Abstract
Neuronal cell cultures offer a crucial tool to mechanistically analyse regeneration in the nervous system. Despite the increasing importance of zebrafish (Danio rerio) as an in vivo model in neurobiological and biomedical research, in vitro approaches to the nervous system are lagging far behind and no method is currently available for establishing enriched neuronal cell cultures. Here we show that magnetic-activated cell sorting (MACS) can be used for the large-scale generation of neuronal-restricted progenitor (NRP) cultures from embryonic zebrafish. Our findings provide a simple and semi-automated method that is likely to boost the use of neuronal cell cultures as a tool for the mechanistic dissection of key processes in neuronal regeneration and development.
Neural repair and regeneration in the central nervous system (CNS) is a promising topic in regenerative medicine, with targets ranging from the treatment of spinal cord injuries to that of stroke and degenerative brain diseases such as Alzheimer and Parkinson's1. Significant progress in developing new therapeutic strategies might be achieved by studying the zebrafish, a vertebrate whose CNS has a much higher regenerative capacity than that of humans or of other mammals2. The regulation and maintenance of adult neurogenic regions in the brain of this fish and its ability to even completely regenerate injured brain regions already make the zebrafish an outstanding in vivo model to study the processes of neural development, adult neurogenesis and neural regeneration in vertebrates2,3,4,5.
In order to further dissect molecular mechanisms involved in the regenerative capacities, working on neuronal cell cultures would be a powerful additional tool. However, despite the enormous success of zebrafish as an in vivo model system, only a few attempts have been reported so far describing the effective culture of primary neuronal cells from embryonic to adult zebrafish6,7,8,9,10. Moreover, the challenging and time-consuming methods currently used for manual dissection of embryonic neural tissues only permit the processing of a limited number of embryos. Furthermore, these do not yet allow the robust establishment of standardised neuronal cultures but rather result in mixed cell cultures6,7,8,9 even when amended with fluorescence-activated cell sorting8. In mammals, enriched neuronal cell cultures can be reliably generated by using magnetic-activated cell sorting (MACS). Since the polysialilated form of the neural cell adhesion molecule (PSA-NCAM) is a distinct marker of immature neuronal-restricted progenitors (NRPs)11,12,13, MACS with microbeads conjugated to an antibody against PSA-NCAM can be used to generate cultures of mammalian NRPs14,15, which subsequently differentiate into neurons but not glial cells11,12,13.
Here we show for the first time the successful application of a MACS based technique in zebrafish. By using a semi-automated dissociation process along with anti-PSA-NCAM microbeads, we isolated immature neuronal cells from a large number of embryonic zebrafish. Our simple, cheap and reproducible method allows the large-scale generation of enriched and viable in vitro cultures of zebrafish NRPs and lays the ground for the establishment of differentiated neuronal cell cultures that will be useful to study neurogenesis or axonal regeneration.
Results
Primary cell cultures derived from zebrafish embryos contain few neural cells
To establish neuronal cell cultures from zebrafish, we first dissociated sterilized zebrafish embryos at 30 hours post fertilization (hpf) into a single cell suspension by applying a semi-automated and standardised protocol (see Methods). We then cultured the cells on laminin in a defined serum-free medium especially formulated for neural cell cultivation. Since we used entire zebrafish embryos, the cultivation of the dissociated cells resulted in heterogeneous cell cultures with various cell morphologies (Fig. 1a). As in blastula-derived cell cultures6, the embryonic cells also started to form interconnected cell aggregates after a few days in vitro (Fig. 1a). The embryonic cells were cultured in a medium that promotes growth and survival of neural cells. Additionally, laminin was employed as a substrate that enhances neural differentiation and survival16. Nevertheless, only a small proportion of cells could be identified as neuronal after one week of culture and only single cells both within and beyond the aggregates expressed neuronal and glial markers (Fig. 1b, c).
Figure 1. Without further treatment zebrafish embryonic cell cultures contain only few neuronal cells.
(a) Embryonic zebrafish at 30 hours post fertilization (hpf) were dissociated into a single cell suspension by a semi-automated dissociation process (see Methods). The primary cells were cultured on laminin (50.000 cells/cm2) in a defined serum-free MACS Neuro Medium. Representative light microscope images of the in vitro cultures show cells with different morphologies, which started to form cell aggregates that became interconnected by neurites after a few days in vitro. (b) Immunofluorescent staining of 48 h and (c) 7 d primary cultures identified few neuronal (neurofilament, NF) and glial (glial fibrillary acid protein, GFAP) cell types located within these aggregates. Nuclei were stained with DAPI (blue). All scale bars, 50 μm.
Isolation of PSA-NCAM positive cells from embryonic zebrafish by using MACS
As illustrated by Fig. 1, a method is needed to specifically enrich neuronal cells in zebrafish embryonic cell populations. To separate neuronal cells from the heterogeneous single cell suspension, we attempted to use magnetic-activated cell sorting (Fig. 2a) with anti-PSA-NCAM microbeads, a cost-efficient technique that is widely applied in mammals14,15. Because the antibody used in this magnetic-based isolation targets polysialic acid (PSA) and because PSA-NCAM is expressed in both embryonic17,18 and adult zebrafish CNS19,20 we were hoping that anti-PSA-NCAM microbeads could be applicable in zebrafish as well. To test this assertion, we used the same PSA-NCAM antibody that would later be conjugated to the microbeads to detect PSA-NCAM immunoreactivity on paraffin sections from embryonic zebrafish at 30 hpf. The findings clearly demonstrated the expression of PSA-NCAM in cells of the developing zebrafish CNS (Fig. 2b,c), raising hope that anti-PSA-NCAM microbeads could be used to sort neuronal cells in zebrafish.
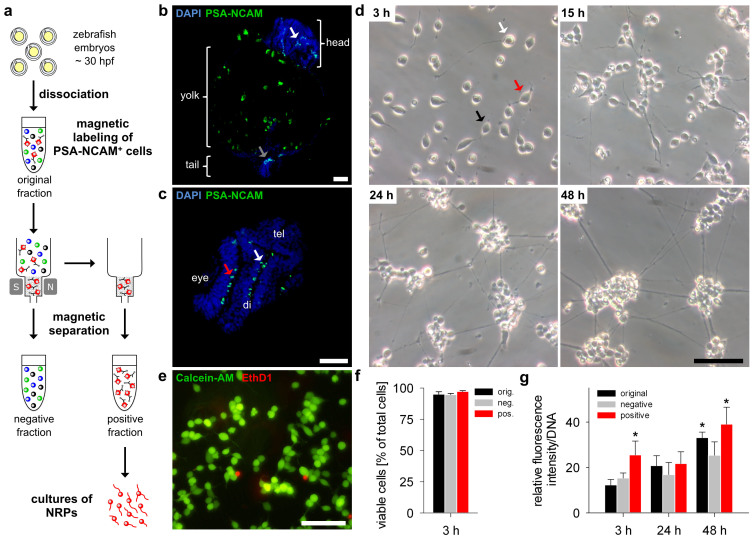
Figure 2. Homogeneous and viable cell cultures of MACS-isolated PSA-NCAM positive cells from embryonic zebrafish.
(a) Schematic representation of magnetic-activated cells sorting (MACS) via magnetic anti-PSA-NCAM microbeads. The single cell suspension of dissociated embryonic zebrafish was incubated with anti-PSA-NCAM microbeads and loaded onto a MACS Column. The magnetically labelled cells were retained in the magnetic field of a MACS Separator, whereas the unlabelled cells flowed through the column (negative fraction). The labelled PSA-NCAM positive cells were then flushed out and collected (positive fraction). (b) Coronal and (c) transversal paraffin sections of embryonic zebrafish (30 hpf) showing PSA-NCAM expression (green) in the tail (grey arrow) as well as in several regions of the brain (white arrows) and the eyes (red arrow). Note the autofluorescence of the yolk. Nuclei were stained with DAPI (blue). tel, telencephalon; di, diencephalon. (d) Primary cultures of adherent cells from the positive fraction (50.000 cells/cm2) showed unipolar (white arrow), bipolar (black arrow) and multipolar (red arrow) morphologies and formed interconnected cell aggregates after 24 h. (e) Viability was assessed by using the Live/Dead assay showing live cells stained with calcein (green) and dead cells with EthD-1 (red). All scale bars, 25 μm. (f) Cells from each fraction showed high viability at 3 h in vitro (n = 3, mean ± SD). (g) Metabolic activity of cells from each fraction was detected by a resazurin-based assay at 3 h, 24 h and 48 h in vitro (n = 5, mean ± SD, P < 0.001). See Fig. 3 for further characterization of PSA-NCAM positive cells and their viability.
When we thus performed a magnetic-based separation, we obtained a positive cell fraction with magnetically labelled cells and an unlabelled negative cell fraction (Fig. 2a). We cultured cells from each fraction (original, negative, positive) on laminin in a defined serum-free medium for further in vitro characterization. Since the isolated cells were exposed to mechanical and physical stress during dissociation and magnetic separation, it was crucial to test first the viability of the cells in the positive fraction. Light microscopic examination revealed that the initially round shaped cells of the positive fraction became adherent and extended long neurites resulting in unipolar, bipolar or multipolar morphologies similar to NRPs in mammalian cell cultures11. The cells were migratory and started to reorganize in small clusters and to form discrete cell aggregates that were interconnected by bundles of neurites after 24 to 48 h of culture (Fig. 2d). Furthermore, the apparently homogeneous neuronal cultures formed from the positive fraction showed high viability and metabolic activity (Fig. 2e–g). In contrast, primary cultures from the original and the negative fraction appeared more heterogeneous and showed lower metabolic activity (Fig. 2g).
Generation of highly enriched NRP cultures
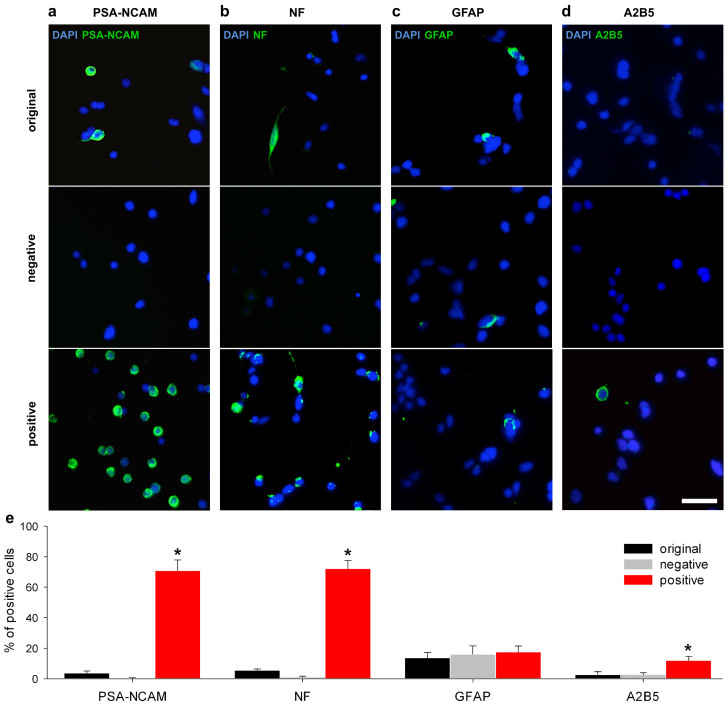
The findings thus suggest that our magnetic microbead-based isolation technique indeed generated enriched and homogeneous NRP cultures. To further test this, we identified NRPs in the positive fraction and quantified the purity of the enriched cultures by monitoring the expression of PSA-NCAM (Fig. 3a) as well as of non-phosphorylated neurofilaments (NF; Fig. 3b), a pan-neuronal intermediate filament found specifically in adult and developing neurons, including neuronal progenitor cells. These assays proved that magnetic separation with anti-PSA-NCAM microbeads indeed led to a significant enrichment of PSA-NCAM positive cells in the positive fraction (70.9 ± 7.1% after 3 h and 77.0 ± 9.1% after 15 h, respectively; P < 0.001) compared to the original fraction (3.8 ± 1.3% after 3 h and 3.2 ± 2.1% after 15 h, respectively). As a control the negative fraction showed the depletion of PSA-NCAM+ cells (0.4 ± 0.3% after 3 h and 0.9 ± 0.6% after 15 h, respectively; Fig. 3e). Beside the expression of PSA-NCAM, we were also able to detect the expression of NFs (Fig. 3b). We demonstrated the significant enrichment of NF positive cells in the positive fraction (72.3 ± 5.2%; P < 0.001) compared to the original fraction (5.4 ± 1.0%). As a control, the negative fraction showed a reduction of neuronal cells (1.1 ± 0.7%; Fig. 3e).
Figure 3. Enrichment of immature neuronal-restricted progenitors using PSA-NCAM mediated magnetic cell separation.
Cells from the original, the negative and the positive fraction were cultured on laminin (50.000 cells/cm2). After 3 h in vitro, the expression of (a) polysialilated-neural cell adhesion molecules (PSA-NCAM), (b) neurofilaments (NF), (c) glial fibrillary acid proteins (GFAP) and (d) A2B5 was analysed immunocytochemically in each cell fraction. Nuclei were stained with DAPI (blue). Scale bar, 25 μm. (e) The positive fraction showed a significantly higher content of PSA-NCAM- and NF-positive cells (n = 5, mean ± SD, P < 0.001). GFAP was detected in glial cells in all fractions (n = 5, mean ± SD, P = 0.41). A2B5 on the surface of glial-restricted progenitors (GRPs) is only detected in the positive fraction (n = 5, mean ± SD, P = 0.001).
We subsequently characterized the remaining 27% of NF-negative cells. Since we used a culture medium that promotes the survival of neural cells, we hypothesized that the majority of the non-neuronal cells were glial cells. We determined the number of glial cells in the positive fraction by their expression of glial fibrillary acid protein (GFAP; Fig. 3c), an intermediate filament that is expressed in mature astrocytes as well as in neural or glial progenitor cells21. As PSA-NCAM can also be expressed in oligodendrocyte progenitors15,22, we identified these and other glial progenitors by using an antibody against a ganglioside-specific antigen (A2B5; Fig. 3d), a marker for glial-restricted progenitors (GRPs)23 that has already been described in the brain of other teleost fish24,25. The staining revealed that most of the NF negative cells expressed GFAP (17.5 ± 4.0%) and A2B5 (11. 9 ± 2.7%) (Fig. 3e). Taken together, the glial assays suggest that the remaining, non-NF positive cells in our cultures were predominantly PSA-NCAM positive GRPs.
Overall, the immunocytochemical analysis of the MACS-isolated positive cell population showed that our novel magnetic cell sorting method worked, allowing us to successfully generate viable, enriched NRP cultures from embryonic zebrafish.
Enriched NRP cultures form neuronal aggregates in vitro
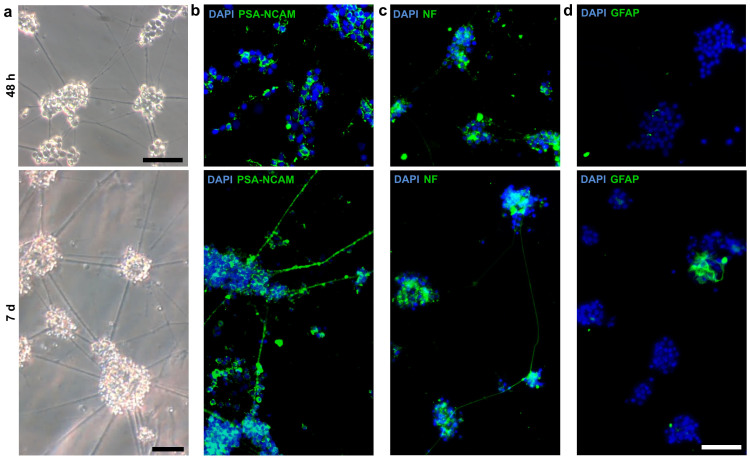
As our objective was to generate long-term neuronal cell cultures from zebrafish, we next aimed to characterize the cells in our NRP cultures beyond 3 h in vitro. Therefore, cells from the neuronal enriched positive fraction were cultured for 2 and 7 d, respectively. As already shown, the cells in the positive fraction started to form aggregates that became interconnected by neurites within 2 d (Fig. 2d). During the following days, both the size of the aggregates and the density of neurites extending from the aggregates increased (Fig. 4a). Since we started with cell cultures containing predominantly NRPs (Fig 3a, d), we expected that the majority of the cells forming these aggregates are still neuronal after 7 d in vitro. To confirm this assumption, we investigated the cellular composition of the aggregates by immunocytochemistry. Consistent with our findings at 3 h (Fig. 3), the cells showed positive immunostaining for PSA-NCAM and NF (Fig. 4b, c), whereas GFAP staining was evident in only a few cells (Fig. 4d).
Figure 4. Spontaneously formed aggregates predominantly contain neuronal cells.
(a) NRP cultures from embryonic zebrafish were cultured for 2 and 7 d, respectively. Representative images show the formation of cell aggregates that became interconnected by neurites. Both the size of the aggregates and the density of neurites extending from the aggregates increased until day 7. Immunocytochemical analyses demonstrated the expression of (b) polysialilated-neural cell adhesion molecule (PSA-NCAM) and (c) neurofilaments (NF) in most of the cells within the aggregates at 2 and 7 d in vitro. (d) Only a few cells showed the expression of glial fibrillary acid proteins (GFAP). Nuclei were stained with DAPI (blue). All scale bars, 50 μm.
The predominant expression of PSA-NCAM along with NF validates the neuronal composition of the aggregates after 2 as well as 7 d in vitro (Fig. 4c, d). Overall, zebrafish NRPs can be enriched by MACS and form in vitro neuronal aggregates, in contrast to heterogeneous cell aggregates developed without MACS.
Neuronal network formation induced by retinoic acid
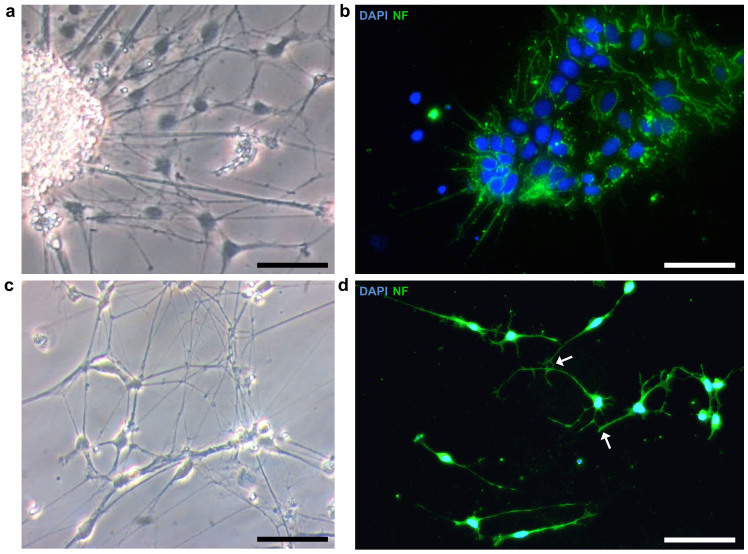
In mouse26, rat11,13 or human27 homogenous cell populations of neuronal-restricted progenitors can be used to develop cell cultures of differentiated and functional neurons. Here, retinoic acid (RA) induces the in vitro differentiation into multiple neuronal cell types. To test whether our NRPs isolated from zebrafish embryos would similarly respond to RA, we grew enriched NRP cultures in a differentiation medium containing 1 μM RA. After several days under these conditions, several cells began to leave the aggregates, forming a monolayer and displaying neuronal morphologies with small extending processes (Fig. 5a). As expected, the immunocytochemical analysis of these cells revealed the expression of NFs (Fig. 5b). During the next weeks, further neuronal maturation and the formation of neuronal networks of NF expressing cells were observed (Fig. 5c, d). In addition, NF positive cells seemed to be connected to adjacent neurons indicating the formation of synapses (Fig. 5d). Therefore, the development of neuronal networks provides evidence suggesting that RA promotes the differentiation of NRPs into mature neurons in zebrafish as well.
Figure 5. Formation of neuronal networks induced by retinoic acid (RA).
(a) NRPs were cultured in a differentiation medium containing retinoic acid (1 μM in DMSO). After 7 d in vitro many cells left the neuronal aggregates, formed adherent monolayers and extended small processes. (b) The immunocytochemical staining of cells forming these monolayers revealed the expression of neurofilaments (NF). Nuclei were stained with DAPI (blue). (c) After two weeks in vitro further neuronal maturation and the formation of neuronal networks were observed. (d) The NF positive neurons seemed to be connected to adjacent neurons indicating the formation of synapses (white arrows). Nuclei were stained with DAPI (blue). All scale bars, 50 μm.
Taken together, our findings suggest that our method has produced viable neuronal cells that not only respond appropriately to RA differentiation cues but form typical morphologies and interconnections.
Discussion
The currently available methods for establishing neuronal cell cultures in zebrafish are insufficient for large-scale experiments. The labour-intensive and time-consuming microdissection of neural tissues only allow the processing of a small number of embryos and the resulting cultures contain only a small fraction of neuronal cells6,7,8,9. We have employed a semi-automated dissociation that allows the simultaneous processing of a large number of zebrafish embryos in less than an hour. Nevertheless, culturing the resulting cell population led to a heterogeneous primary cell culture, similarly as in blastula-derived cell cultures6. We therefore used magnetic-activated cell sorting (MACS) to specifically enrich neuronal cells in zebrafish cell populations. We show that magnetic anti-PSA-NCAM microbeads are applicable for zebrafish and can easily be used to separate neuronal-restricted progenitors (NRPs) for subsequent long-term neuronal cell cultures. Because MACS is comparatively simple and cost-effective to establish, compared e.g. to fluorescent-activated cell sorting (FACS), our methods would be suitable for many labs. If a higher purity is required, our findings suggest that a first major step should be the depletion of PSA-NCAM expressing glial-restricted progenitors (GRPs) by using MACS along with anti-A2B5 microbeads.
In summary, we have established a novel easily applicable large-scale method that many labs could use to generate enriched and viable standardised neuronal cell cultures from embryonic zebrafish in only 2 hours. Large-scale in vivo screens of chemical libraries are a powerful tool in studies on neural degeneration, regeneration and neural development in zebrafish28,29. However, many of this studies, like the search for intrinsic and extrinsic factors that underlie the ability of zebrafish neurons to functionally regenerate axons30 or reestablish neuronal circuits and behavior31, are likely to benefit from an in vitro system that allows drug screening experiments. In general, our technique should be particularly useful for in vitro studies to address the mechanistic basis of the high neuroregenerative potential in zebrafish. Particularly, we expect that findings from zebrafish NRPs will facilitate the improvement of therapeutic strategies, such as cell-replacement therapies, where cultured NRPs could be a promising exogenous source of replacement neurons12,27,32.
Methods
Fish strain and maintenance
Zebrafish (Danio rerio) wild-type Tuebingen (Tu) strain were maintained at 28.5°C under standard conditions33. Fertilized eggs were collected and washed with 0.5% bleach solution for 1 min. Until dissociation, the embryos were kept and raised in E3 embryo medium, as described33. The embryonic development was staged by hours post fertilization (hpf) according to Kimmel et al.34. Since the expression of PSA-NCAM is highest from 27–40 hpf17, neuronal-restricted progenitors were isolated from prim-15 (30 hpf) stage embryos.
Isolation and cell culture of neuronal-restricted progenitors (NRPs)
The embryos were sterilized prior to dissociation with a 70% ethanol solution for 5 sec and rinsed three times in sterile E3 embryo medium. All subsequent steps were performed under sterile conditions. First, the embryos were pretreated with a dilute solution of pronase E (2 mg/ml in E3 embryo medium; Sigma) for 1 min to facilitate the dechorionation during dissociation and washed again three times. The embryos were then dissociated automatically into a single cell suspension with the MACS Neural Tissue Dissociation Kit (T) (Miltenyi Biotec) in gentleMACS C tubes on the gentleMACS Dissociator (Miltenyi Biotec) according to the manufacturer's recommended protocol. Briefly, up to 1000 sterilized and pronase-treated embryos were transferred into one gentleMACS C-Tube (Miltenyi Biotec) containing Hanks' balanced salt solution without calcium or magnesium (HBSS w/o; PAA Laboratories). HBSS w/o was aspirated completely and the dissociation was started by adding 1950 μl of enzyme mix 1 and by using the defined gentleMACS program m_brain_01 on the gentleMACS Dissociator. The sample was incubated for 15 min at 37°C followed by the gentleMACS program m_brain_02. After adding 30 μl of enzyme mix 2, the sample was incubated for 10 min at 37°C followed by the gentleMACS program m_brain_03. Subsequent to an additional incubation for 10 min at 37°C, the sample was applied to a cell strainer placed on a 50 ml tube. The cell strainer was washed with 10 ml of HBSS and the resulting single cell suspension was centrifuged at 300 × g for 10 min.
PSA-NCAM+ NRPs were then purified with magnetic-activated cell sorting (MACS) and MACS anti-PSA-NCAM Microbeads (Miltenyi Biotec) according to the manufacturer's recommended protocol. Briefly, 107 cells were resuspended in 70 μl of cold separation buffer (phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 2 mM EDTA; both from Sigma-Aldrich), incubated for 15 min at 4°C with 20 μl of anti-PSA-NCAM Microbeads per 70 μl of cell suspension, washed by adding 2 ml of separation buffer and centrifuged at 300 × g for 10 min. Cells were resuspended in 500 μl of separation buffer and applied onto a MACS Column (MS type; Miltenyi Biotec) placed in the magnetic field of a MACS Separator (Miltenyi Biotec). The flow-through was collected as the unlabelled negative fraction. The column was then washed three times with 500 μl of separation buffer and the retained magnetically labelled cells were flushed out with 1 ml of separation buffer as the positive fraction (Fig 2a). Cells from the original, the negative and the positive fraction were counted using a particle size counter (Z2, Beckman Coulter GmbH) and seeded onto laminin-coated (50 μg/ml; Sigma) plastic plates or sterilized glass slides for immunocytochemistry (50.000 cells/cm2). Cells from each fraction were cultured in serum-free MACS Neuro Medium (Miltenyi Biotec) supplemented with 1 × MACS NeuroBrew-21 (Miltenyi Biotec), 1% GlutaMAX and 1% penicillin–streptomycin (Gibco). Cultures were incubated at 28.5°C and 5% CO2 in a humidified environment.
To induce neuronal differentiation, retinoic acid (1 μM in DMSO; Sigma) was added to the medium 1 day after seeding. Half of the medium was changed every two to three days.
Immunocytochemistry
All staining procedures were carried out at room temperature. Cultures from the original, the negative and the positive cell fraction were chemically fixed for 10 min using a 4% formaldehyde solution in PBS and washed three times with PBS. In order to detect cytoskeletal components, cells were permeabilized with 0.1% Triton-X 100 in PBS for 5 min. Unspecific binding was blocked with PBS containing 5% normal goat serum (NGS; Sigma-Aldrich) for 1 h. Samples were incubated with a mouse monoclonal antibody against polysialilated neuronal cell adhesion molecule (PSA-NCAM, 1:100; Miltenyi Biotec), non-phosphorylated neurofilaments (NF, clone SMI-311, 1:1000; abcam), glial fibrillary acid protein (GFAP, clone ZRF-1, 1:1000; abcam) or A2B5 (clone 105, 1:500; Sigma) for 1 h. After washing three times with PBS, cells were incubated with an Alexa Fluor 488-conjugated goat anti-mouse secondary antibody (1:400, Invitrogen) for 1 h. Slides were washed three times in PBS and mounted using ProLong Gold antifade reagent with 4',6-diamidino-2-phenylindole (DAPI; Invitrogen). Immunocytochemically and immunohistochemically stained slides were visualized using a fluorescent microscope (Axioplan 2 imaging, Zeiss). Representative images were recorded digitally using a ProgRes C14 camera system (ProgRes C14, Jenoptik AG) in combination with a capture and image analysis software (AnalySIS Version 3.2). To estimate the purity of the positive fractions the percentage of PSA-NCAM and NF positive stained cells were calculated for each cell fraction. Cells were counted in five randomly chosen microscope fields with at least 150 cells taken from five independent experiments and averaged. The percentage of stained cells was defined as the number of cells positive for PSA-NCAM, NF, GFAP or A2B5 divided by the total number of cells. Cell counting was performed using ImageJ software (Version 1.47, Rasband).
Immunohistochemistry
For immunohistochemical detection of PSA-NCAM in paraffin embedded sections, zebrafish embryos (30 hpf) were first dechorionated using two fine forceps and fixed overnight at 4°C using a 4% formaldehyde solution in PBS. The embryos were orientated in a same direction and embedded in 1% agarose to fix their position. Both dehydration through a series of graded ethanol and embedding in paraffin was done automatically by a tissue processing center (TPC 15 Duo, Medite). The paraffin block was cut on a rotary microtome (Biocut 2030, Reichert-Jung) into 5 μm sections, which were mounted on glass slides covered with poly-L-lysine (Thermo Scientific). Paraffin sections were deparaffined in Roticlear (Carl Roth GmbH) and rehydrated in a graded ethanol series (100%, 100%, 100%, 80%, 75%, 50% and distilled water for 5 min each). For heat-induced epitope retrieval sections were incubated for 20 min with HIER H buffer (Thermo Scientific) at 95°C in a steamer. The subsequent immunofluorescent staining protocol to detect PSA-NCAM expression was performed as described above.
Cell viability
Viability of isolated primary cells was assessed after 3 h using a Live/Dead two-colour fluorescence assay according to the manufacturer's protocol (Molecular Probes). Cells from each fraction were grown on laminin coated glass slides. After washing with PBS, cells were exposed to 2 μM ethidium homodimer-1 (EthD-1) and 1 μM calcein-AM in PBS for 30 min at 28.5°C in the incubator. To quantify the viability, the percentage of live cells stained with calcein (green) and dead cells stained with EthD-1 (red) were calculated for each cell fraction. Cells were counted in five randomly chosen microscope fields with at least 200 cells taken from three independent experiments and averaged. The percentage of viable cells was defined as the number of calcein-stained cells divided by the total number of cells. Cell counting was performed using ImageJ software (Version 1.47, Rasband). Dye uptake was detected by using a fluorescent microscope (Axioplan 2 imaging, Zeiss).
Viability of cultured cells was also determined by using a metabolic resazurin assay (Sigma-Aldrich). Cells of each fraction were cultured in 96 well plates (15.000 cells/well). Resazurin dye solution (4 μl) was added to each well at 3 h, 24 h and 48 h in vitro. After 3 h of incubation at 28.5°C, the bioreduction of resazurin into red-fluorescent resorufin by living cells was measured at each time point by fluorescence intensity at 590 nm (excitation 560 nm) on a microplate reader (PolarSTAR OPTIMA, BMG-Labtech). Subsequent to the resazurin assay, the total DNA content was quantified in each well using the Quant-iT™ PicoGreen®dsDNA assay kit (Invitrogen) in accordance with the manufacturer's protocol.
Data analysis and statistics
All data was normally distributed (Shapiro–Wilk, P > 0.05) and presented as mean values ± standard deviations (SD). The statistical significance between groups of data was evaluated by one-way ANOVAs followed by Tukey post hoc tests. Values of P < 0.05 were considered statistically significant.
Author Contributions
G.W. performed and analysed experiments, D.S. and G.W. developed tools, G.W. and S.S. wrote the manuscript.
Acknowledgments
This work is supported by a Reinhart-Koselleck project of the Deutsche Forschungsgemeinschaft (S.S.) and by grants from the Friedrich-Baur Foundation (S.S.). We thank Antje Halwas (Schuster lab) for excellent technical help.
References
- Vishwakarma S. K., Bardia A., Tiwari S. K., Paspala S. A. B. & Khan A. A. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. J. Adv. Res. 5, 277–294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C., Kaslin J., Kroehne V. & Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 72, 429–61 (2012). [DOI] [PubMed] [Google Scholar]
- Kroehne V., Freudenreich D., Hans S., Kaslin J. & Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 138, 4831–41 (2011). [DOI] [PubMed] [Google Scholar]
- Kishimoto N., Shimizu K. & Sawamoto K. Neuronal regeneration in a zebrafish model of adult brain injury. Dis. Model. Mech. 5, 200–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis N. et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338, 1353–6 (2012). [DOI] [PubMed] [Google Scholar]
- Ghosh C., Liu Y., Ma C. & Collodi P. Cell cultures derived from early zebrafish embryos differentiate in vitro into neurons and astrocytes. Cytotechnology 23, 221–30 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. S. Preparation of dissociated zebrafish spinal neuron cultures. Methods Cell Sci. 23, 205–9 (2001). [DOI] [PubMed] [Google Scholar]
- Sakowski S. A. et al. A novel approach to study motor neurons from zebrafish embryos and larvae in culture. J. Neurosci. Methods 205, 277–82 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. et al. Primary neuron culture for nerve growth and axon guidance studies in zebrafish (Danio rerio). PLoS One 8, e57539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapanes-Castillo A. et al. Characterization of a novel primary culture system of adult zebrafish brainstem cells. J. Neurosci. Methods 223, 11–9 (2014). [DOI] [PubMed] [Google Scholar]
- Mayer-Proschel M., Kalyani A. J., Mujtaba T. & Rao M. S. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron 19, 773–85 (1997). [DOI] [PubMed] [Google Scholar]
- Yang H. et al. Region-specific differentiation of neural tube-derived neuronal restricted progenitor cells after heterotopic transplantation. Proc. Natl. Acad. Sci. U. S. A. 97, 13366–71 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyani A. J., Piper D., Mujtaba T., Lucero M. T. & Rao M. S. Spinal cord neuronal precursors generate multiple neuronal phenotypes in culture. J. Neurosci. 18, 7856–68 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-S. et al. Highly pure and expandable PSA-NCAM-positive neural precursors from human ESC and iPSC-derived neural rosettes. PLoS One 7, e39715 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenfaden R., Desoeuvre A., Bosio A., Virard I. & Cremer H. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol. Cell. Neurosci. 32, 187–98 (2006). [DOI] [PubMed] [Google Scholar]
- Barros C. S., Franco S. J. & Müller U. Extracellular matrix: functions in the nervous system. Cold Spring Harb. Perspect. Biol. 3, a005108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx M., Rutishauser U. & Bastmeyer M. Dual function of polysialic acid during zebrafish central nervous system development. Development 128, 4949–58 (2001). [DOI] [PubMed] [Google Scholar]
- Langhauser M. et al. Ncam1a and Ncam1b: two carriers of polysialic acid with different functions in the developing zebrafish nervous system. Glycobiology 22, 196–209 (2012). [DOI] [PubMed] [Google Scholar]
- Adolf B. et al. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–93 (2006). [DOI] [PubMed] [Google Scholar]
- März M. et al. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 58, 870–88 (2010). [DOI] [PubMed] [Google Scholar]
- Middeldorp J. & Hol E. M. GFAP in health and disease. Prog. Neurobiol. 93, 421–43 (2011). [DOI] [PubMed] [Google Scholar]
- Ben-Hur T., Rogister B., Murray K., Rougon G. & Dubois-Dalcq M. Growth and Fate of PSA-NCAM+ Precursors of the Postnatal Brain. J. Neurosci. 18, 5777–5788 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. S. & Mayer-Proschel M. Glial-Restricted Precursors Are Derived from Multipotent Neuroepithelial Stem Cells 1. Dev. Biol. 63, 48–63 (1997). [DOI] [PubMed] [Google Scholar]
- Wen C.-M., Wang C.-S., Chin T.-C., Cheng S.-T. & Nan F.-H. Immunochemical and molecular characterization of a novel cell line derived from the brain of Trachinotus blochii (Teleostei, Perciformes): A fish cell line with oligodendrocyte progenitor cell and tanycyte characteristics. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 156, 224–31 (2010). [DOI] [PubMed] [Google Scholar]
- Jeserich G. & Stratmann A. In vitro differentiation of trout oligodendrocytes: evidence for an A2B5-positive origin. Brain Res. Dev. Brain Res. 67, 27–35 (1992). [DOI] [PubMed] [Google Scholar]
- Mujtaba T. et al. Lineage-Restricted Neural Precursors Can Be Isolated from Both the Mouse Neural Tube and Cultured ES Cells. Dev. Biol. 127, 113–127 (1999). [DOI] [PubMed] [Google Scholar]
- Zou Q. et al. Direct conversion of human fibroblasts into neuronal restricted progenitors. J. Biol. Chem. 289, 5250–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Martin C. et al. High-throughput in vivo vertebrate screening. Nat. Methods 7, 634–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke G. J. & Currie P. D. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 8, 353–67 (2007). [DOI] [PubMed] [Google Scholar]
- Becker T. & Becker C. G. Axonal regeneration in zebrafish. Curr. Opin. Neurobiol. 27, 186–91 (2014). [DOI] [PubMed] [Google Scholar]
- Bhatt D. H., Otto S. J., Depoister B. & Fetcho J. R. Cyclic AMP-induced repair of zebrafish spinal circuits. Science 305, 254–8 (2004). [DOI] [PubMed] [Google Scholar]
- Bonner J. F. et al. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J. Neurosci. 31, 4675–86 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C. & Dahm R. Zebrafish: a practical approach. (Oxford University Press, Oxford., 2002). [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. & Schilling T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 (1995). [DOI] [PubMed] [Google Scholar]