Abstract
Avian leukosis virus subgroup J (ALV-J) is an avian oncogenic retrovirus that can induce various clinical tumors and has caused severe economic losses in China. To improve our understanding of the host cellular responses to virus infection and the pathogenesis of ALV-J infection, we applied isobaric tags for relative and absolute quantification (iTRAQ) labeling coupled with multidimensional liquid chromatography-tandem mass spectrometry to detect the protein changes in DF-1 cells infected and mock-infected with ALV-J. A total of 75 cellular proteins were significantly changed, including 33 upregulated proteins and 42 downregulated proteins. The reliability of iTRAQ-LC MS/MS was confirmed via real-time PCR. Most of these proteins were related to the physiological functions of metabolic processes, biosynthetic processes, responses to stimuli, protein binding, signal transduction, cell cytoskeleton, and so forth. We also found some proteins that play important roles in apoptosis and oncogenicity. The differentially expressed proteins identified may provide valuable information to elucidate the pathogenesis of virus infection and virus-host interactions.
1. Introduction
The J subgroup of avian leukosis virus (ALV-J), which belongs to the Retroviridae family, was first isolated from white-meat-type chickens in the United Kingdom in 1988 [1]. It can predominantly lead to myeloid leukosis (ML) and immunosuppression effects in both naturally and experimentally infected chickens [2, 3]. In China, ALV-J-associated myeloid leukosis in chickens was first reported in 1999 [4]. ALV-J can induce various tumors, growth retardation, and production problems. In addition, in recent years, it has become widespread in many parts of our country and leads to severe economic losses in the poultry industry.
The pathogenesis of virus infection and the mechanism through which the virus interacts with host cells remain unclear. During virus infection, the proteins of host cells may be significantly changed. It is now possible to use proteomic techniques to identify the changes in protein abundance that indicate host cellular responses to virus infection and provide useful information to obtain a better understanding of the pathogenesis of virus infection [5–8]. Kvaratskhelia et al. [9] applied enzymatic digestion coupled with mass spectrometry (MS) to detect the sites of glycosylation on the surface of avian leukosis virus subgroup A (ALV-A) and found that carbohydrates may play an important role in receptor binding.
To explore the possible mechanisms of virus infection, we used isobaric tags for relative and absolute quantification (iTRAQ) combined with multidimensional liquid chromatography (LC) and tandem MS analysis to perform a quantitative proteomic analysis of DF-1 cells infected with ALV-J [10]. To the best of our knowledge, no previous study had used the iTRAQ LC-MS/MS proteomics strategy to investigate the differently expressed proteins in ALV-J-infected DF-1 cells. The iTRAQ labeling technology could greatly increase the identification sensitivity and quantitation accuracy of proteomic analyses through a multiplexed quantitation strategy [11]. The results showed that 75 proteins were significantly changed after ALV-J infection. These changed proteins may provide valuable information to study the molecular mechanisms underlying ALV-J pathogenesis.
2. Materials and Methods
2.1. Reagents
The iTRAQ Reagent Multi-Plex Kit was acquired from Applied Biosystems (Foster City, CA, USA). A multidimensional liquid chromatographer (RIGOL 3220) was purchased from RIGOL, and the chromatographic column (Agela, C18 chromatographic column, 250 × 4.6 mm i.d., filler particles diameter: 5 μm) was acquired from Agela Co., Ltd. (Tianjin, China). The LC-MS/MS instrument (Q-Exactive) was obtained from Thermo Fisher Scientific.
2.2. Cell Culture and Virus Infection
DF-1 cells (ATCC accession number: CRL-12203) were cultured in Dulbecco's modified Eagle medium (DMEM; HyClone, Beijing, China) supplemented with 10% fetal bovine serum (FBS) and 100 μg/mL streptomycin and penicillin at 37°C in a 5% CO2 atmosphere. ALV-J strain HPRS-103 (GenBank: Z46390) was kindly provided by Professor Venugopal Nair. DF-1 cells cultured in flasks to approximately 80% confluence were infected with 0.5 mL of 103.5/mL 50% tissue culture infectious doses (TCID50) of ALV-J for 144 h. Uninfected DF-1 cells served as mock-infected cells.
2.3. Indirect Immune Fluorescence Assay (IFA)
At 144 h after infection, the infected DF-1 cells were washed twice with PBS and fixed with anhydrous ethanol for 20 min. The fixed cells were then incubated with mouse anti-P27 monoclonal antibody (prepared in our lab) at 37°C for 60 min. After washing three times with PBST (0.01 M PBS, pH 7.2, 0.05% Tween 20), the cells were incubated with goat anti-mouse IgG conjugated to FITC (Sigma, USA) at 37°C for another 60 min. Finally, the cells were observed under a Carl Zeiss Vision microscope (ZEISS Axio Observer D1) after three washes with PBST.
2.4. Protein Extraction, Digestion, and Labeling with iTRAQ Reagents
Infected and mock-infected DF-1 cells were washed twice with PBS. The cells were lysed in a lysis buffer (9 M urea, 4% CHAPS, 1% DTT, and 1% IPG buffer). The mixtures were centrifuged at 15,000 g and 4°C for 15 min. The supernatant was collected, and the protein concentration was determined using the Bradford protein assay [12] (Bio-Rad Laboratories). Then, 100 μg of protein was mixed overnight with four volumes of cold (−20°C) acetone and then dissolved using the dissolution buffer. After being reduced, alkylated, and digested with trypsin, the samples were labeled following the manufacturer's instructions described in the iTRAQ protocol. The labeled samples were pooled for further analysis.
2.5. LC-MS/MS and Database Searches
The iTRAQ-labeled sample mixtures were then fractionated by strong cation exchange (SCX) chromatography on a high-performance liquid chromatography (HPLC) system (RIGOL 3220; Beijing, China) using a chromatographic column (Agela, C18 chromatographic column, 250 × 4.6 mm i.d., filler particles diameter: 5 μm; Tianjin, China). Mobile phase A consisted of 2% ACN-98% H2O (pH 10.0), and mobile phase B consisted of 98% ACN-2% H2O (pH 10.0). The solvent gradient was as follows: 5%–8% B for 1 min, 8%–32% B for 24 min, 32%–95% B for 2 min, 95% for 4 min, and 95%–5% B for 1 min. The column temperature was 45°C, the flow rate was 0.7 mL/min, and the detection wavelength was 214 nm. Peptides were collected every minute within the effective gradient from 8% to 32%. A total of 27 fractions were collected and then dried.
The dried fractions were dissolved in 1.9% ACN/98% H2O/0.1% FA aqueous solution and combined into nine samples. The samples were centrifuged at 12,000 ×r for 3 min, and the supernatant was collected. The supernatant was then analyzed using the EASY-nLC-1000 liquid phase interfaced with a Q Exactive mass spectrometer (Thermo Fisher). The chromatographic conditions are as follows: liquid phase, EASY-nLC-1000; enriching column, C18, 5 μm, ID100 μm, 20 mm in length; separation column, C18, 3 μm, ID75 μm, 120 mm in length; mobile phase A, 1.9% ACN + 98% H2O + 0.1% FA; mobile phase B, 98% ACN + 1.9% H2O + 0.1% FA; and flow rate, 450 nl/min.
Elution Conditions. See Table 1.
Table 1.
| Time | 0 | 24 | 30 | 31 | 38 |
|---|---|---|---|---|---|
| B% | 3 | 16 | 30 | 90 | 90 |
The data were acquired at 38 min. The spray voltage was 2.0 KV, the capillary temperature was 320°C, the collision energy was 30, and the acquisition quality range was 300–1400 da.
The relative quantification and protein identification were performed with the Protein Discoverer software (version 1.2) using the built-in mascot as the search engine.
2.6. Real-Time PCR
The primers (Table 2) were synthesized by BoShi Biotechnology Company (Harbin, China). The gene was amplified from the genomic DNA of DF-1 cells by polymerase chain reaction (PCR). The PCR-amplified products were separated in a 2% agarose gel and then purified using a DNA gel extraction kit (Axygen Biotechnology Limited, Hangzhou City, China). The products were then ligated into the pZeroBack/blunt vector (Tiangen Biotech Co., Ltd., Beijing, China), and the sequence was verified. The plasmid DNA was used as the standard to construct the standard curve via SYBR Green real-time PCR. The total cellular RNA of the infected or mock-infected DF-1 cells was extracted using the RNeasy Mini Kit (QIAGEN, China) according to the manufacturer's protocol. Reverse transcription was performed using a PrimeScript II First-Strand cDNA Synthesis Kit (TaKaRa, China) as described in the protocol. The real-time PCR was performed using the Roche LightCycler 480 real-time PCR System.
Table 2.
Primer sequences for real-time PCR.
| Gene | Sequence | Size |
|---|---|---|
| BLOC1S5 | F-TATATGAGCGGGGCAGGCCCT | 150 bp |
| R-TTCCCCGACATCCTTGAT | ||
|
| ||
| Keratin | F-ATGTCCCGCTCCGTCAGCTTC | 150 bp |
| R-AGAGCCCAGGTTGTAGAGGCT | ||
|
| ||
| HMG14 | F-ATGCCGAAGAGAAAGGTG | 140 bp |
| R-TCAGATTTATCCTTAGCCGCC | ||
|
| ||
| AACS | F-ATGTCCCGCGAGCCCGAGATT | 150 bp |
| R-CACTGACCACTGGTATAAGTC | ||
2.7. Bioinformatics Analysis
The functional annotation of the 75 proteins in DF-1 cells that were significantly changed after infection with ALV-J was performed using the GOSlimViewer tool of the AgBase database (http://www.agbase.msstate.edu/) [13]. In addition, we aimed to determine how ALV-J interacts with the host cellular proteins and how it affects the function of host cells. The identified proteins were inputted into the STRING database to obtain the protein-protein interaction network [14, 15] (http://string.embl.de/).
3. Results
3.1. Confirmation of ALV-J Infection in DF-1 Cells by IFA
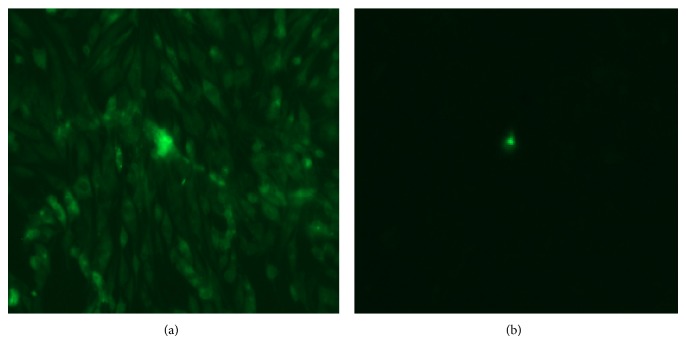
To confirm that the DF-1 cells were infected by ALV-J, IFA was used to detect the viral P27 antigen. The results showed clear green fluorescence in ALV-J-infected DF-1 cells 144 h after infection, whereas the uninfected DF-1 cells exhibited no green fluorescence (Figure 1).
Figure 1.
Identification of DF-1 cells infected with ALV-J by IFA. (a) DF1 cells infected with ALV-B. (b) Normal uninfected DF1 cells.
3.2. Protein Profile Obtained by iTRAQ LC-MS/MS Analysis
To explore the differences in the protein expression levels after virus infection, the total proteins of ALV-J-infected and mock-infected DF-1 cells were extracted for iTRAQ-LC-MS/MS analysis. A total of 1091 proteins were detected, including 75 proteins in DF-1 cells that were significantly changed infection with ALV-J for 144 h (Table 3). These differently expressed proteins were divided into two clusters: upregulated and downregulated. The number of upregulated proteins was 33, whereas the number of downregulated proteins was 42.
Table 3.
List of significant differentially expressed proteins identified by iTRAQ analysis of DF-1 cells infected with ALV-J.
| Accession number | Protein name | Protein score | Fold change in expression | Protein MW | Protein PI |
|---|---|---|---|---|---|
| Cluster 1: tendency for upregulation (33) | |||||
| O73612 | Ephrin-B1 GN=EFNB1 | 34.98 | 1.667 | 36.8 | 8.87 |
| F1P187 | Gephyrin (fragment) GN=GPHN | 0.00 | 1.560 | 77.4 | 5.38 |
| P12274 | Nonhistone chromosomal protein HMG-14B GN=HMG14 | 0.00 | 1.473 | 11.2 | 9.63 |
| E1BTX9 | Serine/threonine-protein phosphatase | 39.24 | 1.429 | 73.4 | 8.34 |
| P08286 | Histone H1.10 | 476.50 | 1.315 | 22.0 | 11.18 |
| E1C281 | PHD finger protein 6 GN=PHF6 | 0.00 | 1.281 | 41.0 | 8.62 |
| Q5ZJ02 | DBIRD complex subunit ZNF326 GN=ZNF326 | 45.04 | 1.280 | 63.5 | 5.78 |
| Q5ZIK4 | Protein yippee-like GN=YPEL5 | 0.00 | 1.276 | 13.8 | 7.31 |
| Q5F3J5 | Proteasome activator complex subunit 3 GN=PSME3 | 143.05 | 1.274 | 29.5 | 6.19 |
| Q5F3Z5 | DnaJ homolog subfamily B member 6 GN=DNAJB6 | 0.00 | 1.263 | 36.7 | 8.84 |
| F1NB51 | Zinc finger E-box-binding homeobox 1 GN=ZEB1 | 41.75 | 1.258 | 123.1 | 5.02 |
| F1NLA7 | Zinc finger CCCH domain-containing protein 11A GN=ZC3H11A | 43.83 | 1.254 | 79.0 | 8.16 |
| F1P5W3 | Ephrin-B1 (Fragment) GN=EFNB1 | 34.98 | 1.249 | 32.7 | 8.46 |
| F1NXG2 | WW domain-binding protein 4 (Fragment) GN=WBP4 | 0.00 | 1.235 | 45.2 | 5.73 |
| P08267 | Ferritin heavy chain GN=FTH | 65.41 | 1.226 | 21.1 | 6.21 |
| Q6K1L7 | Probable RNA-binding protein EIF1AD GN=eif1ad | 0.00 | 1.210 | 21.2 | 4.79 |
| F1NEY0 | Syndecan (Fragment) GN=CPQ | 45.65 | 1.208 | 19.9 | 4.70 |
| F1NMD7 | Pre-mRNA-splicing factor RBM22 GN=RBM22 | 21.07 | 1.205 | 46.7 | 8.54 |
| O93481 | Chromobox protein (CHCB2) GN=CBX3 | 0.00 | 1.190 | 19.8 | 5.12 |
| F1NFJ0 | DNA replication licensing factor MCM3 GN=MCM3 | 46.00 | 1.188 | 91.3 | 5.74 |
| E1C9E9 | DCN1-like protein GN=DCUN1D5 | 0.00 | 1.183 | 27.2 | 5.77 |
| F1NAQ1 | Vascular endothelial growth factor A GN=VEGFA | 0.00 | 1.179 | 25.1 | 9.10 |
| F1NLU6 | Enhancer of mRNA-decapping protein 3 GN=EDC3 | 49.54 | 1.175 | 56.0 | 7.17 |
| P16527 | Myristoylated alanine-rich C-kinase substrate GN=MARCKS | 30.67 | 1.174 | 27.7 | 4.44 |
| Q5ZMC9 | Nuclear distribution protein nudE homolog 1 GN=NDE1 | 0.00 | 1.173 | 39.5 | 5.11 |
| Q5ZII6 | Protein kish-A GN=TMEM167A | 27.01 | 1.173 | 8.0 | 8.92 |
| Q5ZIL9 | KIF1-binding protein homolog GN=kbp | 0.00 | 1.167 | 69.0 | 5.21 |
| F1NFP5 | Arginine-tRNA ligase, cytoplasmic GN=RARS | 184.95 | 1.158 | 75.4 | 6.98 |
| E1C4V1 | ATP synthase-coupling factor 6, mitochondrial GN=ATP5J | 111.05 | 1.157 | 12.5 | 9.33 |
| Q90595 | Transcription factor MafF GN=MAFF | 37.09 | 1.155 | 16.6 | 9.74 |
| R4GJF8 | TAR DNA-binding protein 43 GN=TARDBP | 131.97 | 1.155 | 42.2 | 6.19 |
| Q6B7Z6 | Polymyositis/scleroderma autoantigen 1 GN=EXOSC9 | 0.00 | 1.151 | 49.3 | 5.54 |
| E1C7X8 | S-adenosylmethionine synthase GN=LOC427292 | 14.44 | 1.150 | 43.2 | 6.62 |
|
| |||||
| Cluster 2: Tendency to down-regulation (42) | |||||
| R4GKA6 | Collagen alpha-2(VI) chain GN=COL6A2 | 181.18 | 0.850 | 102.4 | 5.48 |
| E1BXS2 | Guanine nucleotide-binding protein G(i) subunit alpha-1 GN=GNAI1 | 147.69 | 0.850 | 40.4 | 5.97 |
| Q90927 | Nuclear factor 1 GN=cNFI-A4 | 0.00 | 0.850 | 54.6 | 8.31 |
| E1BUI0 | tRNA pseudouridine synthase (Fragment) GN=PUSL1 | 55.04 | 0.849 | 33.7 | 9.64 |
| Q90617-3 | Isoform LAMP-2C of Lysosome-associated membrane glycoprotein 2 GN=LAMP2 | 149.29 | 0.849 | 46.4 | 6.43 |
| Q90733 | COUP transcription factor 2 GN=NR2F2 | 0.00 | 0.848 | 45.4 | 8.28 |
| P12957-2 | Isoform Brain l-cad of Caldesmon GN=CALD1 | 0.00 | 0.847 | 58.8 | 8.44 |
| F1N9D8 | Cathepsin B GN=CTSB | 133.09 | 0.847 | 37.6 | 5.86 |
| A4GTP0 | Galectin | 66.44 | 0.846 | 25.7 | 8.27 |
| Q08392 | Glutathione S-transferase | 108.74 | 0.846 | 25.3 | 8.88 |
| F1N965 | Frizzled-7 GN=FZD7 | 0.00 | 0.842 | 62.7 | 7.99 |
| E1C3U7 | Lysyl oxidase homolog 2 GN=LOXL2 | 0.00 | 0.842 | 86.9 | 6.49 |
| F1NGX1 | Integrin alpha-V GN=ITGAV | 172.35 | 0.841 | 114.3 | 5.58 |
| E1BRJ4 | DNA-directed RNA polymerase GN=POLR3B | 0.00 | 0.840 | 127.4 | 8.54 |
| Q8AXV1 | Endophilin-A1 GN=SH3GL2 | 65.55 | 0.839 | 39.9 | 5.47 |
| F1NMF6 | Procollagen-lysine,2-oxoglutarate 5-dioxygenase 1 GN=PLOD1 | 198.96 | 0.837 | 84.3 | 6.74 |
| F1P2F0 | Collagen alpha-3(VI) chain GN=COL6A3 | 642.87 | 0.836 | 339.4 | 6.68 |
| R4GFM0 | FERM, RhoGEF and pleckstrin domain-containing protein 1 GN=FARP1 | 0.00 | 0.835 | 119.5 | 8.15 |
| F1N8G4 | Diphthamide biosynthesis protein 2 GN=DPH2 | 0.00 | 0.834 | 52.1 | 5.54 |
| H9L0H3 | Alpha-actinin-4 (Fragment) GN=ACTN4 | 515.20 | 0.834 | 71.6 | 6.09 |
| Q5F4B1 | Phosphoglycolate phosphatase GN=PGP | 39.98 | 0.833 | 33.0 | 5.73 |
| P56673 | Pituitary homeobox 1 GN=PITX1 | 20.32 | 0.833 | 34.5 | 9.11 |
| Q90611 | 72 kDa type IV collagenase GN=MMP2 | 267.53 | 0.829 | 74.9 | 5.49 |
| F1NME2 | Integrin beta GN=ITGB5 | 51.04 | 0.828 | 88.4 | 6.71 |
| F1NBZ7 | Serine/threonine-protein phosphatase GN=PPP3CA | 0.00 | 0.827 | 60.6 | 5.83 |
| P51890 | Lumican GN=LUM | 44.33 | 0.826 | 38.6 | 6.52 |
| B3TZC1 | PNPLA7 GN=PNPLA7 | 0.00 | 0.821 | 147.6 | 7.17 |
| Q5ZLG0 | Acetoacetyl-CoA synthetase GN=AACS | 189.47 | 0.818 | 74.3 | 6.49 |
| F1NLD4 | Inhibitor of nuclear factor kappa-B kinase subunit alpha GN=CHUK | 0.00 | 0.817 | 86.1 | 6.32 |
| F1NFE0 | Collagen alpha-1(VI) chain GN=COL6A1 | 110.52 | 0.812 | 107.9 | 5.90 |
| F1N9N4 | Stathmin-3 GN=NPC2 | 34.96 | 0.808 | 16.2 | 6.51 |
| F1NPX5 | SH3 domain-binding glutamic acid-rich-like protein (Fragment) GN=SH3BGRL | 106.54 | 0.802 | 12.9 | 4.88 |
| P01038 | Cystatin | 39.09 | 0.799 | 15.3 | 7.69 |
| Q8QG94 | Suppressor of fused GN=SUFU | 46.78 | 0.786 | 53.7 | 5.33 |
| Q90Y35-2 | Isoform 2 of Zinc finger protein 622 GN=ZNF622 | 0.00 | 0.783 | 42.6 | 6.65 |
| F1NMZ3 | Hemoglobin subunit epsilon GN=HBE | 37.75 | 0.761 | 16.6 | 8.91 |
| Q5ZK77 | Biogenesis of lysosome-related organelles complex 1 subunit 5 GN=BLOC1S5 | 34.23 | 0.759 | 22.6 | 7.06 |
| F1P0D2 | Glutamine synthetase (Fragment) GN=LOC417253 | 0.00 | 0.758 | 44.9 | 7.02 |
| F1NJT4 | Fibronectin GN=FN1 | 1373.06 | 0.752 | 259.0 | 6.11 |
| Q155F6 | Tumor necrosis factor-inducible protein 6 GN=TNFIP6 | 44.59 | 0.747 | 30.7 | 6.02 |
| F1NJT3 | Fibronectin GN=FN1 | 1373.06 | 0.727 | 273.1 | 5.64 |
| O93532 | Keratin, type II cytoskeletal cochlear | 95.26 | 0.693 | 53.8 | 6.10 |
Fold change = infected/control. Fold change >1 indicates upregulation, and fold change <1 indicates downregulation.
3.3. Functional Classifications of the Identified Proteins
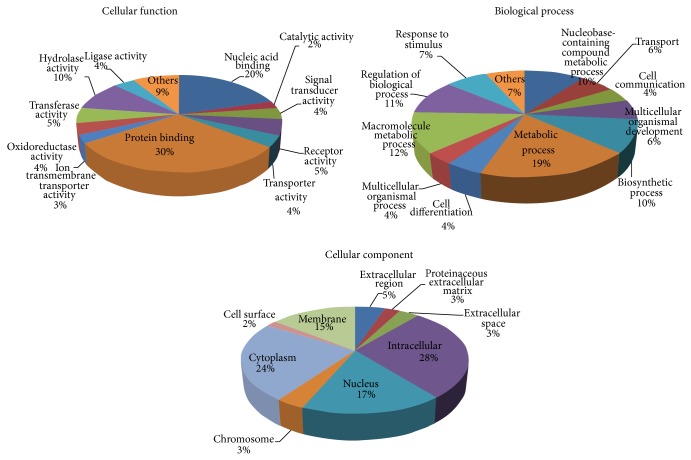
To annotate the functions of the 75 significantly changed proteins identified in our study, the proteins were submitted to GORetriever (http://www.agbase.msstate.edu/) for analysis. Three types of annotations were obtained using the website: molecular functions, biological processes, and cellular components.
The biological process annotation revealed that the significantly changed proteins were involved in metabolic process (19%), macromolecule metabolic process (12%), regulation of biological process (11%), biosynthetic processes (10%), nucleobase-containing compound metabolic process (10%), response to stimulus (7%), and various other activities (31%) (Figure 2, biological process).
Figure 2.
Functional annotation of the differently expressed proteins according to their biological process, molecular function, and cellular component.
The molecular function annotation revealed that these differently expressed proteins were involved in protein binding (30%), nucleic acid binding (21%), hydrolase activity (11%), transferase activity (5%), receptor activity (5%), oxidoreductase activity (4%), and various other activities (24%) (Figure 2, molecular function).
The cellular component annotation revealed that the altered proteins were associated with the following cellular components: intracellular (28%), cytoplasm (24%), nucleus (17%), membrane (15%), extracellular region (5%), chromosome (3%), and various others (8%) (Figure 2, cellular component).
3.4. Validation of the iTRAQ Data by Real-Time PCR
To confirm the results of the differentially expressed proteins identified by iTRAQ LC-MS/MS analysis, real-time PCR was performed to detect the transcript expression levels of the genes after ALV-J infection. We generated four standard curves to determine the gene expression of BLOC1S5, keratin, HMG14, and AACS in ALV-J-infected and mock-infected DF-1 cells. The results showed that HMG14 was upregulated (Figure 3), whereas BLOC1S5, AACS, and keratin were downregulated (Figure 3). The RT-PCR results were consistent with the results of the iTRAQ LC-MS/MS analysis (Table 3), confirming that the iTRAQ data were reliable.
Figure 3.
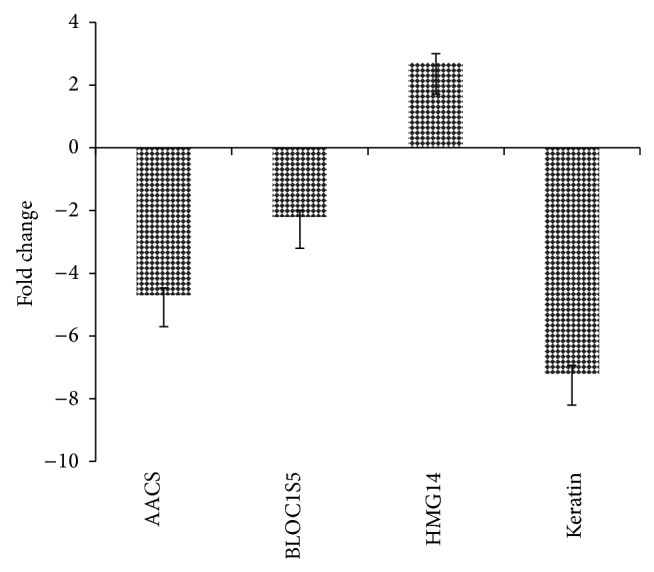
Transcriptional profiles of the significantly changed proteins in ALV-J-infected DF-1 cells. The error bars represent the standard deviations.
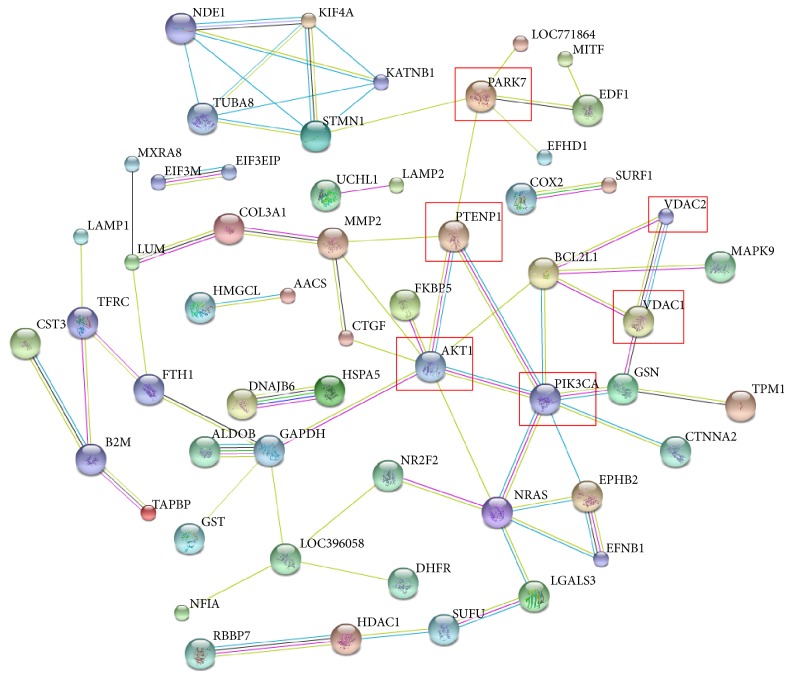
3.5. Protein-Protein Interaction Analysis
The mechanism through which the virus interacts with host cells remains unclear, and oncogenicity is an important index of the pathogenicity of ALV-J. During virus infection, some proteins of host cells may be significantly changed. As a result, the functions of the changed proteins will also be altered. In our study, we aimed to determine whether the significantly changed proteins that were identified have some relationship with apoptosis or ALV-J-induced oncogenicity. We searched the STRING database to analyze the protein-protein interactions between the differently expressed proteins and PARK7, PTENP1, AKT1, PIK3CA (PI3K), and VDAC (Figure 4). These proteins are known to have some relationship with tumor-associated process and apoptosis. The protein-protein interaction networks may provide valuable information to further investigate the possible mechanism of ALV-J-induced oncogenicity.
Figure 4.
The protein-protein interaction between the identified proteins and the tumor- or apoptosis-associated proteins analyzed by the STRING software. An edge was drawn with up to seven differently colored lines, representing the existence of the seven types of evidence used for predicting the associations: a red line indicates the presence of fusion evidence; a yellow line indicates text mining evidence; a purple line indicates experimental evidence; a blue line indicates cooccurrence evidence; a light blue line indicates database evidence; a green line indicates neighborhood evidence; a black line indicates coexpression evidence.
4. Discussion
Proteomics is a relatively novel technology that has been used for the detection of the host cellular proteins response to virus infection [16, 17]. Isobaric tags for relative and absolute quantification (iTRAQ) combined with multidimensional liquid chromatography (LC) and tandem MS analysis are a powerful tool for quantitative proteomic analysis that has been widely applied in many studies [18–20]. In this study, we first applied the iTRAQ approach to identify the differential protein expression profiles of DF-1 cells infected with ALV-J. Using the iTRAQ LC-MS/MS technology, the significantly changed proteins were mostly associated with metabolic process, signal transducer activity, cell cytoskeleton, oxidoreductase activity, response to stimulus, and immune responses. In addition, some apoptosis and tumor-associated proteins (VEGF-A, ACTN4, and METAP2) were also identified by the iTRAQ LC-MS/MS technology.
4.1. Alterations of Tumor-Associated Proteins
Vascular endothelial growth factor A (VEGF-A) is an important inducer of angiogenesis [21]. As has been shown in many reports, upregulated VEGF-A can induce tumor formation via some unique signaling pathways [22, 23]. In addition, VEGF-A, which is known as a positive regulator, contributes to tumor growth and promotes tumor formation [24, 25]. Previous studies described a threshold level of proteins to promote tumorigenesis, which indicated that the expression level of one protein needs to reach the threshold level before promoting tumorigenesis [26, 27]. Studies in our lab showed that the increased replication of ALV-J increased the expression of VEGF-A, indicating an increased opportunity for ALV-J to push the expression level of VEGF-A to reach the threshold level to promote tumorigenesis [27]. In this study, we found that VEGF-A is overexpressed in DF-1 cells after infection with ALV-J. The results further suggested that VEGF-A is closely associated with ALV-J-induced tumorigenesis and may also suggest a novel molecular mechanism for better understanding of the higher oncogenicity of ALV-J.
Alpha-actinins (ACTNs) were classified into cytoskeleton proteins, while ACTN4 has some other unique functions, such as signal transduction, protein expression regulation, and nuclear transport. Histological analyses of cancer tissues showed a strong correlation between ACTN4 expression and tumorigenesis in several types of cancers [28–30]. Furthermore, upregulated ACTN4 in cancer cells has been suggested as a biomarker for drug resistance and malignant cell invasion [31–34]. Previous studies showed that ALV-J infection in DF-1 cells led to rapid increase in Akt phosphorylation and the phosphorylation of Akt was PI3K-dependent [35]. PI3K/Akt pathway also regulates viral replication of ALV-J [35]. Furthermore, AKT interacts with ACTN4 and ACTN4 is a functional partner of AKT [36]. Therefore, the upregulated ACTN4 observed in this study may be associated with tumorigenesis induced by ALV-J through PI3K/Akt pathway. This may provide useful information to elucidate the mechanism of ALV-J induced tumorigenesis and may also become potential therapeutic targets to control ALV-J infections.
METAP2 was considered to have some relationship with angiogenesis inhibition [37]. In addition, METAP2 can block B cell differentiation into plasma cells [38]. Some viruses, whose primary target cells are B cells, can clinically induce tumor formation. Therefore, downregulation of METAP2 in this study may influence the function of B cells, which may provide evidence to explain why ALV-J infection can result in immune suppression and tumorigenesis.
4.2. Redox Regulation
Peroxiredoxins (PRDXs), a family of peroxidases as antioxidant enzymes, can support tumor maintenance and survival through protecting cells from apoptosis induced by oxidative stress [39–41]. A previous study indicated that liver cells transfected with PRDX6 siRNA resulted in an increase in peroxide-induced cytotoxicity by apoptosis, which implies that decrease of PRDX6 promotes apoptosis [42]. Therefore, downregulated PRDX6 in this study suggests that ALV-J infection may weaken the antiapoptotic function of PRDX6.
In addition, PRDX1 was found to be upregulated in this study. A previous study indicated that the mice lacking PRDX1 have several malignant cancers, including sarcomas, carcinomas, and lymphomas [43]. These malignancies are associated with low expression of PRDX1, which suggests that PRDX1 may function as a tumor suppressor [43]. Studies also indicated that PRDX1 interacts with the c-Myc oncogene and can inhibit its transcriptional activity [44] and high expression of PRDX1 appears to be associated with less aggressive breast cancers [45]. Therefore, upregulation of PRDX1 in this study may result from the defense of host cells responses to the ALV-J infection.
4.3. Cytoskeleton Proteins and ALV-J Infection
Cytoskeleton proteins are involved in the maintenance of cell morphology, regulation of protein synthesis, endocytosis, cell movement, and cell-to-cell attachment [46, 47]. As determined through iTRAQ LC-MS/MS analysis, some cytoskeleton proteins were identified to be significantly changed in DF-1 cells after infection with ALV-J. Isoform 2 of the F-actin-capping protein subunit beta isoforms 1 and 2 (CAPZB) can regulate the growth of actin filaments, and actin filaments play a vital role in the maintenance of cell morphology [48]. Furthermore, actin-related protein 3 (ACTR3) and actin-related protein 5 (ACTR5) were also found to be changed. The low expression of these proteins revealed that the cytoskeletal proteins were disrupted during infection with ALV-J. In addition, the differential expression of these proteins may be due to the interaction between the virus and host cellular proteins after infection with ALV-J.
It has been reported that keratins have become the standard detection marker for tumor cells and were also the most common marker to identify tumor cells [49]. Previous studies showed that before tumor cells got the ability to migrate and invade the host, they need to undergo epithelial-mesenchymal transition, during which the cytoskeletons are rearranged and epithelial markers, such as keratins, claudins, and E-cadherin, are observed to be downregulated [49–52]. Immunohistochemical analysis showed that low expression of keratin was associated with a higher tumor grade in breast cancer [52]. Previous study indicated that acetoacetyl-CoA synthetase (AACS) was found in tumor tissues and plays important roles in metabolic processes of tumors [53]. Whether or not the downregulated keratin and AACS in this study were associated with tumorigenesis induced by ALV-J infection needs to be further investigated.
5. Conclusions
In summary, our study was the first to use iTRAQ LC-MS/MS to detect cellular responses to ALV-J infection in DF-1 cells. A total of 75 significantly changed proteins were identified. These differently expressed proteins may provide useful information for elucidating the molecular mechanism underlying the interaction between ALV-J and DF-1 cells and will also facilitate our understanding of the pathogenesis of ALV-J infection.
Supplementary Material
List of differentially expressed proteins identified by iTRAQ analysis of DF-1 cells infected with ALV-J. Fold change=infected/control. Fold change >1 indicates up regulation, and fold change <1 indicates down regulation.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (31372437 and 31201923) and the Earmarked Fund for the Modern Agro-industry Technology Research System (no. nycytx-42-G3-01).
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Payne L. N., Howes K., Gillespie A. M., Smith L. M. Host range of Rous sarcoma virus pseudotype RSV(HPRS-103) in 12 avian species: support for a new avian retrovirus envelope subgroup, designated J. Journal of General Virology. 1992;73(part 11):2995–2997. doi: 10.1099/0022-1317-73-11-2995. [DOI] [PubMed] [Google Scholar]
- 2.Payne L. N., Gillespie A. M., Howes K. Recovery of acutely transforming viruses from myeloid leukosis induced by the HPRS-103 strain of avian leukosis virus. Avian Diseases. 1993;37(2):438–450. doi: 10.2307/1591671. [DOI] [PubMed] [Google Scholar]
- 3.Witter R. L. Avian tumor viruses: persistent and evolving pathogens. Acta Veterinaria Hungarica. 1997;45(3):251–266. [PubMed] [Google Scholar]
- 4.Du Y., Cui Z., Qin A. Subgroup J of avian leukosis viruses in China. China Poultry. 1999;3:1–4. [Google Scholar]
- 5.Narayan R., Gangadharan B., Hantz O., et al. Proteomic analysis of HepaRG cells: a novel cell line that supports hepatitis B virus infection. Journal of Proteome Research. 2009;8(1):118–122. doi: 10.1021/pr800562j. [DOI] [PubMed] [Google Scholar]
- 6.Alfonso P., Rivera J., Hernáez B., Alonso C., Escribano J. M. Identification of cellular proteins modified in response to African swine fever virus infection by proteomics. Proteomics. 2004;4(7):2037–2046. doi: 10.1002/pmic.200300742. [DOI] [PubMed] [Google Scholar]
- 7.Toda T., Sugimoto M., Omori A., Matsuzaki T., Furuichi Y., Kimura N. Proteomic analysis of Epstein–Barr virus-transformed human B- lymphoblastoid cell lines before and after immortalization. Electrophoresis. 2000;21(9):1814–1822. doi: 10.1002/(SICI)1522-2683(20000501)21:9<1814::AID-ELPS1814>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Sun J., Jiang Y., Shi Z., et al. Proteomic alteration of PK-15 cells after infection by classical swine fever virus. Journal of Proteome Research. 2008;7(12):5263–5269. doi: 10.1021/pr800546m. [DOI] [PubMed] [Google Scholar]
- 9.Kvaratskhelia M., Clark P. K., Hess S., Melder D. C., Federspiel M. J., Hughes S. H. Identification of glycosylation sites in the SU component of the Avian Sarcoma/Leukosis virus Envelope Glycoprotein (Subgroup A) by mass spectrometry. Virology. 2004;326(1):171–181. doi: 10.1016/j.virol.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Ross P. L., Huang Y. N., Marchese J. N., et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Molecular and Cellular Proteomics. 2004;3(12):1154–1169. doi: 10.1074/mcp.m400129-mcp200. [DOI] [PubMed] [Google Scholar]
- 11.Seggerson K., Tang L., Moss E. G. Two genetic circuits repress the Caenorhabditis elegans heterochronic gene lin-28 after translation initiation. Developmental Biology. 2002;243(2):215–225. doi: 10.1006/dbio.2001.0563. [DOI] [PubMed] [Google Scholar]
- 12.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein—dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Bai J., Lu Q., et al. Two-dimensional liquid chromatography-tandem mass spectrometry coupled with isobaric tags for relative and absolute quantification (iTRAQ) labeling approach revealed first proteome profiles of pulmonary alveolar macrophages infected with porcine circovirus type 2. Journal of Proteomics. 2013;79:72–86. doi: 10.1016/j.jprot.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Thanthrige-Don N., Abdul-Careem M. F., Shack L. A., Burgess S. C., Sharif S. Analyses of the spleen proteome of chickens infected with Marek's disease virus. Virology. 2009;390(2):356–367. doi: 10.1016/j.virol.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Q., Bai J., Zhang L., et al. Two-dimensional liquid chromatography-tandem mass spectrometry coupled with isobaric tags for relative and absolute quantification (iTRAQ) labeling approach revealed first proteome profiles of pulmonary alveolar macrophages infected with porcine reproductive and respiratory syndrome virus. Journal of Proteome Research. 2012;11(5):2890–2903. doi: 10.1021/pr201266z. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell K. L., Frappier L. Viral proteomics. Microbiology and Molecular Biology Reviews. 2007;71(2):398–411. doi: 10.1128/MMBR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng X., Hong L., Shi L., Guo J., Sun Z., Zhou J. Proteomics analysis of host cells infected with infectious bursal disease virus. Molecular and Cellular Proteomics. 2008;7(3):612–625. doi: 10.1074/mcp.M700396-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Seshi B. An integrated approach to mapping the proteome of the human bone marrow stromal cell. Proteomics. 2006;6(19):5169–5182. doi: 10.1002/pmic.200600209. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H., Lv L., Liu H., et al. Profiling the potential biomarkers for cell differentiation of pancreatic cancer using iTRAQ and 2-D LC-MS/MS. Proteomics—Clinical Applications. 2009;3(7):862–871. doi: 10.1002/prca.200800029. [DOI] [PubMed] [Google Scholar]
- 20.Muraoka S., Kume H., Watanabe S., et al. Strategy for SRM-based verification of biomarker candidates discovered by iTRAQ method in limited breast cancer tissue samples. Journal of Proteome Research. 2012;11(8):4201–4210. doi: 10.1021/pr300322q. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biological and Pharmaceutical Bulletin. 2011;34(12):1785–1788. doi: 10.1248/bpb.34.1785. [DOI] [PubMed] [Google Scholar]
- 22.Catalano A., Romano M., Martinotti S., Procopio A. Enhanced expression of vascular endothelial growth factor (VEGF) plays a critical role in the tumor progression potential induced by simian virus 40 large T antigen. Oncogene. 2002;21(18):2896–2900. doi: 10.1038/sj/onc/1205382. [DOI] [PubMed] [Google Scholar]
- 23.Ohtani K., Usuda J., Ichinose S., et al. High expression of GADD-45alpha and VEGF induced tumor recurrence via upregulation of IL-2 after photodynamic therapy using NPe6. International Journal of Oncology. 2008;32(2):397–403. [PubMed] [Google Scholar]
- 24.Lorenzon E., Colladel R., Andreuzzi E., et al. MULTIMERIN2 impairs tumor angiogenesis and growth by interfering with VEGF-A/VEGFR2 pathway. Oncogene. 2012;31(26):3136–3147. doi: 10.1038/onc.2011.487. [DOI] [PubMed] [Google Scholar]
- 25.Oka N., Soeda A., Inagaki A., et al. VEGF promotes tumorigenesis and angiogenesis of human glioblastoma stem cells. Biochemical and Biophysical Research Communications. 2007;360(3):553–559. doi: 10.1016/j.bbrc.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 26.Li Q., Ishikawa T.-O., Oshima M., Taketo M. M. The threshold level of adenomatous polyposis coli protein for mouse intestinal tumorigenesis. Cancer Research. 2005;65(19):8622–8627. doi: 10.1158/0008-5472.CAN-05-2145. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q., Gao Y., Wang Y., et al. A 205-nucleotide deletion in the 3' untranslated region of avian leukosis virus subgroup J, currently emergent in China, contributes to its pathogenicity. Journal of Virology. 2012;86(23):12849–12860. doi: 10.1128/jvi.01113-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda K., Yamada T., Hayashida Y., et al. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology. 2005;128(1):51–62. doi: 10.1053/j.gastro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Welsch T., Keleg S., Bergmann F., Bauer S., Hinz U., Schmidt J. Actinin-4 expression in primary and metastasized pancreatic ductal adenocarcinoma. Pancreas. 2009;38(8):968–976. doi: 10.1097/MPA.0b013e3181b28d6f. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto S., Tsuda H., Honda K., et al. Actinin-4 expression in ovarian cancer: a novel prognostic indicator independent of clinical stage and histological type. Modern Pathology. 2007;20(12):1278–1285. doi: 10.1038/modpathol.3800966. [DOI] [PubMed] [Google Scholar]
- 31.Fellenberg J., Dechant M. J., Ewerbeck V., Mau H. Identification of drug-regulated genes in osteosarcoma cells. International Journal of Cancer. 2003;105(5):636–643. doi: 10.1002/ijc.11135. [DOI] [PubMed] [Google Scholar]
- 32.He J., Whelan S. A., Lu M., et al. Proteomic-based biosignatures in breast cancer classification and prediction of therapeutic response. International Journal of Proteomics. 2011;2011:16. doi: 10.1155/2011/896476.896476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuchi S., Honda K., Tsuda H., et al. Expression and gene amplification of actinin-4 in invasive ductal carcinoma of the pancreas. Clinical Cancer Research. 2008;14(17):5348–5356. doi: 10.1158/1078-0432.CCR-08-0075. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C., Zhong Q., Rhodes L. V., et al. Proteomic analysis of acquired tamoxifen resistance in MCF-7 cells reveals expression signatures associated with enhanced migration. Breast Cancer Research. 2012;14(2, article R45) doi: 10.1186/bcr3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng S.-Z., Cao W.-S., Liao M. The PI3K/Akt pathway is involved in early infection of some exogenous avian leukosis viruses. Journal of General Virology. 2011;92(7):1688–1697. doi: 10.1099/vir.0.030866-0. [DOI] [PubMed] [Google Scholar]
- 36.Ding Z., Liang J., Lu Y., et al. A retrovirus-based protein complementation assay screen reveals functional AKT1-binding partners. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(41):15014–15019. doi: 10.1073/pnas.0606917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvakumar P., Lakshmikuttyamma A., Dimmock J. R., Sharma R. K. Methionine aminopeptidase 2 and cancer. Biochimica et Biophysica Acta—Reviews on Cancer. 2006;1765(2):148–154. doi: 10.1016/j.bbcan.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Priest R. C., Spaull J., Buckton J., et al. Immunomodulatory activity of a methionine aminopeptidase-2 inhibitor on B cell differentiation. Clinical and Experimental Immunology. 2009;155(3):514–522. doi: 10.1111/j.1365-2249.2008.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.So Y. K., Jo H. Y., Mi H. K., et al. H2O2-dependent hyperoxidation of peroxiredoxin 6 (Prdx6) plays a role in cellular toxicity via up-regulation of iPLA2 activity. Journal of Biological Chemistry. 2008;283(48):33563–33568. doi: 10.1074/jbc.m806578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fatma N., Kubo E., Sen M., et al. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Research. 2008;1233:63–78. doi: 10.1016/j.brainres.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann C. A., Fang Q. Are peroxiredoxins tumor suppressors? Current Opinion in Pharmacology. 2007;7(4):375–380. doi: 10.1016/j.coph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Walsh B., Pearl A., Suchy S., Tartaglio J., Visco K., Phelan S. A. Overexpression of Prdx6 and resistance to peroxide-induced death in Hepa1-6 cells: Prdx suppression increases apoptosis. Redox Report. 2009;14(6):275–284. doi: 10.1179/135100009x12525712409652. [DOI] [PubMed] [Google Scholar]
- 43.Neumann C. A., Krause D. S., Carman C. V., et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424(6948):561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 44.Egler R. A., Fernandes E., Rothermund K., et al. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24(54):8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 45.Pei-Jou Chua E.-H. L., Chunhua G., George W.-C. Y., Puay-Hoon T., Boon-Huat B. Clinicopathological correlation of peroxiredoxin I in breast cancer. Proceedings of the World Medical Conference; 2011; Prague, Czech Republic. [Google Scholar]
- 46.Radulovic M., Godovac-Zimmermann J. Proteomic approaches to understanding the role of the cytoskeleton in host-defense mechanisms. Expert Review of Proteomics. 2011;8(1):117–126. doi: 10.1586/epr.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou W., Ke J., Zhang A., et al. Proteomics analysis of differential expression of chicken brain tissue proteins in response to the neurovirulent H5N1 avian influenza virus infection. Journal of Proteome Research. 2010;9(8):3789–3798. doi: 10.1021/pr100080x. [DOI] [PubMed] [Google Scholar]
- 48.Casella J. F., Maack D. J. Purification and initial characterization of a protein from skeletal muscle that caps the barbed ends of actin filaments. The Journal of Biological Chemistry. 1986;261(23):10915–10921. [PubMed] [Google Scholar]
- 49.Joosse S. A., Hannemann J., Spötter J., et al. Changes in keratin expression during metastatic progression of breast cancer: impact on the detection of circulating tumor cells. Clinical Cancer Research. 2012;18(4):993–1003. doi: 10.1158/1078-0432.ccr-11-2100. [DOI] [PubMed] [Google Scholar]
- 50.Thiery J. P., Sleeman J. P. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Reviews Molecular Cell Biology. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 51.Radisky D. C. Epithellial-mesenchymal transition. Journal of Cell Science. 2005;118(19):4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 52.Willipinski-Stapelfeldt B., Riethdorf S., Assmann V., et al. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clinical Cancer Research. 2005;11(22):8006–8014. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- 53.Tisdale M. J. Role of acetoacetyl-CoA synthetase in acetoacetate utilization by tumor cells. Cancer Biochemistry Biophysics. 1984;7(2):101–107. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of differentially expressed proteins identified by iTRAQ analysis of DF-1 cells infected with ALV-J. Fold change=infected/control. Fold change >1 indicates up regulation, and fold change <1 indicates down regulation.