Abstract
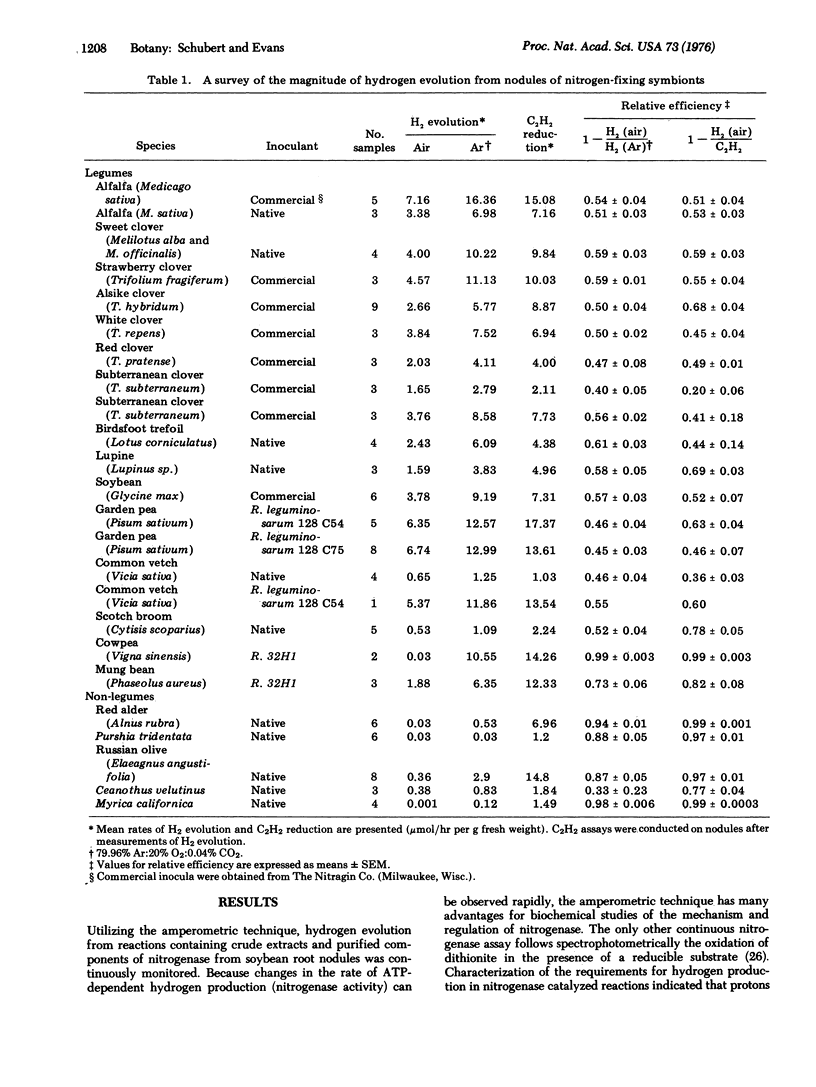
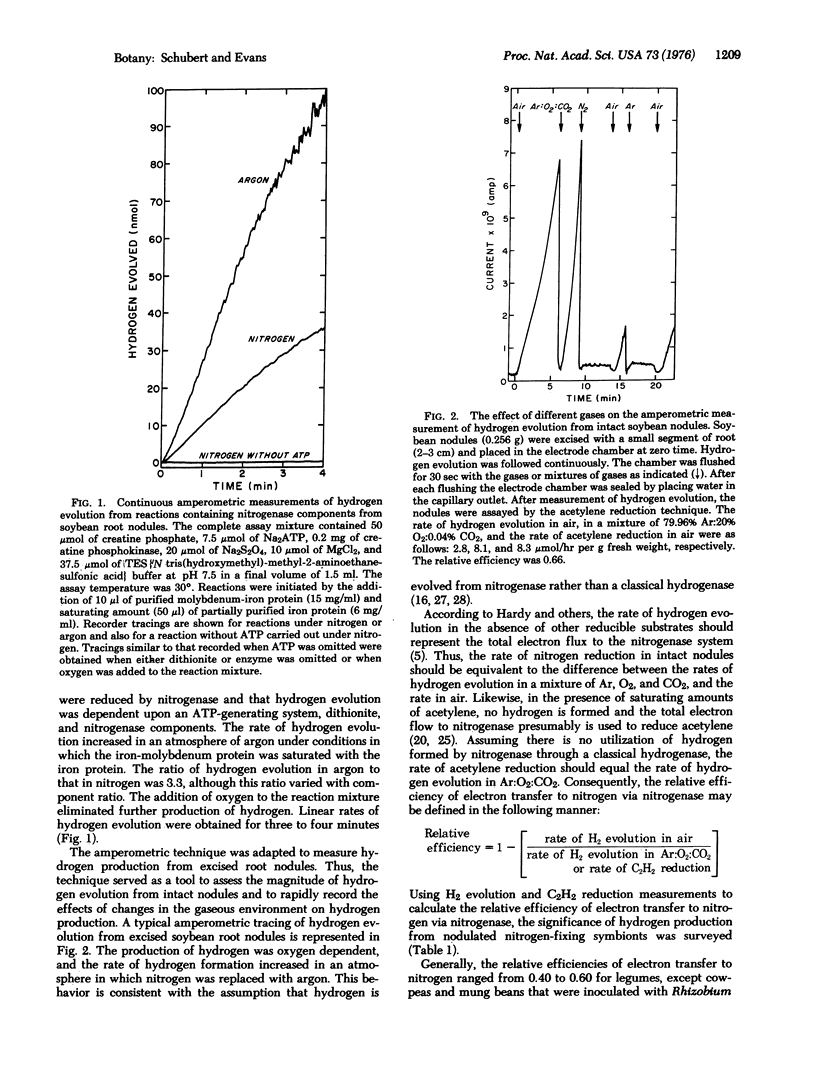
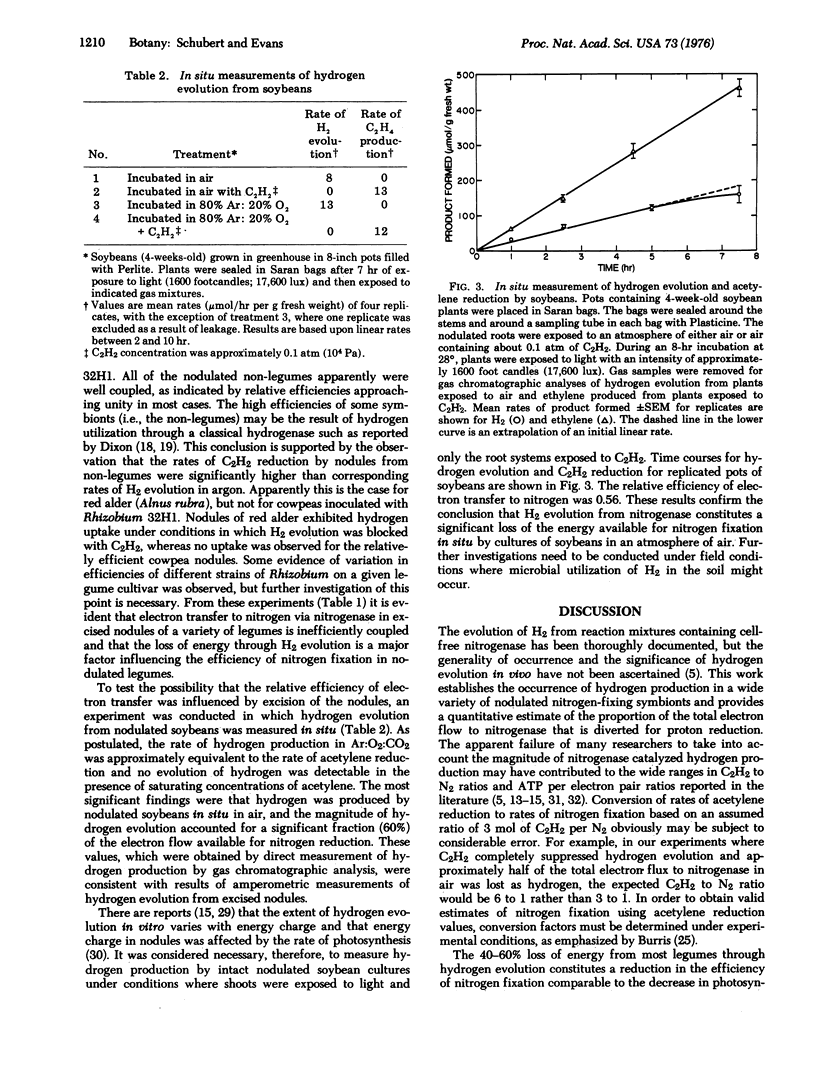
Nitrogenase-dependent hydrogen evolution from detached legume nodules and from reaction mixtures containing cell-free nitrogenase has been well established, but the overall effect of hydrogen evolution on the efficiency of nitrogen fixation in vivo has not been critically assessed. This paper describes a survey which revealed that hydrogen evolution is a general phenomenon associated with nitrogen fixation by many nodulated nitrogen-fixing symbionts. An evaluation of the magnitude of energy loss in terms of the efficiency of electron transfer to nitrogen, via nitrogenase, in excised nodules suggested that hydrogen production may severely reduce nitrogen fixation in many legumes where photosynthate supply is a factor limiting fixation. With most symbionts, including soybeans, only 40-60% of the electron flow to nitrogenase was transferred to nitrogen. The remainder was lost through hydrogen evolution. In situ measurements of hydrogen evolution and acetylene reduction by nodulated soybeans confirmed the results obtained with excised nodules. In an atmosphere of air, a major portion of the total electron flux available for the reduction of atmospheric nitrogen by either excised nodules or intact nodulated plants was utilized in the production of hydrogen gas. Some non-leguminous symbionts, such as Alnus rubra, and a few legumes (i.e., Vigna sinensis) apparently have evolved mechanisms of minimizing net hydrogen production, thus increasing their efficiency of electron transfer to nitrogen. Our results indicate that the extent of hydrogen evolution during nitrogen reduction is a major factor affecting the efficiency of nitrogen fixation by many agronomically important legumes.
Keywords: legumes, energy
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A., BURNS R. C., LECOMTE J. R. NITROGEN FIXATION: HYDROSULFITE AS ELECTRON DONOR WITH CELL-FREE PREPARATIONS OF AZOTOBACTER VINELANDII AND RHODOSPIRILLUM RUBRUM. Proc Natl Acad Sci U S A. 1965 Mar;53:532–539. doi: 10.1073/pnas.53.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen F. J. Some properties of nitrogen-fixing breis prepared from soybean root nodules. Biochim Biophys Acta. 1966 Dec 28;130(2):304–312. doi: 10.1016/0304-4165(66)90225-x. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L., Appleby C. A. Studies of the physiological role of leghaemoglobin in soybean root nodules. Biochim Biophys Acta. 1973 Jan 18;292(1):271–282. doi: 10.1016/0005-2728(73)90271-5. [DOI] [PubMed] [Google Scholar]
- Burns R. C., Bulen W. A. ATP-dependent hydrogen evolution by cell-free preparations of Azotobacter vinelandii. Biochim Biophys Acta. 1965 Sep 20;105(3):437–445. doi: 10.1016/s0926-6593(65)80229-6. [DOI] [PubMed] [Google Scholar]
- Ching T. M., Hedtke S., Russell S. A., Evans H. J. Energy State and Dinitrogen Fixation in Soybean Nodules of Dark-grown Plants. Plant Physiol. 1975 Apr;55(4):796–798. doi: 10.1104/pp.55.4.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L. C., Shah V. K., Brill W. J. Nitrogenase. VII. Effect of component ratio, ATP and H2 on the distribution of electrons to alternative substrates. Biochim Biophys Acta. 1975 Sep 22;403(1):67–78. doi: 10.1016/0005-2744(75)90009-1. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in pea root nodule bacterioids. Arch Mikrobiol. 1968;62(3):272–283. doi: 10.1007/BF00413898. [DOI] [PubMed] [Google Scholar]
- HOCH G. E., SCHNEIDER K. C., BURRIS R. H. Hydrogen evolution and exchange, and conversion of N2O to N2 by soybean root nodules. Biochim Biophys Acta. 1960 Jan 15;37:273–279. doi: 10.1016/0006-3002(60)90234-1. [DOI] [PubMed] [Google Scholar]
- HYNDMAN L. A., BURRIS R. H., WILSON P. W. Properties of hydrogenase from Azotobacter vinelandii. J Bacteriol. 1953 May;65(5):522–531. doi: 10.1128/jb.65.5.522-531.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield K. L., Bulen W. A. Adenosine triphosphate requirement of nitrogenase from Azotobacter vinelandii. Biochemistry. 1969 Dec;8(12):5103–5108. doi: 10.1021/bi00840a064. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Havelka U. D. Nitrogen fixation research: a key to world food? Science. 1975 May 9;188(4188):633–643. doi: 10.1126/science.188.4188.633. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., Burris R. H. Nitrogenase-catalyzed reactions. Biochim Biophys Acta. 1972 Nov 17;283(2):339–350. doi: 10.1016/0005-2728(72)90250-2. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., Chen C. H., Burris R. H. Inhibition of nitrogenase-catalyzed reductions. Biochim Biophys Acta. 1973 Jan 18;292(1):256–270. doi: 10.1016/0005-2728(73)90270-3. [DOI] [PubMed] [Google Scholar]
- Klucas R. V., Koch B., Russell S. A., Evans H. J. Purification and Some Properties of the Nitrogenase From Soybean (Glycine max Merr.) Nodules. Plant Physiol. 1968 Dec;43(12):1906–1912. doi: 10.1104/pp.43.12.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch B., Evans H. J., Russell S. Properties of the nitrogenase system in cell-free extracts of bacteroids from soybean root nodules. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1343–1350. doi: 10.1073/pnas.58.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljones T., Burris R. H. ATP hydrolysis and electron transfer in the nitrogenase reaction with different combinations of the iron protein and the molybdenum-iron protein. Biochim Biophys Acta. 1972 Jul 12;275(1):93–101. doi: 10.1016/0005-2728(72)90027-8. [DOI] [PubMed] [Google Scholar]
- Ljones T., Burris R. H. Continuous spectrophotometric assay for nitrogenase. Anal Biochem. 1972 Feb;45(2):448–452. doi: 10.1016/0003-2697(72)90206-0. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E. Components of cell-free extracts of Clostridium pasteurianum required for ATP-dependent H2 evolution from dithionite and for N2 fixation. Biochim Biophys Acta. 1966 Sep 26;127(1):18–25. doi: 10.1016/0304-4165(66)90470-3. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. A new procedure for assay of bacterial hydrogenases. J Bacteriol. 1956 Jan;71(1):70–80. doi: 10.1128/jb.71.1.70-80.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Ortiz J. M., Burris R. H. Interactions among substrates and inhibitors of nitrogenase. J Bacteriol. 1975 Aug;123(2):537–545. doi: 10.1128/jb.123.2.537-545.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Healey F. P., Myers J. Amperometric measurement of hydrogen evolution in chlamydomonas. Plant Physiol. 1971 Jul;48(1):108–110. doi: 10.1104/pp.48.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Improving the efficiency of photosynthesis. Science. 1975 May 9;188(4188):626–633. doi: 10.1126/science.188.4188.626. [DOI] [PubMed] [Google Scholar]



