical assessment that decreases the average time to compile, analyse, and interpret variant data7. In this report, Duzkale et al. propose several items that should be analysed to classify the pathogenicity of each genetic variation. However, they do not achieve an accurate numeric scale of pathogenicity, thus the pathogenic classification remains ambiguous. Another recent report highlights the current challenges in investigating causality of human sequence variants, suggesting that each variation be assessed by combining different tools to determine disease contributions8. In the present report we propose a scale to stratify pathogenicity for genetic variations associated with channelopathies. We integrate multiple parameters to reach a more definite conclusion for use in the clinic.
Channelopathies
Channelopathies are electrical disorders in structurally normal hearts caused by pathogenic variations in genes encoding cardiac ionic channels or regulatory proteins. These alterations modify the ionic balance of the electrical component of cardiac function and cause life-threatening arrhythmias9.
Long QT syndrome
LQTS is a genetic disorder characterized by a prolongation of the QT interval on the ECG (QTc > 480 ms). The clinical presentation can be variable, ranging from asymptomatic to episodes of syncope and SCD due to ventricular tachyarrhythmia (torsade de pointes) in the setting of a structurally normal heart. The syncopal episode may be induced by exertion, and emotional or auditory stimuli. SCD is the first event in 5% of asymptomatic LQTS individuals. The estimated prevalence is 1/2500, but the penetrance of the disease is not 100%10. In 1993, the first clinical diagnosis of LQTS was published11. At present, genetic analyses are included in the scoring criteria for diagnosis5. Thus, individuals and family members at risk with a normal ECG may be identified through genetic testing. Currently, nearly 1200 pathogenic variations have been identified in 15 genes (KCNQ1, KCNH2, SCN5A, ANK2, KCNE1, KCNE2, KCNJ2, CACNA1C, CAV3, CALM1, CALM2, SCN4B, AKAP9, SNTA1, and KCNJ5)12. Combined, these genes contribute to approximately 80%–85% of all LQT cases. However, 70%–75% of LQT cases are attributable to pathogenic variations only in 3 genes, KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3), with KCNQ1 responsible for around 35% of cases. Genetic testing for LQTS has a major role in the diagnosis of index cases, risk stratification, family screening, and therapeutic strategies, but only for a small number of pathogenic variations13. Most identified genetic variations remain untranslated to the clinic.
Brugada syndrome
BrS is an inherited disease described 20 years ago and characterized by an ECG pattern consisting of coved-type ST-segment elevation in atypical right-bundle branch block in leads V1 to V3 (type-1)14. BrS patients have an increased risk for SCD resulting from episodes of polymorphic ventricular tachyarrhythmia15. BrS accounts for 4%–12% of overall SCD and 20% of SCDs with normal hearts. The reported prevalence is 1 to 5/10000 in Europe and 12/10000 in Southeast Asia, with a ratio of 8:1 men:women affected. BrS typically affects young male adults (30–40 years old), and SCD typically occurs during sleep15. To date, more than 300 pathogenic variations in 16 genes (SCN5A, GPD1-L, SCN1B, SCN2B, SCN3B, KCNE3, KCNE5, KCNJ8, KCND3, CACNA1C, CACNB2b, CACNA2D1, RANGRF, HCN4, SLMAP and TRPM4) have been associated with BrS16. However, genetic testing only identifies the pathogenic cause in 35%–40% of clinically diagnosed cases of BrS. Approximately 25%–30% of diagnosed patients carry a pathogenic variation in the SCN5A gene17.
Catecholaminergic Polymorphic Ventricular Tachycardia
CPVT is a familial disease characterized by severe arrhythmias under adrenergic stimulation, such as exercise or emotional stress. The resting ECG is usually normal, but exercise testing induces ventricular arrhythmia in 75%–100% of patients. In many cases, the first manifestation of CPVT is the death of the patient. CPVT is associated with high mortality (around 30% by the age of 30 years), and the estimated prevalence is 1/1000018. To date, more than 100 pathogenic variations have been identified in five genes, contributing to nearly 60% of all clinically diagnosed cases (RYR2, CASQ2, KCNJ2, TRDN, CALM1, and CALM2). The RYR2 gene, encoding the ryanodine receptor (autosomal dominant), is responsible for nearly 50% of all cases12.
Short QT syndrome
First reported in 200019, SQTS is considered the most lethal channelopathy; it has a high familial incidence of palpitations or syncope, and SCD, typically during childhood, is often the only phenotypic manifestation. It is characterized by a short QT interval on the ECG (QTc < 325 ms) with a high sharp T wave20. The incidence and prevalence are difficult to determine due to limited data. So far, pathogenic variations have been reported in 4 genes (KCNQ1, KCNH2, KCNJ2, and CACNA2D1), and these account for nearly 50% of clinically diagnosed SQT cases12.
Methods
In 2008, the American College of Medical Genetics and Genomics (ACMG) published guidelines for genetic variant interpretation21. However, most of the genetic tools outlined have been improved since 2008, and additional ones have been developed. Most of the current genetic data generated by high-throughput technologies has identified exonic common variants previously identified in the general population (minor allele frequency, MAF > 1%). Much of the remainder is classified as being of unknown significance, with only a small number of variants classified as pathogenic. To supplement the ACMG guidelines, we propose to incorporate new information to stratify the level of pathogenicity into 5 groups: pathogenic, probably pathogenic, unknown significance, probably non-pathogenic, and non-causal/benign.
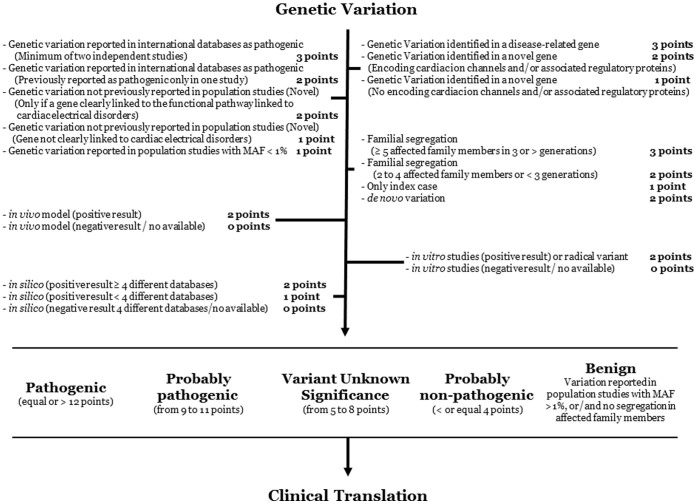
Our scale of pathogenicity (Figure 1) is based on the analysis of several items:- Clinical ascertainment (disease-associated gene or candidate gene) and determination of the genetic isoform in which the variation was identified;- Whether the variation has been previously identified in international databases;- Family segregation (identification of de novo variation may occur); and- In vitro, in vivo, and in silico evaluation.
Figure 1. Scale classification workflow.
The proposed items should be analysed to classify the variation (gene, variation, family segregation, in vitro, in vivo, and in silico studies). MAF: Minor Allele Frequency.
We have categorized the scoring using a similar approach to that in the LQTS Diagnostic Criteria11. Our scores range from 0 to 15 points (Figure 1). Each item receives a score between 0 and 3 points, and the total of the assessed items determines whether the exonic genetic variation could be considered pathogenic (≥ 12 points), probably pathogenic (from 9 to 11 points), unknown significance (from 5 to 8 points), probably non-pathogenic (≤ 4 points), and benign. This last group comprises genetic variations considered as common in the global population (MAF > 1%). This fact implies that our score is useful only for non-silent variants identified in dominant disorders, in concordance to the diseases assessed in the present report. Finally, before final genetic interpretation of a variant, it is crucial to know the genetic isoform and tissue expression in order to perform an appropriate translation into clinical practice.
Results
Clinical ascertainment
The use of genetics as a clinical diagnostic tool must always consider the context of the clinical case. Therefore, genetics should complement, and never supplant, the clinical investigation. The advent of NGS, with its constant discovery of new associations of genes with diseases, prompts the need for the confirmation of pathogenicity in more patients and families. This is especially true in the study of SCD, in which segregation analyses are limited by the sizes of the families due to the sudden death of some affected individuals at a young age22. Without a clear clinical ascertainment, and a clear correlation between the disease and the genetic variant, causality cannot be conclusive.
Some proportion of the SCD cases arising from unknown genetic causes could be explained by pathogenic genetic variations in genes not yet associated with the disease. We hypothesize that the genes most likely to be associated with such cases are other genes encoding cardiac ion channels and/or regulatory proteins associated with them, independently of the cardiac channelopathy diagnosed. Recently, through the development and improvement of NGS technologies, several genes previously associated with only one channelopathy have been linked to other channelopathies; for example, the SCN5A gene contributes to both BrS and LQTS23.
- Genetic variation identified in a disease-related gene 3 points
- Genetic variation identified in a novel gene 2 points
(Encoding cardiac ion channels and/or associated regulatory proteins)
- Genetic variation identified in a novel gene 1 point
(Not encoding cardiac ion channels and/or associated regulatory proteins)
Genetic databases
Several genetic databases are available, such as PubMed (www.ncbi.nlm.nih.gov/pubmed), the Exome Variant Server (http://evs.gs.washington.edu/EVS/), the 1000 Genomes Project (www.1000genomes.org), and the ClinSeq Project (www.genome.gov/clinseq). These databases catalog all the genetic data generated by published large-scale population studies, including pathogenic, potentially pathogenic, genetic variant of unknown significance (GVUS), and common genetic variations in the population. Other databases focus only on potential pathogenic variations [such as ClinVar (www.clinvar.com) and HGMD (www.hgmd.cf.ac.uk)]; however, caution should be exercised when regarding these classifications as not all characterizations are exhaustive and some of these classified variants could be benign. We believe that, for each reported pathogenic variation, the published source should be carefully analysed and interpreted. Importantly, only if a genetic variation has been reported as pathogenic in more than one study could it be suggested as damaging.
In our proposed stratification, this item determines if the variation is novel (never identified so far) or already reported. If reported, the variation could be considered pathogenic, of unknown significance, or neutral/non-causal. In addition, the genetic variation could be classified as a common (MAF > 1%) or rare (MAF < 1%) variation in the global population. (MAF < 1% does not imply certain pathogenicity).
- Genetic variation reported in international databases as pathogenic 3 points
(Minimum of two independent studies)
- Genetic variation reported in international databases as pathogenic 2 points
(Previously reported as pathogenic only in one study)
- Genetic variation not previously reported in population studies (Novel) 2 points
(Only if a gene is clearly linked to the functional pathway(s) in cardiac electrical disorders)
- Genetic variation not previously reported in population studies (Novel) 1 point
(Gene not clearly linked to cardiac electrical disorders)
- Genetic variation reported in population studies with MAF < 1% 1 point
One of the main limitations in genetic scenario is the previous report that a variant is pathogenic. Although there are several genetic variants already catalogued, recent whole exome/genome studies have demonstrated a large number of false-positive pathogenic variants that have been incorrectly classified24,25,26.
Family segregation
Family history of syncope/SCD is a significant risk factor for other syncope/SCD in the family5. For this reason, familial genetic screening is recommended. However, the lack of information about pathogenicity of most known genetic variants implies that genetic screening does not always help to clarify the risk of syncope/SCD in a family.
In our proposed stratification, familial testing enables discernment of whether a variation is de novo or inherited. If de novo, the score should be high (2 points) because de novo variations are strongly associated with pathogenicity27. If a variant is suspected to be inherited, as many relatives as possible should be tested to clarify the role of variant. We believe that family segregation is the most important parameter that helps to clarify a variant's contribution, but should not be the only one investigated. For cases in which a clinically affected family member does not carry the potentially pathogenic variation identified in the index case (negative segregation), the genetic variation could be discarded, or at least ruled out as the main contributor to the disease, since it could be a modifier of the phenotype. If an asymptomatic family member carries the potentially pathogenic variation, the variation should not be discarded because of the possibility for incomplete penetrance. Therefore, in our stratification to determine pathogenicity, the presence of a minimum of 5 clinically affected carriers in at least 3 generations28 denotes high genotype-phenotype correlation (3 points). A lower number of affected carriers is given a score of 2 points; when the index case alone carries the variant, 1 point is assigned.
- Familial segregation (≥ 5 affected family members in 3 or more generations) 3 points
- Familial segregation (2 to 4 affected family members or < 3 generations) 2 points
- Only index case 1 point
- de novo variation 2 points
In vivo studies
Experimental studies in animals are often used to recapitulate the phenomena underlying both normal and abnormal human biology. However, both economic and technological limitations prohibit the development of an animal model for each genetic variation. In our proposed scale, if an animal model is available for a variant and it recapitulates the human phenotype (positive result), 2 points are tallied due to the complexity of obtaining the data. We have not designated a score of 3 points in this category because the biological mechanisms exhibited in an animal model do not faithfully represent the mechanistic pathway(s) observed in humans. If an animal model is available but does not recapitulate the human phenotype (negative result) and/or no model is available, 0 points are given.
- in vivo model (positive result) 2 points
- in vivo model (negative result/not available) 0 points
In vitro studies
In vitro studies are one of the main experimental confirmations of pathogenicity in cardiac channelopathies. However, it is not feasible to perform in vitro studies to characterize each identified genetic variation. Even when available, some in vitro functional data not accurately reflect in vivo physiology29. This phenomenon occurs because in vitro studies are performed in heterologous systems that do not include all the necessary biological partners that modify the final phenotype.
Therefore, in our proposed stratification, if a cellular model is available for a variant and it mimics the biological mechanism(s) observed in vivo in humans (positive result), 2 points are tallied. In particular, “radical” genetic variations (nonsense and indels) have been assumed as pathogenic/deleterious because the protein will be altered; in consequence, 2 points are given in all these cases. As with the animal models, a score of 3 points is excluded from this category because in vitro studies cannot faithfully reflect the in vivo mechanistic pathway(s) in humans. If a cellular model is available but does not recapitulate the biological mechanism(s) observed in vivo in humans (negative result) or no model is available, 0 points are given.
- in vitro studies (positive result) or radical variation 2 points
- in vitro studies (negative result/not available) 0 points
In silico studies
Several bioinformatic tools have been developed in recent years to predict pathogenicity [such as PROVEAN (http://provean.jcvi.org/index.php), Condel ( http://bg.upf.edu/fannsdb/), SIFT (http://sift.jcvi.org/), Polyphen2 (http://genetics.bwh.harvard.edu/pph2/), Mutation Taster (www.mutationtaster.org), and Mutation Assessor (www.mutationassessor.org)]. These computational tools predict the impact of the genetic variant on the gene/protein sequence, based on gene alteration, type of variation, protein structure, biochemical properties of amino acids, and even evolutionary sequence conservation between species. However, these items are not all included within one bioinformatic database. In addition, all genetic variations cannot be found in all bioinformatic databases. Consequently, splicing changes are identified in specific databases [such as Human Splicing Finder (http://www.umd.be/HSF/), NNSplice (www.fruitfly.org/seq_tools/splice.html), and GeneSplicer (www.ccb.jhu.edu/software/genesplicer)], just as intronic variations are identified in others [such as Alamut (www.interactive-biosoftware.com/alamut/doc/2.0/splicing.html)].
In our proposed scale, 0 points are assigned if bioinformatic analyses are not available for a variant in any of the computational databases, or, if they are available but all in silico analyses showed a neutral/benign prediction. To include the largest quantity of bioinformatic data possible, 2 points are assigned if more than 4 different databases agree on the predicted pathogenicity (deleterious, probably, and/or possibly pathogenic) and 1 point if less than 4 databases agree on the predicted pathogenicity of the variant. This last point includes, for example, 3 pathogenic and 1 benign, 2 pathogenic and 2 benign, or 4 benign predictions. This threshold is based on the fact that computational systems do not include all biological elements existing in humans. Regarding “radical” genetic variations, including nonsense genetic variations and frameshift/in-frame genetic variations, no database analyses them because it is assumed that the protein product will be altered; 2 points are given in all these cases. We do not assign a score 3 points because an in silico prediction cannot faithfully reflect a human mechanistic pathway, and the use of bioinformatic tools can produce erroneous conclusions regarding pathogenicity, as we reported recently30. A positive in silico result means that pathogenicity is predicted; in contrast, a negative result means that a neutral/benign variation is predicted.
- in silico (positive result in ≥ 4 different databases) 2 points
- in silico (positive result in < 4 different databases) 1 point
- in silico (negative result in 4 different databases/not available) 0 points
Discussion
To validate our scale, we analysed several genetic variants localised in diverse genes associated and non-associated with different channelopathies (Table 1).
Table 1. Examples of the stratified scoring system. LQT: Long QT Syndrome. BrS: Brugada Syndrome. CPVT: Catecholaminergic Polymorphic Ventricular Tachycardia. GVUS: Genetic Variant Unknown Significance.
| Disease | Variation | Isoform | Disease-associated | Reported | Familial Segregation | In vivo | In vitro | In silico | Score | Classification of Variant |
|---|---|---|---|---|---|---|---|---|---|---|
| LQT | KCNQ1_p.R452W | NM_000218.2 | 3 | 2 | 1 | 0 | 0 | 2 | 8 | GVUS |
| LQT | KCNQ1_p.Y315S | NM_000218.2 | 3 | 3 | 2 | 2 | 2 | 2 | 14 | Pathogenic |
| LQT | KCNH2_p.E58A | NM_000238.3 | 3 | 2 | 1 | 0 | 0 | 1 | 7 | GVUS |
| LQT | KCNH2_p.G628S | NM_000238.3 | 3 | 2 | 2 | 2 | 2 | 2 | 13 | Pathogenic |
| CPVT | RyR2_p.R1013Q | NM_001035.2 | 3 | 2 | 1 | 0 | 0 | 1 | 7 | GVUS |
| CPVT | RyR2_p. V2475F | NM_001035.2 | 3 | 3 | 1 | 2 | 2 | 2 | 13 | Pathogenic |
| BrS | SCN5A_p.V1340I | NM_198056.2 | 3 | 2 | 1 | 0 | 2 | 1 | 9 | Probably Pathogenic |
| BrS | SCN5A_p.R104Q | NM_198056.2 | 3 | 3 | 2 | 0 | 2 | 2 | 12 | Pathogenic |
| BrS | SCN5A_p.N70K | NM_198056.2 | 3 | 1 | 1 | 0 | 0 | 1 | 6 | GVUS |
| BrS | PKP2_p.Q62K | NM_004572.3 | 1 | 2 | 1 | 0 | 0 | 1 | 5 | GVUS |
| LQT | SCN1Bb_p.P213T | NM_199037.3 | 2 | 2 | 1 | 0 | 2 | 2 | 9 | Probably Pathogenic |
For LQTS, we analysed genetic variations localized in two different genes, KCNQ1 and KCNH2. The first variation analysed was KCNQ1_p.R452W (c.1354C > T). This genetic variation has been previously associated with the disease31 -CM055335- (3 points), reported as pathogenic but only detected in the index case (2 point). No in vivo or in vitro studies were performed or identified. In silico analysis revealed four databases with damaging prediction (2 points). The total score of 8 points in this case indicates that the variant remains classified as a GVUS; this is in accordance with a recent report classifying KCNQ1_p.R452W as a variant of unknown significance32. Thus, this GVUS should be considered carefully in clinical practice. We believe that GVUS should be further analysed in relatives, since family segregation and genotype-phenotype correlation are required to clarify the pathogenic role of a variant.
The second variation was KCNQ1_p.Y315S (c.944A > C), previously associated with the disease33 -CM970823- (3 points) and reported as pathogenic in different studies (3 points). Familial segregation was detected in less than 4 relatives (2 points), and expression of the variant in rabbit heart produced transgenic rabbits with an LQT phenotype34 (2 points). In vitro analysis revealed a pathogenic role (2 points), and in silico analysis revealed 4 databases with damaging prediction (2 points). The total score of 14 points supports its classification as pathogenic in LQTS. In cases in which a pathogenic variation has been identified, current clinical/genetic guidelines should be followed both in the index case and in family members.
In KCNH2, also associated with LQTS, we analysed 2 genetic variations. The first one was KCNH2_p.E58A (c.173A > C), which was previously associated with the disease35 -CM057129- (3 points), reported as pathogenic only in one study (2 points), and was detected only in the index case (1 point). No in vivo or in vitro studies were identified, and in silico analysis revealed 3 databases with pathogenic prediction (1 point). The total score of 7 points indicates that the variant is likely a GVUS. Hence, further studies such as family segregation and in vivo and/or in vitro analyses should be performed to clarify its role in LQTS. The last variation associated with LQTS that we analysed was KCNH2_p.G628S (c.1882G > A), which was reported to be associated with the disease36 -CM950710- (3 points) and as pathogenic in more than one study (2 points). Familial segregation was identified in < 4 relatives (2 points), and expression in rabbit heart produced transgenic rabbits with an LQT phenotype34 (2 points). In vitro analysis revealed a pathogenic role (2 points), and in silico analysis revealed 4 databases with damaging prediction (2 points). A total score of 13 points supports the classification of this variant as pathogenic in LQTS.
For CPVT, 2 variations were analysed. The first one was RyR2_p.R1013Q (c.3038G > A), previously associated with the disease37 -CM097930- (3 points) reported as pathogenic but only detected in the index case (2 point). Familial segregation was identified only in the index case (1 point) and no in vivo or in vitro studies were performed. In silico analysis revealed two databases with damaging prediction (1 point). A score of 7 points suggests classification as a GVUS, in accordance with a recent report32. The second was RyR2_p.V2475F (c.7423G > T), previously reported associated with the disease37 -CM097960- (3 points), and with a deleterious effect in more than one study (3 points). Familial segregation was identified only in the index case (1 point), and in vivo studies yielded a positive result (2 points)38. In vitro studies also showed a positive effect (2 points), and in silico analysis revealed 4 databases with damaging prediction (2 points). The total score of 13 points supports classification of this variant as pathogenic in CPVT.
Finally, three variations were analysed for BrS. The first one was SCN5A_p.V1340I (c.4018G > A), previously associated with the disease17 -CM100703- (3 points), and with a deleterious effect reported in only one publication (2 points). It was identified only in the index case (2 points), and no in vivo studies were performed. In vitro studies showed a functional effect (2 points), and in silico analysis revealed three databases with damaging prediction (1 point). A score of 9 points indicates classification of this variant as probably pathogenic. Therefore, further studies should be performed to clarify its role in BrS.
The second variation analysed in BrS was SCN5A_p.R104Q (c.311G > A), previously reported associated with the disease39 -CM014904- (3 points), and with a deleterious effect in some studies (3 points). Positive familial segregation was identified in less than 4 relatives (2 points) but no in vivo studies were performed. In vitro studies showed a functional effect (2 points), and in silico analysis revealed four databases with damaging prediction (2 points). The total score of 12 points indicates pathogenicity.
The last variation showing a disease-association gene was SCN5A_p.N70K (c.210T > G), previously reported to be associated with the disease17 -CM100623- (3 points), and with a deleterious effect but only in one report (2 points). It was identified only in the index case (1 point), and no in vivo or in vitro studies were performed. In silico analysis revealed two databases with damaging prediction (1 point). The total score of 7 points supports classification of this variant as a GVUS.
To further assess our scale of pathogenicity, we include some variation in genes showing a reported association with the disease. Hence, we analyze PKP2_p.Q62K in a case of suspicious BrS. The genetic variation is located in a novel gene which encodes a desmosomal protein (1 point). The variation is already reported only in one study as pathogenic (associated with arrhythmogenic cardiomyopathy) (2 points), but only identified in the index case (1 point). Neither in vivo nor in vitro studies were performed (0 points respectively), and in silico analysis revealed two databases with damaging prediction (1 point). The total score of 5 points indicates GVUS, in concordance to published studies40.
We also analyzed SCN1Bb_p.P213T in a case of suspicious LQT. The genetic variation is located in a novel gene which encodes an ion channel regulatory protein (2 points). The variation is novel but the gene is linked to functional pathways of cardiac electrical disorders (2 points). No segregation was reported (1 point), and no in vivo studies are available (0 points). Both in vitro and in silico studies showed positive results (2 points). Therefore, total score of 9 points indicates probably pathogenic, as suggested in a recent publication41.
Conclusions
Determining the pathogenic role of genetic variants in channelopathies associated with SCD is necessary for improved clinical diagnosis and therapy of inherited arrhythmogenic diseases. To date, most identified genetic variants are considered rare variants of ambiguous clinical significance.
In the present report we propose a first approach in stratification of pathogenicity for genetic variations associated with channelopathies. Using several genetic tools available in the biomedical field, we propose a stratified scale of pathogenicity for variants identified in genes associated with SCD. We bring together multiple well accepted parameters that should be used together to reach a more definite clinical conclusion. We believe that this stratification may help determine the pathogenicity of genetic variants and will help clinicians understand the limitations of genetics and to better decide on therapeutic measures to prevent syncopal episodes and SCD.
Limitations
However, some limitations have to be acknowledged in the use of this approach. First, we propose the scale using several tools, but not all tools are available for each variant. It is not prudent to classify a variant using only one of these tools. Second, as genetic information is progressively becoming available, it is possible that new genetic/clinical data can change the pathogenicity level in a near future. We recommend reassessing the results on a regular basis. Finally, expertise is crucial in the handling of the databases and interpretation of the information. Thus, translation into clinical practice should be performed by consensus of a group of experts in different clinical and basic disciplines.
Author Contributions
O.C., C.A., A.I. wrote the manuscript. O.C., A.F., R.B. designed the study. All authors reviewed the manuscript.
Acknowledgments
The present study was supported by the Instituto de Salud Carlos III, Fondo Investigación Sanitaria FIS-PI2011/01826, and Obra Social “La Caixa”.
References
- Sharma S., Whyte G. & McKenna W. J. Sudden death from cardiovascular disease in young athletes: Fact or fiction? Br J Sports Med. 31, 269–276 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzamendi D. et al. Increase in sudden death from coronary artery disease in young adults. Am Heart J. 161, 574–580 (2011). [DOI] [PubMed] [Google Scholar]
- Pachon M. & Almendral J. Sudden death: Managing the patient who survives. Heart. 97, 1619–1625 (2011). [DOI] [PubMed] [Google Scholar]
- Raffan E. & Semple R. K. Next generation sequencing--implications for clinical practice. Br Med Bull. 99, 53–71 (2011). [DOI] [PubMed] [Google Scholar]
- Priori S. G. et al. Hrs/ehra/aphrs expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromesexpert consensus statement on inherited primary arrhythmia syndromes: Document endorsed by hrs, ehra, and aphrs in may 2013 and by accf, aha, paces, and aepc in june 2013. Heart Rhythm. 10, 1932–63 (2013). [DOI] [PubMed] [Google Scholar]
- Boycott K. M., Vanstone M. R., Bulman D. E. & MacKenzie A. E. Rare-disease genetics in the era of next-generation sequencing: Discovery to translation. Nature reviews. Genetics. 14, 681–691 (2013). [DOI] [PubMed] [Google Scholar]
- Duzkale H. et al. A systematic approach to assessing the clinical significance of genetic variants. Clin Genet. 84, 453–463 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. J. et al. Tryptophan biosynthesis protects mycobacteria from cd4 t-cell-mediated killing. Cell. 155, 1296–1308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano O. et al. Genetics and cardiac channelopathies. Genet Med. 12, 260–267 (2010). [DOI] [PubMed] [Google Scholar]
- Roden D. M. Clinical practice. Long-qt syndrome. N Engl J Med. 358, 169–176 (2008). [DOI] [PubMed] [Google Scholar]
- Schwartz P. J., Moss A. J., Vincent G. M. & Crampton R. S. Diagnostic criteria for the long qt syndrome. An update. Circulation. 88, 782–784 (1993). [DOI] [PubMed] [Google Scholar]
- Campuzano O. et al. Negative autopsy and sudden cardiac death. Int J Legal Med. 128, 599–606 (2014). [DOI] [PubMed] [Google Scholar]
- Schwartz P. J., Crotti L. & Insolia R. Long-qt syndrome: From genetics to management. Circ Arrhythm Electrophysiol. 5, 868–877 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugada P. & Brugada J. Right bundle branch block, persistent st segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 20, 1391–1396 (1992). [DOI] [PubMed] [Google Scholar]
- Berne P. & Brugada J. Brugada syndrome 2012. Circ J. 76, 1563–1571 (2012). [DOI] [PubMed] [Google Scholar]
- Campuzano O., Brugada R. & Iglesias A. Genetics of brugada syndrome. Curr Opin Cardiol. 25, 210–5 (2010). [DOI] [PubMed] [Google Scholar]
- Kapplinger J. D. et al. An international compendium of mutations in the scn5a-encoded cardiac sodium channel in patients referred for brugada syndrome genetic testing. Heart Rhythm. 7, 33–46 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylanen K., Poutanen T., Hiippala A., Swan H. & Korppi M. Catecholaminergic polymorphic ventricular tachycardia. Eur J Pediatr. 169, 535–542 (2010). [DOI] [PubMed] [Google Scholar]
- Gussak I. et al. Idiopathic short qt interval: A new clinical syndrome? Cardiology. 94, 99–102 (2000). [DOI] [PubMed] [Google Scholar]
- Patel C, Yan G. X. & Antzelevitch C. Short qt syndrome: From bench to bedside. Circ Arrhythm Electrophysiol. 3, 401–408 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. S. et al. Acmg recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. 10, 294–300 (2008). [DOI] [PubMed] [Google Scholar]
- Pilmer C. M., Kirsh J. A., Hildebrandt D., Krahn A. D. & Gow R. M. Sudden cardiac death in children and adolescents between 1 and 19 years of age. Heart Rhythm. 11, 239–245 (2014). [DOI] [PubMed] [Google Scholar]
- Song W. & Shou W. Cardiac sodium channel nav1.5 mutations and cardiac arrhythmia. Pediatr Cardiol. 33, 943–949 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsgaard L. et al. High prevalence of genetic variants previously associated with lqt syndrome in new exome data. Eur J Hum Genet. 20, 905–908 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risgaard B. et al. High prevalence of genetic variants previously associated with brugada syndrome in new exome data. Clin Genet. 84, 489–495 (2013). [DOI] [PubMed] [Google Scholar]
- Jabbari J. et al. New exome data question the pathogenicity of genetic variants previously associated with catecholaminergic polymorphic ventricular tachycardia. Circ Cardiovasc Genet. 6, 481–489 (2013). [DOI] [PubMed] [Google Scholar]
- Ku C. S., Vasiliou V. & Cooper D. N. A new era in the discovery of de novo mutations underlying human genetic disease. Hum Genomics. 6, 27 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattenberry E. et al. A comprehensive next generation sequencing-based genetic testing strategy to improve diagnosis of inherited pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 98, E1248–1256 (2013). [DOI] [PubMed] [Google Scholar]
- North K. N. et al. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat Genet. 21, 353–354 (1999). [DOI] [PubMed] [Google Scholar]
- Jadon D. et al. Exploring ankylosing spondylitis-associated erap1, il23r and il12b gene polymorphisms in subphenotypes of psoriatic arthritis. Rheumatology (Oxford). 52, 261–266 (2013). [DOI] [PubMed] [Google Scholar]
- Tester D. J. & Ackerman M. J. Genetic testing for cardiac channelopathies: Ten questions regarding clinical considerations for heart rhythm allied professionals. Heart Rhythm. 2, 675–677 (2005). [DOI] [PubMed] [Google Scholar]
- Dorschner M. O. et al. Actionable, pathogenic incidental findings in 1,000 participants' exomes. Am J Hum Genet. 93, 631–640 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donger C. et al. Kvlqt1 c-terminal missense mutation causes a forme fruste long-qt syndrome. Circulation. 96, 2778–2781 (1997). [DOI] [PubMed] [Google Scholar]
- Brunner M. et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long qt syndrome. J Clin Invest. 118, 2246–2259 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano C. et al. Genetic testing in the long qt syndrome: Development and validation of an efficient approach to genotyping in clinical practice. Jama. 294, 2975–2980 (2005). [DOI] [PubMed] [Google Scholar]
- Curran M. E. et al. A molecular basis for cardiac arrhythmia: Herg mutations cause long qt syndrome. Cell. 80, 795–803 (1995). [DOI] [PubMed] [Google Scholar]
- Medeiros-Domingo A. et al. The ryr2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long qt syndrome: A comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 54, 2065–2074 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza R. et al. Heterogeneity of ryanodine receptor dysfunction in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 112, 298–308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Nissenbaum E. et al. Genetic analysis of brugada syndrome in israel: Two novel mutations and possible genetic heterogeneity. Genet Test. 5, 331–334 (2001). [DOI] [PubMed] [Google Scholar]
- Cerrone M. et al. Missense mutations in plakophilin-2 can cause brugada syndrome phenotype by decreasing sodium current and nav1.5 membrane localization. Heart Rhythm. 10, 1743 (2013). [Google Scholar]
- Riuro H. et al. A missense mutation in the sodium channel beta1b subunit reveals scn1b as a susceptibility gene underlying long qt syndrome. Heart Rhythm. 11, 1202–1209 (2014). [DOI] [PubMed] [Google Scholar]