Summary
Background
There is limited research characterizing the HIV care continuum with population-based data in sub-Saharan Africa. The objectives of this study were to: 1) describe engagement in care among all known HIV-positive adults in one sub-county of western Kenya; and 2) determine the time to and predictors of linkage and engagement among adults newly diagnosed via home-based counseling and testing (HBCT).
Methods
AMPATH (Academic Model Providing Access to Healthcare) has provided HIV care in western Kenya since 2001 and HBCT since 2007. Following a widespread HBCT program in Bunyala sub-county, electronic medical records (EMR) were reviewed to identify uptake of care among individuals with previously known (self-reported) infection and new (identified by HBCT) HIV diagnoses as of June 2014. Engagement in HIV care was defined as an initial encounter with an HIV care provider. Cox regression analysis was used to examine the predictors of engagement among those newly diagnosed.
Findings
Of the 3,482 infected adults identified, 61% had previously known infections, among whom 84% (n = 1778/2122) had ever had at least one clinical encounter within AMPATH. While 73% were registered in the EMR, only 15% (n = 209/1360) of the newly diagnosed had seen a clinician over a median of 3·4 years. The median time to engagement among the newly diagnosed was 60 days (interquartile range: 10–411 days).
Interpretation
Engagement in care was high among those who at the time of HBCT were already known HIV-positive, but few who were newly diagnosed in HBCT saw an HIV care provider.
Funding
This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through USAID under the terms of Cooperative Agreement No. AID-623-A-12-0001. The HBCT program was supported by grants from Abbott Laboratories, the Purple ville Foundation, and the Global Business Coalition. Abbott Laboratories provided test kits and logistical support. Further support was provided by the National Institute of Mental Health (K01MH099966, PI: Genberg) and the Bill and Melinda Gates Foundation. The contents of this study are the sole responsibility of the authors and do not necessarily reflect the views of USAID, NIMH, BMGF, or the United States Government.
Keywords: Linkage to HIV care, Engagement in HIV care, HIV treatment-as-prevention, Home-based HIV counseling and testing, sub-Saharan Africa, Implementation research
INTRODUCTION
As antiretroviral treatment (ART) programs expand in resource-limited settings, early identification and engagement in care of individuals with HIV is critical to optimizing treatment outcomes. Home-based counseling and testing (HBCT) successfully identifies HIV earlier in the course of disease progression compared with other testing modalities.1,2 However the benefits of HBCT for improving patient outcomes will not be fully realized unless those diagnosed engage with care in a timely manner.
Recent systematic reviews in sub-Saharan Africa demonstrated inadequate linkage to care following HIV testing.3–5 Testing and treatment programs are not often connected in practice, creating structural barriers for patients and limiting patient tracking by the healthcare system. While recent studies have begun to include the critical first step in the cascade (i.e., between testing and linkage to care), much of the existing research on linkage to care has focused on individuals testing via voluntary counseling and testing (VCT) or provider-initiated testing (PITC). Individuals testing via VCT or PITC may be a select group and the prevalence and predictors of linkage to care following diagnoses through these testing programs may not reflect that of the general population of individuals living with HIV in a community.
Because it is delivered to every household in a geographic area, HBCT can provide population-level data on HIV prevalence and linkage to and engagement in HIV care. Population-level data allows not only a current estimate of ART use, but also the ability to understand losses in care starting at the point of HIV testing among all those living with HIV in a geographic area, not just those accessing HIV testing. Data on this part of the cascade from population-based cohort studies is underrepresented in the current literature.6,7 From the perspective of the implementation of treatment-as-prevention (TasP), HBCT also provides insight into the effort required to engage all HIV-positive individuals in a given geographic area in care.
The objective of this study was to use HBCT data from Bunyala, a high prevalence sub-county in western Kenya,2,8 together with clinical data from AMPATH (Academic Model Providing Access to Healthcare), the largest HIV care provider in the region, to measure engagement in the early phases of HIV care at the population-level. Using this population-based framework, we focused on the steps of the HIV care continuum through initiation of ART. The specific objectives of this study were:1) to describe the proportion that had ever engaged in care among all individuals known to be HIV-positive at the time of HBCT; and 2) to prospectively determine the time to and predictors of engagement among all individuals newly identified as HIV-positive during HBCT.
METHODS
Study Setting and Design
This study used data collected through the AMPATH program in western Kenya.8 Briefly, AMPATH is a large clinical care system that has enrolled over 150,000 individuals into HIV care since 2001.9,10 AMPATH implemented HBCT, including community mobilization, in-home HIV testing and counseling with rapid results, and passive referrals to care in six catchment areas from 2007 through 2012.2,8 The goal of the HBCT initiative was to provide HIV testing to all adults (aged 13 and older) living in the catchment areas. Data presented here are from one high prevalence sub-county, Bunyala, where HBCT was conducted from December 2009 to February 2011. Bunyala is located in Busia County, a predominantly rural area bordering Lake Victoria and Uganda, with a population of 66,723 in 2009.11 Non-HIV-related AMPATH programs, including a primary care initiative, also operate in Bunyala sub-County. Residents of Bunyala may have been registered within AMPATH as part of these other programs.
Trained counselors visited homes and enumerated all household members to determine eligibility for HIV testing. All consenting household members 13 years and older were eligible. HIV testing used parallel tests, the Determine HIV 1/2 rapid assay (Abbott Laboratories, Abbott Park, IL) and SD Bioline HIV-1/2 3·0 rapid assay (Standard Diagnostics Inc., Kyonggi-do, South Korea). Verbal referrals to the nearest HIV clinic were provided to all testing positive.
Counselors collected data from participants on socio-demographic characteristics, previous HIV testing and results, self-reported engagement in care, and current test results using mobile devices. Those who reported a previously diagnosed HIV-infection were offered repeat testing. AMPATH medical records (AMRS) data was reviewed retrospectively, from January 2004 to June 2014, to determine engagement in care outcomes among those previously known and newly diagnosed.12,13 This study was reviewed and approved by research ethics committees at Moi University, Indiana University, and Brown University.
Data from HCT and AMRS were merged using probabilistic matching methods.14,15 Specifically, identifiable information (i.e., names, date of birth, and sex, Location, sub-Location, village) from each data source were compared to determine three groups: 1) individuals testing via HBCT who had data in AMRS, 2) individuals testing via HBCT who did not have data in AMRS, and 3) uncertain matches. The Fellegi-Sunter probabilistic matching method assigned agreement rates for demographic fields and calculated a score indicating the likelihood that a given pair of records was correctly matched.14,15 For individuals who were correctly matched, HBCT data were migrated into AMRS, while new AMRS records were created for those not currently found. All uncertain matches were reviewed manually, with phone contact to verify information as needed, and individually adjudicated. If contact was not made, they were classified as unmatched.
New patients accessing care must first register to provide contact information (e.g., names, date of birth, sex, and village of residence) to AMPATH. We examined registration within AMPATH as the first step of the HIV care cascade. Engagement in care was defined as ever having had an initial clinical encounter with an HIV care provider. For those with previously known infection, engagement in care was examined prior to HBCT. Those with known infection who had not engaged in care at the time of HBCT were examined for prospective engagement. We also determined whether individuals with known HIV-infection had received CD4 testing and initiated ART among those who were eligible. Among the newly diagnosed, we examined engagement in care occurring after the HBCT testing date, defined as above. We also examined the proportion of the newly diagnosed who engaged with care within 90 days of HBCT to allow comparability to other studies on linkage to care. Other metrics included:CD4 testing, median CD4 at enrollment, ART initiation among those who were eligible, and losses or deaths prior to ART initiation.
Statistical Analysis
Standard descriptive statistics described the socio-demographic characteristics of the two study groups (e.g., previously known positive and newly diagnosed through HBCT). Descriptive statistics were used to characterize the initial steps in the HIV care continuum, including registration, engagement in care, receipt of CD4 testing, ART initiation, and losses to the care program and death among those who had not initiated ART.
We estimated adjusted prevalence ratios with a log linear regression model to determine the demographic and socioeconomic factors associated with engagement in care among those with known infection.16 Specifically, we modeled log probability of engagement as a linear function of independent variables and fitted the model using nonlinear least squares.17 Robust standard errors were used in the calculation of p-values and confidence intervals. Factors of interest included: sex, age, number of household members, marital status, employment, average monthly income, and educational attainment. Sex (female/male) and employment (yes/no) were included as dichotomous variables. Age quartiles (13–27, 28–33, 34–42, >43 years), number of household members (1–2, 3–4, >5), marital status (single, married, widowed, cohabitating/separated/divorced), average monthly income (0–1000 Kenyan Shillings (KES), 1001–2000 KES, 2001–3000 KES, 3001+ KES), and educational attainment (none, primary, secondary/tertiary) were included as categorical indicator variables.
Survival analysis methods were used to examine the time from HBCT to engagement in care. Those newly diagnosed were followed from HBCT to engaging with care within AMPATH or administrative censoring (June 2014). Predictors of engagement were examined with Cox regression models. Variables were included in models as described above. Robust standard errors were used in the calculation of p-values and 95% confidence intervals for both models to account for clustering within households. All analysis was conducted using Stata version 13·0 (StataCorp, College Station, TX).
Role of the funding source
The sponsor of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
HBCT reached approximately 85% of the population of Bunyala (n = 58,846/66,723).11 Of the individuals enumerated by HBCT, 96% (n = 56,670) were screened for eligibility. Among those, 24,337 were younger than 13 years and excluded from this analysis. Of the remaining 32,333, 64 refused participation (<1%).
HBCT identified 3,482 HIV-positive adults in Bunyala (estimated prevalence = 11%; males = 10%, females = 12%). At the time of analysis in June 2014, among all those with HIV identified by HBCT in Bunyala sub-county (including both previously known positives and those newly diagnosed), 57% (n = 1979) had ever seen an HIV care provider.
Among those identified as HIV-positive by HBCT, 60% (n = 2077) reported that they had been previously diagnosed and an additional 45 participants did not report HIV-infection, but had received care in AMPATH prior to HBCT. Table 1 presents the socio-demographic characteristics of this group. Sixty-seven percent (n = 1,425) were female, 55% (n = 1,169) were married and over half (n = 1,144) reported average monthly income of 0–1000 KES (up to approximately 12 USD). The median age was 36 years (interquartile range (IQR): 30–45). Thirty-eight percent (n = 809) reported no education, while half had a primary school education (n = 1,057).
Table 1.
Demographic and behavioral characteristics of those with known HIV-infection and those newly diagnosed at the time of home-based counseling and testing (HBCT).
| Characteristic | Known HIV-positive N=2122 n (%) |
Newly diagnosed N=1360 n (%) |
|---|---|---|
| Female | 1425 (67) | 786 (58) |
| Median age, in years (IQR) | 36 (30–45) | 31 (25–38) |
| Median number of household residents (IQR) | 4 (2–5) | 3 (2–5) |
| Marital status | ||
| Single | 250 (12) | 250 (18) |
| Married | 1169 (55) | 785 (58) |
| Widowed | 525 (25) | 172 (13) |
| Cohabiting, separated, divorced | 174 (8) | 153 (11) |
| Missing | 4 (<1) | 0 (0) |
| Occupation | ||
| Farmer | 635 (30) | 330 (24) |
| Self-employed | 480 (23) | 359 (26) |
| Unemployed | 434 (20) | 264 (19) |
| Casual worker | 150 (7) | 133 (10) |
| Other | 405 (19) | 262 (19) |
| Missing | 18 (<1) | 12 (<1) |
| Average monthly income (in KES) | ||
| 0–1000 | 1144 (54) | 648 (48) |
| 1001–2000 | 355 (17) | 260 (19) |
| 2001–3000 | 302 (14) | 221 (16) |
| 3001+ | 187 (9) | 145 (11) |
| Missing | 134 (6) | 86 (6) |
| Educational attainment | ||
| None | 809 (38) | 457 (34) |
| Primary | 1057 (50) | 751 (55) |
| Secondary/Tertiary | 240 (11) | 139 (10) |
| Missing | 16 (1) | 13 (1) |
| Previously tested for HIV | ||
| No previous test | -- | 397 (29) |
| Negative/indeterminate result | 963 (71) | |
| Pregnant* | 84 (6) | 53 (7) |
IQR = interquartile range; KES = Kenyan Shillings (1000 KES = 11–12 USD).
Pregnancy status among 1420 females known to be HIV-positive and 786 newly diagnosed; Missing for 7% of females in the sample.
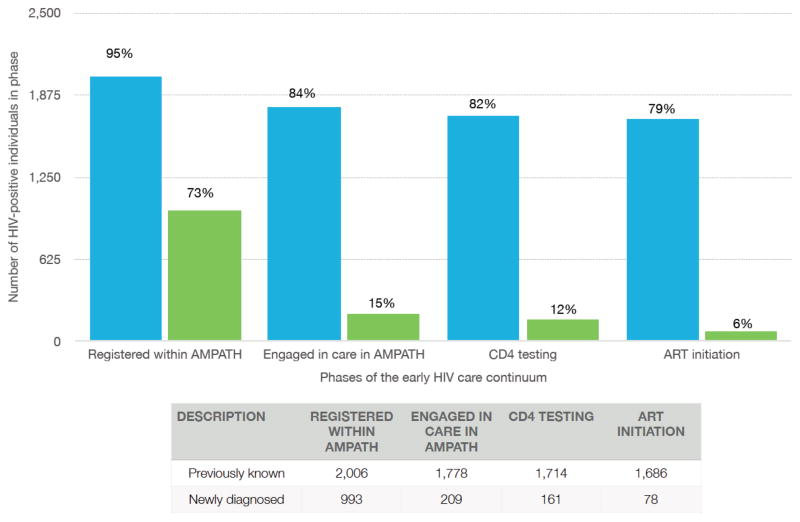
Of those with known HIV-infection at the time of HBCT84% (n = 1787) self-reported ever engaging in care with AMPATH and 9% (n = 185) reported receiving care elsewhere. Figure 1 presents engagement in the early HIV care continuum among those with known HIV-infection. Ninety-five percent (n = 2006) registered within AMPATH and 84% (n = 1778) had a verified visit within AMPATH. Ninety-seven percent (n = 1725/1787) of those who self-reported care within AMPATH had seen an HIV care provider. Of those with known infection who had engaged within AMPATH (n = 1,778), the median time in care was 5 years (IQR: 4–7). Among this group, 98%(n = 1747) received CD4 testing with a median CD4 at enrollment of 385 cells/mm3, and 94% (n = 1,686) initiated ART as of June 2014. Thirty-five (10%) of the 344 individuals who were known to be HIV-positive at the time of HBCT, but had not yet engaged with care, subsequently had a clinical encounter within AMPATH between HBCT and June 2014.
Figure 1.
HIV care among 2,122 adults previously known to be HIV-positive at HBCT (blue bars) and 1,360 newly diagnosed (green bars), in Bunyala sub-County, Kenya, through June 2014.
Table 2 presents the adjusted associations between socio-demographic factors at the time of testing and prior engagement in HIV care among those with self-reported HIV-infection. Males, compared with females, were less likely to have seen a care provider prior to HBCT (adjusted prevalence ratio (APR) = 0·96, 95% confidence interval (CI): 0·92, 0·99). Compared to individuals aged 13–27 years, individuals in all older age categories were more likely to have previously engaged in care. Compared to those with secondary/tertiary education, those with no formal education (APR = 1·17, 95% CI: 1·07, 1·27) or primary education (APR = 1·17, 95% CI: 1·07, 1·26) were more likely to have already seen an HIV care provider. There were no statistically significant associations between prior engagement in care and marital status, average monthly income, or employment status, among those with reported known infection.
Table 2.
Adjusted* prevalence ratios (APR) and 95% confidence intervals (CI) for engagement in HIV care among those previously known to be HIV-positive and adjusted hazard ratios (AHR) and 95% CI of engaging in care following HBCT among those newly diagnosed
| Characteristic | Known HIV-positive n = 2122 |
Newly diagnosed n = 1360 |
|---|---|---|
| Engaged in care, n (%) | 1778 (84) | 209 (15) |
| (APR, 95% CI) | (AHR, 95% CI) | |
| Male | 0·96 (0·92, 0·99) | 0·64 (0·47, 0·89) |
| Age categories (in years) | ||
| 13–27 | 1·00 | 1·00 |
| 28–33 | 1·09 (1·02, 1·17) | 1·40 (0·96, 2·03) |
| 34–42 | 1·15 (1·08, 1·24) | 1·31 (0·88, 1·95) |
| 43 and older | 1·20 (1·12, 1·28) | 1·82 (1·13, 2·92) |
| Number of individuals living in household | ||
| 1–2 | 1·00 | 1.00 |
| 3–4 | 1·07 (1·02, 1·12) | 1·35 (0·94, 1·94) |
| 5+ | 1·04 (0·99, 1·10) | 1·96 (1·36, 2·84) |
| Educational attainment | ||
| None | 1·17 (1·07, 1·27) | 1·22 (0·71, 2·09) |
| Primary | 1·16 (1·07, 1·26) | 1·40 (0·84, 2·34) |
| Secondary/tertiary | 1·00 | 1·00 |
Both models adjusted for all variables in the table, plus average monthly income, employment status, and marital status.
Table 1 displays the socio-demographic characteristics of the 1,360 newly diagnosed. Fifty-seven percent were female (n = 786), with a median age of 31 years (IQR: 25–38). Almost 60% (n = 785) were married. Approximately half (n = 648) earned 0–1000 KES per month and 55% (n=751) reported primary education. Seventy-one percent (n = 963) had previously tested for HIV. Among the newly diagnosed, 73% (n = 993) were registered in the electronic medical records system.
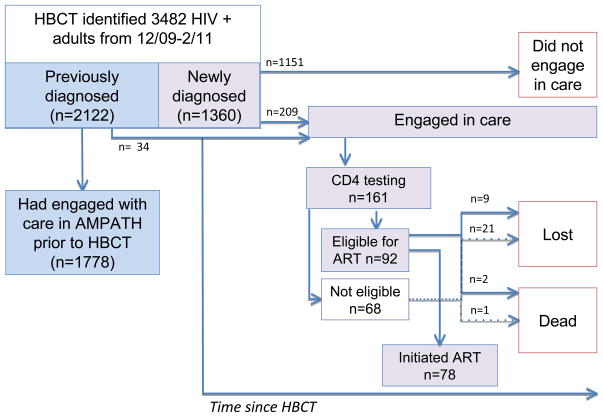
Figure 2 displays prospective engagement in the early part of the HIV care continuum following HBCT. Among those newly diagnosed, 15% (n = 209/1360) prospectively engaged in care. The median time to the initial clinical encounter among those newly diagnosed who saw an HIV care provider was 60 days (IQR: 10–411). Fifty-six percent (n = 116) of those who saw a care provider did so within 90 days of HBCT. Among those who saw a provider, 77% (n = 161) receivedCD4 testing with a median CD4 at enrollment of 436 cells/mm3. As of June 2014, 44% (n = 92) of those who engaged with care were eligible for ART. Among the eligible,85% (n = 78) initiated ART. Among the 14 who were eligible and had not initiated ART, 64% (n = 9) were lost to the care program and 14%(n = 2) died. Among the 68 who met with a care provider, received CD4 testing, and were not eligible for ART at the time of analysis, 31% (n = 21) were lost and 1% (n = 1) died as of June 2014.
Figure 2.
Estimates of the HIV care continuum among 3482 HIV-positive adults in Bunyala sub-County, Kenya, identified or diagnosed via HBCT from December 2009 to February 2011, followed through June 2014.
Table 2 displays the adjusted hazard ratios for prospectively engaging in care among the newly diagnosed. Males, compared to females, were less likely to have seen a care provider following testing (adjusted hazard ratio (AHR) = 0·64, 95% CI: 0·47, 0·89). The oldest (43+ years) were more likely to have met with a care provider compared to the youngest (13–27 years) (AHR = 1·82, 95% CI: 1·13, 2·92). Those with five or more household members were more likely to have seen a care provider (AHR = 1·96, 95% CI: 1·36, 2·84), compared with households of two or fewer. There were no statistically significant associations between marital status, employment, average monthly income, or education and prospective engagement with care following HBCT.
DISCUSSION
This study is among the first to provide prospective estimates of the early phases of the HIV care continuum from a population-based cohort testing for HIV in sub-Saharan Africa. In this high HIV prevalence area of Kenya, we found that 57% of HIV-positive individuals aged at least 13 years in the sub-county had seen an HIV care provider, and 53% were receiving ART. Notably, 84% of individuals in the sub-county who at the time of HBCT knew they were positive were already in care, a finding comparable to recent national estimates from the Kenya AIDS Indicator Survey.18 However, 39% of infected individuals in the sub-county did not know they were HIV-positive until tested through HBCT. While HBCT successfully identified nearly all individuals who were unaware of their infection, among those newly diagnosed during HBCT, only 15%met with an HIV care provider over the 3·4 years following the test. The estimates of engagement in care presented here may be more accurate reflections of the true engagement in the HIV care continuum among all those who are HIV-positive within this community, since they include nearly universal case-finding in the sub-county and the initial losses occurring immediately following diagnosis among those who never enroll in care.
The estimates of engaging in (or linking to) care following testing presented here are lower than what has been observed following other types of testing (i.e., VCT, PITC, mobile VCT) in other settings3,20–23. However, direct comparisons between these testing venues are inappropriate, as are direct comparisons of engagement in care between individuals previously diagnosed and those newly diagnosed. Individuals testing via VCT or PITC, and individuals who already knew they were positive, are likely to have substantially different characteristics related to engagement in care, compared with those identified via HBCT. The main difference is that those testing via VCT have already demonstrated motivation for accessing care by self-selecting into testing, and those testing via PITC are, by definition, a sub-population of those already linked to the healthcare system. Those testing via HBCT, by contrast, represent a nearly complete sample of the entire HIV-positive population in a geographic area of the country. Simple comparisons in rates of engagement in care between modes of diagnosis are therefore not meaningful because the health-seeking behaviors of people among them are fundamentally different. One previous study in South Africa demonstrated higher linkage to care following HBCT than ours, however this small study was restricted to a small geographic area, enrollees were included only if living within walking distance to an HIV care facility, received food packages upon enrolment, relied on self-reported assessment of engagement in care, and had intensive follow-up visits to the home following testing.19 Our findings are more likely reflective of a real-world situation.
Although comparisons of rates are not possible, it is likely more challenging for patients to engage in care following HBCT compared with those testing in clinical facilities or VCT. HBCT is universally administered and most of the diagnosed are healthy and asymptomatic. They maybe less ready to receive test results and may be less motivated to make the initial link to care. There is evidence to suggest that patients testing through different programs vary with respect to subsequent engagement in care. In South Africa those who tested via VCT were less likely to be lost from pre-ART care compared with those testing via PITC, possibly because self-referred VCT testers were more motivated to remain engaged with care.24 Because HBCT also identifies individuals earlier,2,25 HBCT testers may have lower perceived risk prior to testing and lower perceived need for care after testing, reinforced by their lack of physical symptoms.26 Additional research is needed to understand and address the structural, psychosocial and other potential barriers to engaging in care for healthy individuals diagnosed with HIV specifically following HBCT. Adapting strategies for delivering care to a mostly healthy population of newly diagnosed individuals, such as community-based care and task shifting,27,28 is urgently needed. The delivery of care for predominantly healthy individuals newly diagnosed in community-based settings should also work to strengthen the connections between testing and care programs to minimize losses occurring in the first step of the HIV care continuum.
Our study is the first to provide population-based estimates of engagement in the early phases of the continuum of HIV care in sub-Saharan Africa. The early phases of the HIV care continuum include the steps from testing through the initiation of ART.5,29 Most studies provide estimates of patient losses that occur from the point of enrolling in care onward. Further many estimates of the cascade that do not rely on cohort data may not account for patients cycling back into care after they drop out.30 There is a need for standard definitions and applications of the HIV care cascade to enable comparisons across studies. For example, although it has been suggested that registration be used as a universal marker of engagement in care, registration in a program can have many meanings. In our case, a non-HIV related program registered many community members in the electronic medical records system, so while a high proportion of HIV-infected individuals in Bunyala were technically registered in care, very few of them had actually seen a clinician.
The implications of these findings suggest that efforts are needed to improve engagement in care particularly among those newly diagnosed, and especially in the context of “test and treat” strategies.31 Doing so will be necessary to ensure the effectiveness of TasP. Early initiation of ART leads to improved clinical outcomes for patients with HIV and recent research has also demonstrated the impact of ART coverage on decreased individual risk for HIV acquisition,32 suggesting that the benefits of more timely initiation of ART can not only improve individual patient outcomes, but also have real-world impact on population health in sub-Saharan Africa. HBCT can achieve high uptake of testing and identifies individuals early in the course of HIV.2,33 Future research is needed to develop and implement programs and interventions such as point-of-care CD4 testing and active follow-up of those testing positive to effectively link and engage HIV-positive individuals to care from population-based testing efforts such as HBCT.19,34–36
Results from both regression analyses confirmed findings from previous research,37 that additional efforts are needed to engage men and young people in care in this setting. It is possible that alternative strategies beyond HBCT are needed to capture more difficult to engage populations. Among those with known infection, individuals with higher levels of education were less likely to have been engaged in care. This may reflect that those of higher socioeconomic status may seek care outside of the AMPATH network through private practitioners, or that they were more likely to be employed and therefore not able to engage with care during clinic hours. No other demographic factors predicted engagement in care among those with known infection or engagement in care following HBCT among the newly diagnosed. Additional research is needed to understand what motivates engagement in care for the management of chronic conditions such as HIV and for people whose health has been preserved through the early identification of their HIV infection.
Efforts are also needed to retain in care those who link from the community. Among the newly diagnosed that did engage with care following HBCT in this study, 14% (n = 30/209) were lost from the care program, many before initiating ART, and three percent of those who were eligible for ART died. Additional research is needed to follow this cohort of newly diagnosed individuals over time and determine the retention outcomes and factors that predict longer-term retention in care. Additional work is also needed to identify those who did not engage in care following HBCT to understand the barriers and to determine the most effective interventions for promoting timely engagement in HIV care.
There are several limitations to this study. Although the measurement of the outcome was an improvement over self-report as seen in prior studies,19,37 it is possible that there were errors, either false positive or false negatives, in the merging of HBCT and AMRS, leading to misclassification of the outcome. In particular it is possible that there were individuals who engaged in care who were not correctly identified in AMRS (for example, if they used a different name when enrolling in care compared to that used for HBCT), and therefore we may have underestimated engagement. However we employed a manual review of all uncertain matches to reduce the number of false negative and false positive matches. It is also possible that patients sought care from sources other than AMPATH. The impact of those seeking care elsewhere is likely to be limited in this study, however, given that AMPATH is the majority provider in the area, catchment areas are defined by the Ministry of Health, and the self-reported data suggested that only 8% of those previously diagnosed sought care somewhere other than AMPATH. Previous diagnosis was self-reported and due to social desirability, individuals may have chosen not to disclose a previously known infection if they had not already accessed care. This reporting bias may have led to counting those with previously known infection as newly diagnosed. In the cross-sectional analysis among individuals who were previously diagnosed, survival bias likely led to overestimates in engagement in care since those captured must have survived long enough to participate in HBCT. We are missing those who had tested at similar times, but died prior to our study. Individuals may also have died or migrated following HBCT. As has been demonstrated in other settings following ART initiation,38,39 substantial mortality or transfers would suggest that the current study underestimates the true amount of engagement in care.
This study provides critical data on engagement in care among all individuals living with HIV in one sub-county in western Kenya. Over three years after HBCT, 57% of the HIV-infected population had seen an HIV care provider: 84% of those who knew they were infected prior to HBCT and only 15% of those newly diagnosed at the time of HBCT. Existing estimates of losses to follow-up further along in the care continuum may underestimate the true losses occurring because they are not accounting for those who are not currently seeking care (e.g., lost after diagnosis, but before enrolling in care). Research to understand the structural and other barriers to engaging with HIV care and interventions to overcome them, particularly among individuals who are newly diagnosed with HIV, are urgently needed.40 For programs to deliver both the individual and population benefits of ART, additional efforts on the entire community, strengthening ties between testing and care programs, and those who are newly diagnosed are urgently needed.
Panel: Research in context.
Search Strategy: We searched PubMed for articles published in English through July 2014 that included the following terms: HIV, linkage, engagement, cascade, continuum, and retention in the abstract. One systematic review examined retention in care between testing and treatment in sub-Saharan Africa found no studies following one cohort of patients through testing to treatment.3 Some research has examined linkage to care following HIV testing in sub-Saharan Africa, however these studies have focused primarily on individuals testing via voluntary counseling and testing (VCT) or provider-initiated counseling and testing (PITC).
Interpretation: Our findings indicate a high level of care and ART coverage among persons who at the time of home-based counseling and testing (HBCT) already knew they were HIV positive. However, we found that individuals newly diagnosed through HBCT had poor uptake of care in spite of high levels of registration in the clinic. These data suggest first that registration in care is not a reliable measure of linkage to care, and second, that creative and innovative strategies are needed to support individuals who are newly diagnosed through community-based case-finding initiatives to engage with care.
Footnotes
Parts of this data were presented at the following meetings: Controlling the HIV Epidemic with Antiretrovirals: From Consensus to Implementation Evidence Summit (International Association of Providers of AIDS Care (IAPAC) and British HIV Association (BHIVA), September 22–24, 2013 in London, UK and the 9th International Conference on HIV Treatment and Prevention Adherence, June 8–10, 2014 in Miami, Florida, USA.
Conflict of interest: The authors declare no conflicts of interest.
Contributions
Becky L. Genberg conducted the data analysis, the literature review, and drafted the first version of the manuscript. Paula Braitstein conceived and oversaw the study. Violet Naanyu, Juddy Wachira, Joseph Hogan, Samson Ndege, and Paula Braitstein were involved in the study design, data collection, and interpretation of the data. Edwin Sang, Monicah Nyambura, Michael Odawa, and Corey Duefield assisted with data analysis and data management. All authors contributed to the revision of the manuscript and approved the final version for publication.
Declaration of interests
We declare that we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health. 2009;14:849–855. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 2.Wachira J, Kimaiyo S, Ndege S, Mamlin J, Braitstein P. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in western Kenya? Clin Infect Dis. 2012;54:275–281. doi: 10.1093/cid/cir789. [DOI] [PubMed] [Google Scholar]
- 3.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: A systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: A systematic review. AIDS. 2012;26:2059–2067. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 5.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: A systematic review. J Int AIDS Soc. 2012;15:17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Cock KM. Plus ca change … antiretroviral therapy, HIV prevention, and the HIV treatment cascade. Clin Infect Dis. 2014;58:1012–1014. doi: 10.1093/cid/ciu026. [DOI] [PubMed] [Google Scholar]
- 7.Suthar AB, Ford N, Bachanas PJ, et al. Towards universal voluntary HIV testing and counselling: A systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10:e1001496. doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimaiyo S, Were MC, Shen C, et al. Know your epidemic: Home-based counseling and testing in western Kenya. East African Medical Journal. 2010;87:100–108. doi: 10.4314/eamj.v87i3.62195. [DOI] [PubMed] [Google Scholar]
- 9.Inui TS, Nyandiko WM, Kimaiyo SN, et al. AMPATH: Living proof that no one has to die from HIV. J Gen Intern Med. 2007;22:1745–1750. doi: 10.1007/s11606-007-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einterz RM, Kimaiyo S, Mengech HN, et al. Responding to the HIV pandemic: The power of an academic medical partnership. Acad Med. 2007;82:812–818. doi: 10.1097/ACM.0b013e3180cc29f1. [DOI] [PubMed] [Google Scholar]
- 11. [accessed September 20, 2014];Kenya open data. vol 1A: Summary population distribution by district. 2009 www.opendata.go.ke/Population/Vol-1-A-Summary-Population-Distribution-by-Distric/jizy-xanw.
- 12.Tierney WM, Rotich JK, Hannan TJ, et al. The AMPATH medical record system: Creating, implementing, and sustaining an electronic medical record system to support HIV/AIDS care in western Kenya. Stud Health Technol Inform. 2007;129:372–376. [PubMed] [Google Scholar]
- 13.Siika AM, Rotich JK, Simiyu CJ, et al. An electronic medical record system for ambulatory care of HIV-infected patients in Kenya. Int J Med Inform. 2005;74:345–355. doi: 10.1016/j.ijmedinf.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Jaro M. Probabalistic linkage of large public health data files. Stat Med. 2007;14:5–7. doi: 10.1002/sim.4780140510. [DOI] [PubMed] [Google Scholar]
- 15.Fellegi I, Sunter A. A theory for record linkage. J Am Stat Assoc. 1969;64:1183–1210. [Google Scholar]
- 16.Cummings P. Methods for estimating adjusted risk ratios. Stata Journal. 2009;9:175–196. [Google Scholar]
- 17.Lumley T, Kronmai R, Ma S. Relative risk regression in medical research: Models, contrasts, estimators, and alorithms. University of Washington; Jul, 2006. Working paper 293. UW Biostatistics Working Paper Series. [Google Scholar]
- 18.Wafula R, Masyuko S, Ng’ang’a L, et al. Engagement in HIV care among Kenyan adults and adolescents: Results from a national population-based survey. J Acquir Immune Defic Syndr. 2014;66:S98–105. doi: 10.1097/QAI.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune DeficSyndr. 2013;64:e1–8. doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clouse K, Pettifor AE, Maskew M, et al. Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary health care clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2013;62:e39–46. doi: 10.1097/QAI.0b013e318273ac48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayigamba FR, Bakker MI, Fikse H, Mugisha V, Asiimwe A, Schim van der Loeff MF. Patient enrolment into HIV care and treatment within 90 days of HIV diagnosis in eight Rwandan health facilities: A review of facility-based registers. PLoS One. 2012;7:e36792. doi: 10.1371/journal.pone.0036792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassett IV, Regan S, Luthuli P, et al. Linkage to care following community-based mobile HIV testing compared with clinic-based testing in Umlazi Township, Durban, South Africa. HIV Med. 2014;15:367–372. doi: 10.1111/hiv.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govindasamy D, van Schaik N, Kranzer K, Wood R, Mathews C, Bekker LG. Linkage to HIV care from a mobile testing unit in South Africa by different CD4 count strata. J Acquir Immune DeficSyndr. 2011;58:344–352. doi: 10.1097/QAI.0b013e31822e0c4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Losina E, Bassett IV, Giddy J, et al. The “ART” of linkage: Pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5:e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menzies N, Abang B, Wanyenze R, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23:395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 26.Fagan JL, Beer L, Garland P, et al. The influence of perceptions of HIV infection, care, and identity on care entry. AIDS Care. 2012;24:737–746. doi: 10.1080/09540121.2011.630360. [DOI] [PubMed] [Google Scholar]
- 27.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): A pragmatic, parallel, cluster-randomised trial. Lancet. 2012;380:889–898. doi: 10.1016/S0140-6736(12)60730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Decroo T, Telfer B, Biot M, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete province, Mozambique. J Acquir Immune DeficSyndr. 2011;56:e39–44. doi: 10.1097/QAI.0b013e3182055138. [DOI] [PubMed] [Google Scholar]
- 29.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallett TB, Eaton JW. A side door into care cascade for HIV-infected patients? J Acquir Immune DeficSyndr. 2013;63:S228–32. doi: 10.1097/QAI.0b013e318298721b. [DOI] [PubMed] [Google Scholar]
- 31.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368:531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 32.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: A systematic review and meta-analysis. PLoS Med. 2012;9:e1001351. doi: 10.1371/journal.pmed.1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: A systematic review. Int Health. 2013;5:169–179. doi: 10.1093/inthealth/iht016. [DOI] [PubMed] [Google Scholar]
- 35.Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: A systematic review. J Int AIDS Soc. 2014;17:18809. doi: 10.7448/IAS.17.1.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larson BA, Schnippel K, Ndibongo B, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: An evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr. 2012;61:e13–7. doi: 10.1097/QAI.0b013e31825eec60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatcher AM, Turan JM, Leslie HH, et al. Predictors of linkage to care following community-based HIV counseling and testing in rural Kenya. AIDS Behav. 2012;16:1295–1307. doi: 10.1007/s10461-011-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune DeficSyndr. 2010;53:405–411. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng EH, Glidden DV, Emenyonu N, et al. Tracking a sample of patients lost to follow-up has a major impact on understanding determinants of survival in HIV-infected patients on antiretroviral therapy in Africa. Trop Med Int Health. 2010;15:63–69. doi: 10.1111/j.1365-3156.2010.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wachira J, Naanyu V, Genberg B, et al. Perceived health facility barriers to HIV linkage and retention in western Kenya. BMC Infect Dis. 2014;14:P30. doi: 10.1186/s12913-014-0646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]