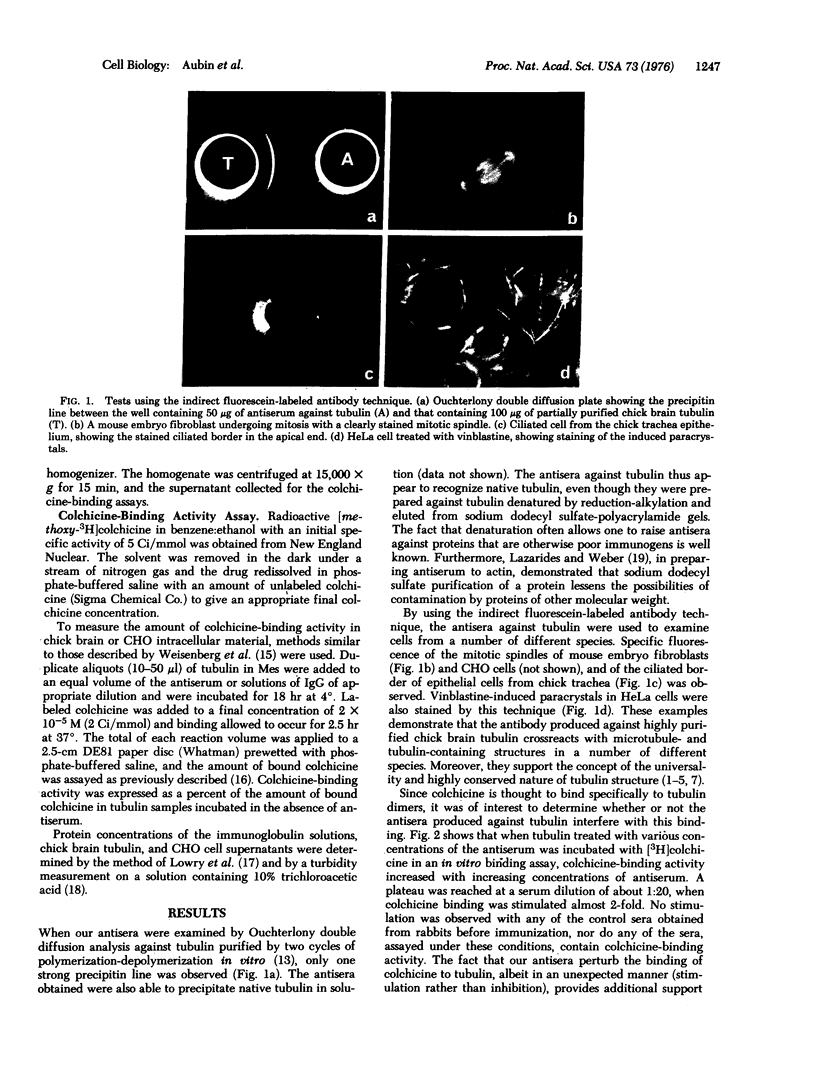
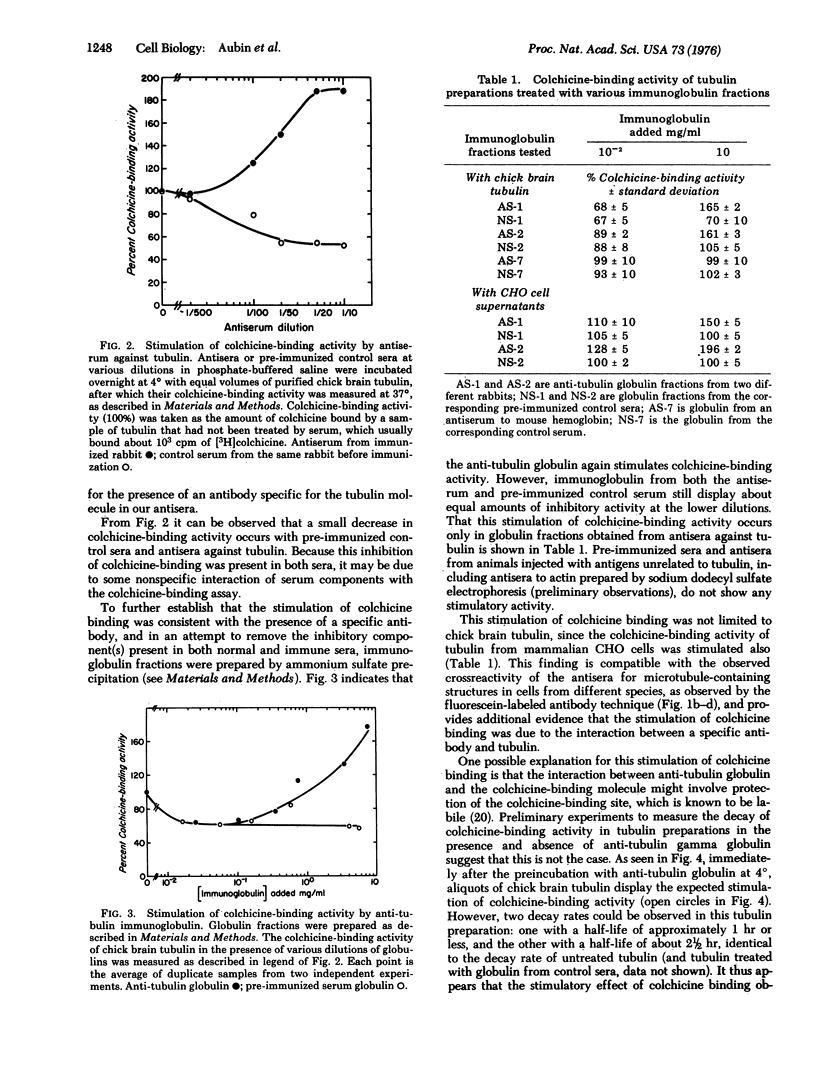
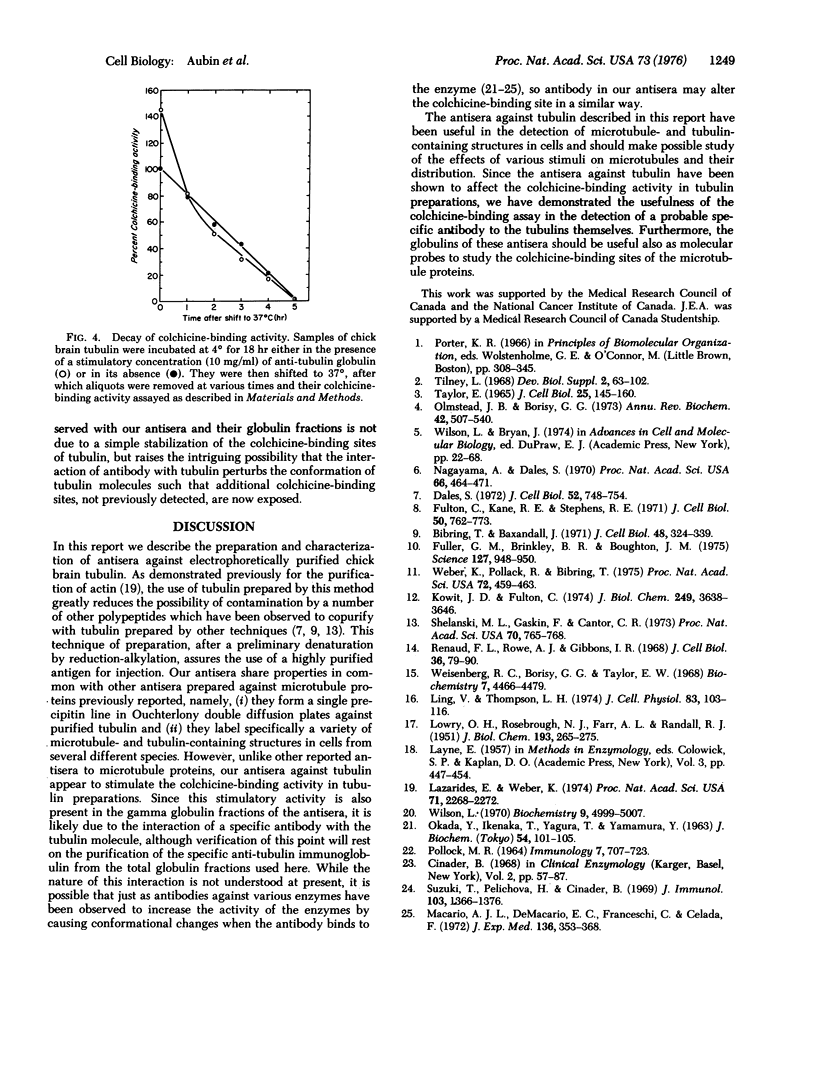
Abstract
Several rabbit antisera have been prepared against reduced and alkylated, electrophoretically purified tubulin isolated from chick brain. These antisera give a single precipitin line in Ouchterlony double diffusion plates when tested against partially purified tubulin, and label specifically microtubule- and tubulin-containing structures, such as mitotic spindles, cilia, and vinblastine-induced crystals, in a variety of cells. The same antisera also display the unique ability to stimulate the colchicine-binding activity of tubulin preparations from chick brain and Chinese hamster ovary tissue culture cells. This specific stimulation of colchicine binding activity is also obtained with the gamma globulin fractions purified by ammonium sulfate precipitation of these antisera.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibring T., Baxandall J. Selective extraction of isolated mitotic apparatus. Evidence that typical microtubule protein is extracted by organic mercurial. J Cell Biol. 1971 Feb;48(2):324–339. doi: 10.1083/jcb.48.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Concerning the universality of a microtubule antigen in animal cells. J Cell Biol. 1972 Mar;52(3):748–754. doi: 10.1083/jcb.52.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Fulton C., Kane R. E., Stephens R. E. Serological similarity of flagellar and mitotic microtubules. J Cell Biol. 1971 Sep;50(3):762–773. doi: 10.1083/jcb.50.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowit J. D., Fulton C. Purification and properties of flagellar outer doublet tubulin from Naegleria gruberi and a radioimmune assay for tubulin. J Biol Chem. 1974 Jun 10;249(11):3638–3646. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Macario A. J., Conway de Macario E., Franceschi C., Celada F. Maturation of the immkune response in vitro. Focal fluctuation and changes in affinity of anti-beta-D-galactosidase activating antibody. J Exp Med. 1972 Aug 1;136(2):353–368. doi: 10.1084/jem.136.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama A., Dales S. Rapid purification and the immunological specificity of mammalian microtubular paracrystals possessing an ATPase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):464–471. doi: 10.1073/pnas.66.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA Y., IKENAKA T., YAGURA T., YAMAMURA Y. IMMUNOLOGICAL HETEROGENEITY OF RABBIT ANTIBODY FRAGMENTS AGAINST TAKA-AMYLASE A. J Biochem. 1963 Jul;54:101–102. doi: 10.1093/oxfordjournals.jbchem.a127737. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. STIMULATING AND INHIBITING ANTIBODIES FOR BACTERIAL PENICILLINASE. Immunology. 1964 Nov;7:707–723. [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Pelichová H., Cinader B. Enzyme activation by antibody. I. Fractionation of immune sera in search for an enzyme activating antibody. J Immunol. 1969 Dec;103(6):1366–1376. [PubMed] [Google Scholar]
- TAYLOR E. W. THE MECHANISM OF COLCHICINE INHIBITION OF MITOSIS. I. KINETICS OF INHIBITION AND THE BINDING OF H3-COLCHICINE. J Cell Biol. 1965 Apr;25:SUPPL–SUPPL:160. doi: 10.1083/jcb.25.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970 Dec 8;9(25):4999–5007. doi: 10.1021/bi00827a026. [DOI] [PubMed] [Google Scholar]