Abstract
Background
Idiopathic nephrotic syndrome (NS) is the most common glomerular disorder of childhood. Invasive biopsy remains the diagnostic method of choice for NS. Prognosis correlates with steroid responsiveness, from sensitive (SSNS) to resistant (SRNS). Neutrophil gelatinase-associated lipocalin (NGAL) has been demonstrated to be a powerful risk marker of chronic kidney disease progression. We set out to determine if urine NGAL can distinguish between patients with SRNS, SSNS, and healthy controls.
Methods
Urine and clinical data were collected from patients at Cincinnati Children's Hospital who were recently diagnosed with active nephrotic syndrome as well as healthy controls. Participants included SRNS (n=15), SSNS (n=14), and healthy controls (n=10). Urinary NGAL was measured by ELISA and normalized to creatinine.
Results
Median NGAL was significantly (p <0.001) higher in SRNS (172.3 ng/ml, IQR 18.8–789) than both SSNS (6.3 ng/ml, IQR 4.9–9.9) and healthy controls (6.5 ng/ml, IQR 4.2–9.1). The area under the curve (AUC) for NGAL to distinguish SRNS from SSNS was 0.91 (p<0.0001). NGAL levels demonstrated a significant negative correlation with glomerular filtration rate (r=−0.5, p<0.001). Results did not change with NGAL corrected for urine creatinine and were independent of the degree of proteinuria.
Conclusions
NGAL levels differentiate SSNS from SRNS and correlate with disease severity in SRNS.
Keywords: NGAL, Nephrotic syndrome, FSGS, MCD, Biomarker
Introduction
Idiopathic nephrotic syndrome (NS) is the most common glomerular disease in children. The two most common histopathological findings on invasive biopsy are minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS). The prognosis of children with NS depends on the underlying histopathology and can be predicted by response to steroid treatment. Steroid-resistant nephrotic syndrome (SRNS) and biopsy-proven focal segmental glomerulosclerosis (FSGS) are significantly associated with poor outcome [1–4]. FSGS is a pathologic diagnosis that is steroid resistant (SRNS) in approximately 70% of cases [5]. While total numbers of patients diagnosed with NS have remained steady, SRNS is on the rise, as marked by the increase in incidence of FSGS in children [6–8]. FSGS is the most common primary glomerular disease leading to end-stage renal disease (ESRD) in children [9]. An additional complication in patients with FSGS leading to ESRD is the high rate of recurrence (30–40%) following transplant [10].
An invasive renal biopsy remains the standard of diagnosis in adults. Children, however, are not typically biopsied at presentation, unless they have atypical features, because response to steroids is a better predictor than histology of long-term prognosis [11]. Single renal biopsies in children tend to under-diagnose FSGS, because of the focal nature of the disease, and their effectiveness in influencing outcome remains under debate [12]. There are currently no diagnostic tests that accurately predict steroid responsiveness in pediatric NS or distinguish SRNS from SSNS. As such, the initial prolonged daily course of high-dose corticosteroids serves both a therapeutic and diagnostic purpose. If physicians had in their arsenal a test that provides additional information regarding a nephrotic syndrome patient’s likelihood to respond to a certain treatment, they could better design a therapeutic strategy for the individual. Therefore, identification of urinary biomarkers that predict steroid responsiveness or differentiate SR/FSGS from SS/MCD would benefit patients with SRNS by potentially avoiding exposure to high-dose corticosteroids.
Neutrophil gelatinase-associated lipocalin (NGAL) is a small protein expressed in neutrophils and various epithelia, especially the distal nephron segments of the kidney. NGAL expression is rapidly upregulated in response to renal injury and has proven to be a robust marker for acute kidney injury (AKI) [13–16]. More recently, the effectiveness of NGAL as a marker of chronic kidney disease (CKD) progression and prognosis has been investigated. Bolignano et al. [17] followed 96 patients with CKD stages 2–4 for 3 months and found that patients whose CKD progressed had higher baseline urine and plasma NGAL levels than non-progressors. NGAL was predictive of progression independent of other markers such as age and estimated glomerular filtration rate (eGFR). We hypothesize that since SRNS has a worse prognosis and is more likely to progress to ESRD than SSNS, NGAL levels will be higher in patients with SRNS than those with SSNS.
In this cross-sectional pilot study of pediatric patients with idiopathic NS, we set out to determine the ability of urinary NGAL levels to differentiate SRNS from SSNS using commercially available assays.
Methods
Patients and study design
Twenty-nine patients between the ages of 2 and 19 years diagnosed with nephrotic syndrome were enrolled in this study along with ten healthy, age-matched controls. Informed consent was obtained from all participants and/or their legal guardians. Patients were enrolled over a period of 24 months. Urine was collected as part of a routine clinic visit, centrifuged for 5 min at 5,000 × g, aliquoted and stored at −80°C. No more than two freeze-thaw cycles were permissible for use in the study. Demographic and clinical data (urinalysis, most recent serum creatinine, steroid-response history, current remission/relapse status) were obtained at the time of enrollment. Patients with a history of gross hematuria, active/recurrent UTI, or nephrotic syndrome secondary to systemic disease were excluded. Creatinine was transformed to GFR by the new Schwartz formula [18] and classified to CKD stage [19]. SRNS was defined when a patient failed to respond to a standard steroid therapy (2 mg/kg/day) for at least 8 weeks. SSNS was defined as the ability to achieve remission in response to the steroid treatment within 8 weeks after initial diagnosis as evidenced by normalization of urine protein to a negative reading on urine dipstick. Normal controls were children who did not have any history or evidence of renal disease.
Urine measurements
Urine NGAL measurements were performed using a commercially available ELISA (Bioporto, Gentofte, Denmark) as published previously [15, 20–22]. The intra- and inter-assay coefficients of variability (CVs) were 5.6% and 6.4%, respectively. Data were analyzed both raw and normalized to urine creatinine, Urine creatinine values were obtained using a calorimetric creatinine kit based on the Jaffe reaction (Enzo Life Sciences, Plymouth Meeting, PA). Intra- and inter-assay CVs for the creatinine assay were 2.4% and 3.15%, respectively. Microalbumin (MALB – mg/dl) was measured by immunoturbidimetry using a Siemens Dimension Xpand Plus HM clinical analyzer (Siemens, Munich, Germany). Intra- and inter-assay CVs for the MALB assay are 0.64% and 1.57%, respectively. Microalbumin was normalized with urine creatinine to estimate levels of proteinuria (mg/g).
Statistical analysis
Statistical analyses were performed using SigmaPlot 12.0 (Systat Software Inc., San Jose, CA). Briefly, medians and interquartile ranges were calculated and groups were compared using non-parametric Mann–Whitney rank-sum analysis with p values less than 0.01 considered significant. A receiver operating characteristic (ROC) curve was also calculated to determine the specificity and sensitivity of NGAL to distinguish SRNS from SSNS patients. We performed subgroup analysis of the SSNS group based on their positive/negative urine dipstick protein test to determine if NGAL levels were different between patients in remission (negative reading) or relapse (positive reading). Correlation data were generated using the Spearman rank correlation test. For categorical demographic and clinical data, Fisher’s exact test was performed.
Results
Patient demographics can be seen in Table 1. One control was excluded due to high levels of urine sediment. SRNS differed from SSNS patients with respect to age (11.5 vs. 5.8 years, p<0.001), diagnosis (FSGS vs. no biopsy, respectively, p<0.001), presence of hypertension (SRNS 53.3%, p=0.002) treatment (SRNS CNI 40%, p=0.02; SRNS MMF 33.3%, p=0.042; SRNS ACEI 33.3%, p=0.042) and serum creatinine (1.62±1.76 vs. 0.47±0.12, respectively, p=0.011). The difference in serum creatinine should be tempered, however, due to the presence of three unusually high levels (3.7–6.2 mg/dl), which raised the average considerably. There was no significant difference in proteinuria (MALB/Cr) between groups. Of the six patients receiving calcineurin inhibitors (CNIs), three were given cyclosporine A (4 mg/kg/day, for an average of 3 months) and three were given tacrolimus (0.2 mg/kg/day, for an average of 2 months) at the time of sampling. Average serum levels were 59.6 ng/ml and 11.4 ng/ml, respectively. None of the other patients had received CNIs at any point in their therapy.
Table 1.
Demographic data for SRNS and SSNS.
| Variable | SRNS (n=15) | SSNS (n=14) (relapse n=9) | p value |
|---|---|---|---|
| Age (years; mean ± SD) | 11.5±5.4 | 5.8±2.4 | 0.001 |
| Sex (%) male | 10 (66.7) | 9 (60) | 1.0 |
| Race (%) | |||
| African American | 7 (46.7) | 8 (57.1) | 1.0 |
| Caucasian | 6 (40) | 5 (35.7) | 1.0 |
| Other | 2 (13.3) | 1 (7.1) | 1.0 |
| Pathology (%) | |||
| FSGS | 13 (86.7) | 0 | 0.001 |
| MCD | 1 (6.7) | 1 (7.1) | 1.0 |
| No biopsy | 1 (6.7) | 13 (92.9) | 0.001 |
| Hypertension (%) | 8 (53.3) | 0 | 0.002 |
| Immunosuppressant (%) | |||
| Steroid | 6 (40) | 10 (71.4) | 0.139 |
| CNI | 6 (40) | 0 | 0.02 |
| MMF | 5 (33.3) | 0 | 0.042 |
| ACEI/ARB (%) | 5 (33.3) | 0 | 0.042 |
| Cr (mg/dl) | 1.62±1.76 | 0.47±0.12 | 0.011 |
| GFR (ml/min/1.73 m2) | 72.21±40.14 | 109.82±37.78 | 0.08 |
| GFR – min/max | 32–228 | 75–174 | N/A |
| MALB/UCr (mg/g) | 26,546.2±42,590.5 | 14,701.3±17,812.0 | 0.368 |
CNI calcineurin inhibitor; MMF mycophenolate mofetil; ACEI angiotensin-converting enzyme inhibitors; Cr serum creatinine; MALB microalbumin; SRNS steroid resistant nephrotic syndrome; SSNS steroid sensitive nephrotic syndrome; MCD minimal change disease
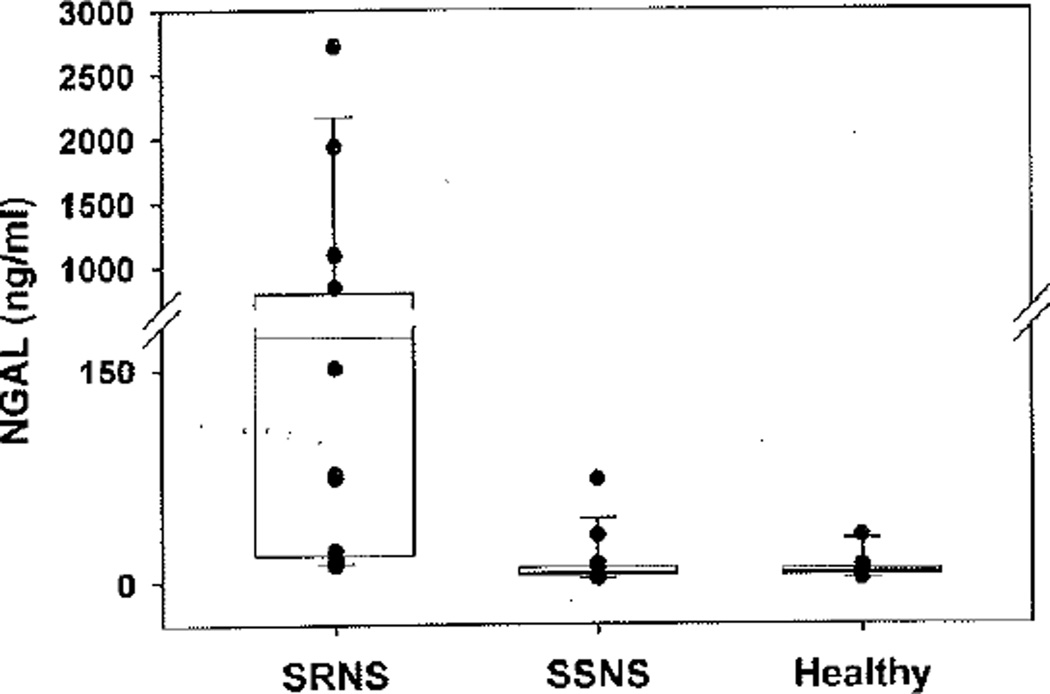
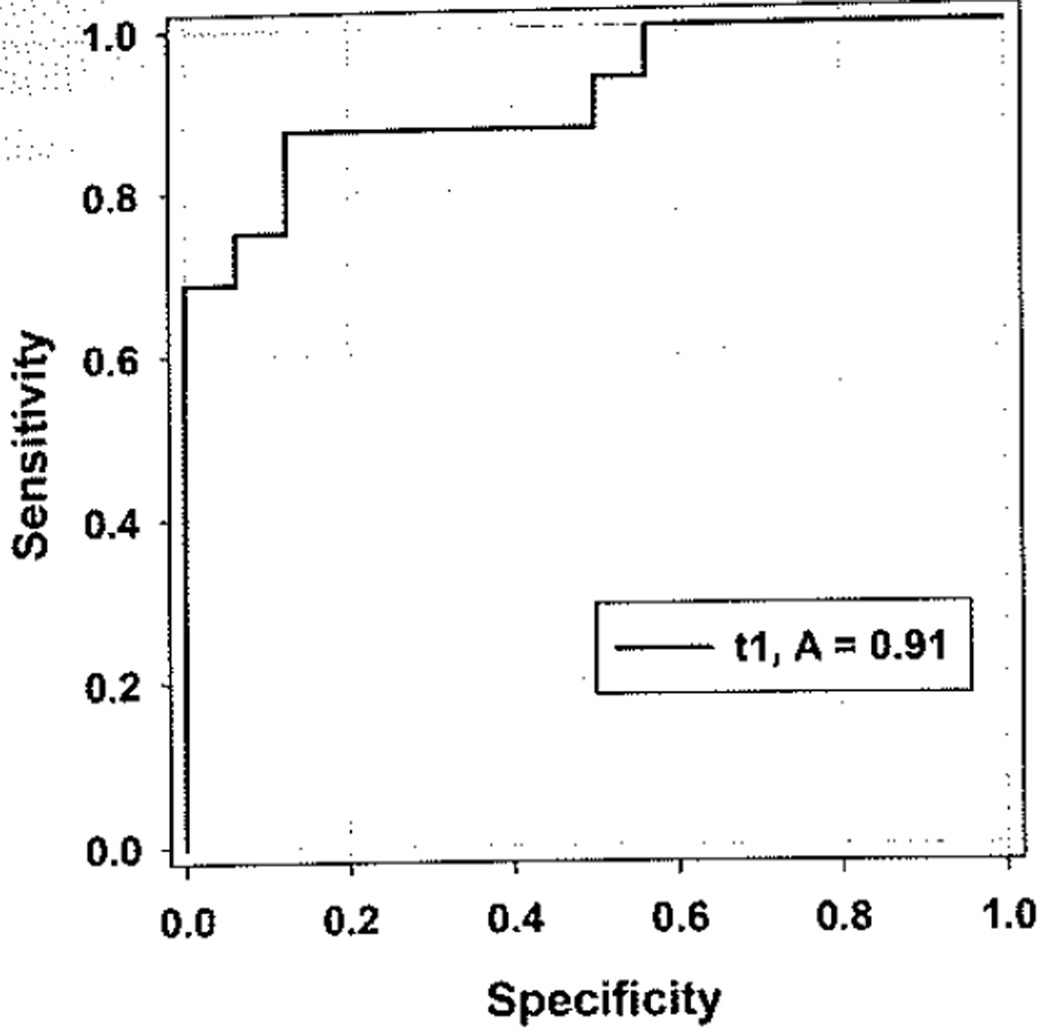
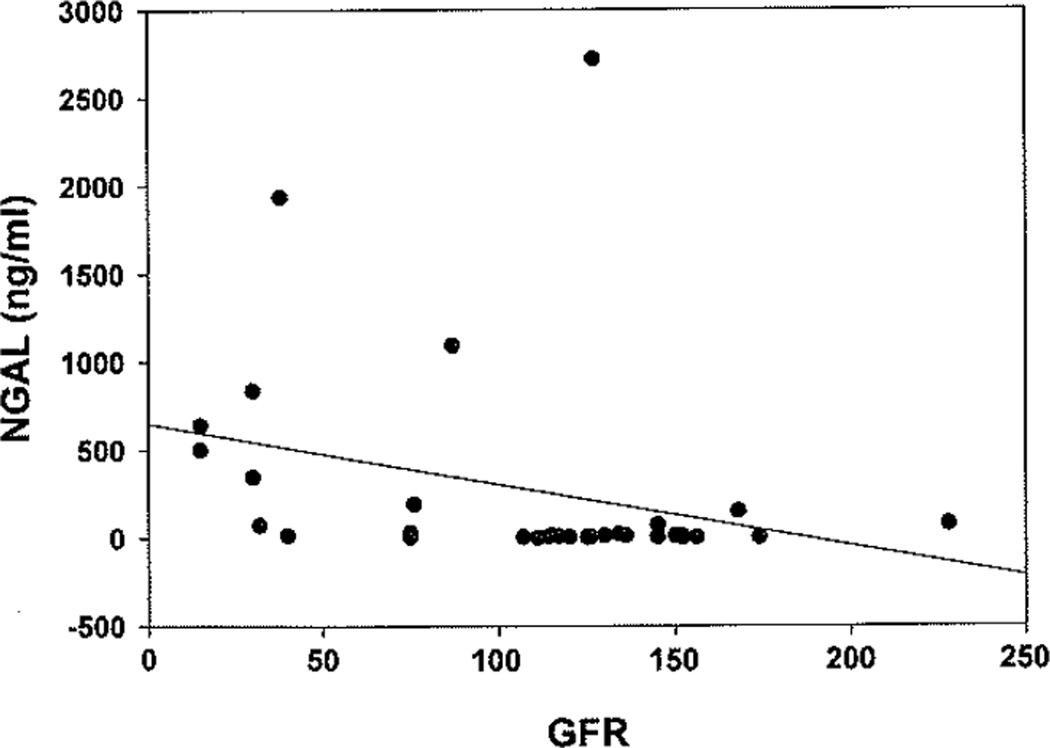
Urine NGAL levels were compared between SSNS, SRNS, and healthy controls (Fig. 1). Median NGAL was significantly (p<0.001) higher in SRNS patients (172.3 ng/ml, IQR 18.8–789) when compared to both patients with SSNS (6.3 ng/ml, IQR 4.9–9.9) and healthy controls (6.5 ng/ml, IQR 4.2–9.1). The results remained significant (p<0.001) when NGAL was indexed to urine creatinine. The area under the ROC curve (AUC) for NGAL to distinguish SRNS from SSNS was 0.91 with a p value of<0.0001, and showed an optimized sensitivity and specificity of 0.88 with a cut-off of 15 ng/mg NGAL/creatinine ratio (Fig. 2). Maximum sensitivity (1.0) and specificity were reached with cut-off values of 8.0 ng/mg and 64.9 ng/mg NGAL/creatinine, respectively. Median NGAL was not significantly different between SSNS and healthy controls. NGAL demonstrated a significant (p<0.001) negative correlation with GFR (r=−0.5), indicative of increased NGAL levels with increased disease severity (Fig. 3). This was not a direct correlation, however, as evidenced by the patient with the highest urine NGAL level (2718.9 ng/ml) who was CKD stage 1 with an eGFR of 127. There was no correlation between NGAL levels and proteinuria (MALB/Cr). The initial NGAL comparison included SSNS patients in both relapse (n=9) and remission (n=5) of proteinuria. To determine if the inclusion of patients in remission falsely impacted the results, a subgroup analysis was performed. Subgroup analysis revealed that median NGAL trended higher in SSNS patients in relapse (n=9; NGAL 9.3 ng/ml, IQR 5.7–22.8) versus remission (n=7; NGAL 5.3 ng/ml, IQR 3.0–6.3), but failed to reach statistical significance (n=16 for SSNS subgroup analysis due to samples from two patients both in remission as well as relapse). NGAL levels between SRNS and SSNS patients remained significantly different (p<0.001) whether the SSNS patients were in relapse or remission.
Fig. 1.
Urine NGAL measurements. Median urine NGAL was significantly higher in SRNS than both SSNS and healthy controls (p<0.001). Vertical dot plot overlays represent individual patient urine NGAL levels. NGAL neutrophil gelatinase-associated lipocalin; SRNS steroid resistant nephrotic syndrome; SSNS steroid sensitive nephrotic syndrome
Fig. 2.
The NGAL/creatinine ratio has a high power to distinguish SRNS from SSNS. ROC analysis revealed an AUC of 0.91 (p<0.0001) for the detection of SRNS. The optimal cut-off value was 15 ng/mg NGAL/Cr with a sensitivity and specificity of 0.88. NGAL neutrophil gelatinase-associated lipocalin; SRNS steroid resistant nephrotic syndrome; SSNS steroid sensitive nephrotic syndrome; RDC receiver opening characteristic
Fig. 3.
Urine neutrophil gelatinase-associated lipocalin (NGAL) correlate with disease severity. Median NGAL shows a significant negative correlation with estimated glomerular filtration rate (eGFR) (r=−0.5, p<0.01), indicating NGAL rises as renal function declines in all nephrotic syndrome (NS) patients
Since CNIs have been shown to increase urine NGAL levels in steroid-dependent nephrotic syndrome [23], we compared NGAL levels in patients who had been treated with CNI (n=6) vs. those SRNS patients receiving other therapies (n=9). NGAL levels were significantly lower in the CNI group than in the other SRNS patients (19.9 ng/ml, IQR 15.4–94.8 vs. 570.9 ng/ml, IQR 163.7–1303.9, p=0.02).
Discussion
Steroid resistance in idiopathic nephrotic syndrome is strongly associated with poor outcomes, including progression to ESRD. Currently, no diagnostic markers exist to distinguish SSNS from SRNS. NGAL has been demonstrated to be a powerful independent risk marker for progression in CKD. In this pilot study, our objective was to determine if urinary NGAL measurements could be used to distinguish SRNS, which has a generally poor prognosis and a high risk for progression from SSNS.
Our results show that NGAL is markedly increased in patients with SRNS versus SSNS patients (in relapse or in remission of proteinuria), and versus healthy controls (p<0.001), These results remained significant after correcting for urine creatinine. NGAL also showed high discriminatory power (AUC 0.91, p<0,0001) between SRNS and SSNS patients. An AUC of 1.0 is indicative of perfect separation, while an AUC of 0.5 has no discriminatory power. These results are consistent with our hypothesis that since nephrotic patients who are steroid resistant have a greater risk for progression, and earlier research [17] found higher levels of NGAL in patients with progressive CKD, patients with SRNS would have higher levels of NGAL than those with SSNS, which is typically not progressive. While we must temper our optimism from a limited pilot study, this is the first urinary marker to our knowledge that successfully distinguishes SRNS from SSNS with a high degree of reliability.
One earlier study successfully utilized proteomic techniques to determine a peptide signature that could classify SSNS and SRNS with great accuracy in a small group of pediatric patients [24]. Major limitations of that study are that (1) the peptides in the signature were not identified, and (2) proteomic signatures have limited clinical value due to the lack of availability of affordable and practical testing procedures. A later attempt was made to determine if a urinary cytokine panel could distinguish steroid responsiveness in pediatric idiopathic NS [25]. While the authors demonstrated that a high-throughput cytokine array, including ICAMI, could distinguish patients with NS from controls, it did not differentiate steroid responsiveness. Similarly, the same study found that urinary excretion of TBF-beta, the activation of which has been associated with SRNS, was higher in FSGS patients than MCD, it failed to differentiate SRNS from SSNS or controls. Nishida et al. [26] found increased serum and urinary NGAL levels in patients with CKD, including SRNS and SSNS, vs, control patients, but did not compare the individual subgroups to each other. A cursory look at the mean urinary NGAL levels in their study reveals higher NGAL in SRNS vs. SSNS, but not to the degree we found in our study, and statistics were not performed on the comparison.
Our results also demonstrated that there was a significant relationship between increasing NGAL levels and severity of disease, as measured by eGFR. This is consistent with other studies showing that urine NGAL levels are inversely associated with renal function, as evidenced by eGFR in CKD [27–29]. This relationship, however, did not appear to be direct, as evidenced by high levels of NGAL appearing in the urine of patients with relatively high eGFRs as well as some patients with both low NGAL levels and low eGFRs. For instance, the patient with the highest urine NGAL level (2,718.9 ng/ml) was only CKD stage 1 and had a relatively high eGFR of 127. While treatment regimens such as calcineurin inhibitors have been found in some instances to increase NGAL levels [23], this patient was being treated with MMF and ACEI. While theoretically these drugs could lead to increased NGAL levels, there is no published evidence directly supporting this possibility. Other factors that are known to lead to increased NGAL levels include various acute kidney injury, infection, inflammation, and certain cancers, but none of these were clinically detected in this particular patient. Our results also did not seem to show a CNI treatment-associated increase in NGAL, as patients receiving these drugs, as a group, had significantly lower NGAL levels than patients receiving other treatments. Possible reasons our results do not reflect the previously published [23] CsA-related NGAL elevation include (1) our patients received lower doses of CsA (4 mg/kg/day vs. 5–6 mg/kg/day) and achieved a generally lower serum concentration (59.6 ng/ml on average, vs. median levels ranging from 65.14–116.83 ng/ml), (2) all patients receiving CsA in our study had biopsy-proven FSGS, while the vast majority in the previous study had MCD (16 MCD, three FSGS), and the overall higher NGAL levels in our FSGS patients may mask any small increase in NGAL resulting from CsA toxicity. Also, while there appeared to be a trend toward increasing NGAL levels in SSNS patients in relapse versus remission, the results failed to reach significance. While this indicates that increasing dysfunction of the kidneys may lead to higher excretion of NGAL, there appear to be additional factors at play that will need to be deciphered with more targeted basic research.
Our study is not without limitations. First, this was a single-center, cross-sectional pilot study with a small group of patients who had already begun treatment at enrollment. This limits the conclusions we can draw about the value of urine NGAL to predict steroid responsiveness in NS patients. Secondly, there is a great degree of variability in both the ages of the patient groups and the NGAL data obtained from the SRNS group. Since the data are not normally distributed, we used non-parametric statistics to analyze our data. This variability is inherent when studying steroid responsiveness in NS and can be magnified in a small patient population. The majority of patients in the SSNS group have MCD and the majority of SRNS patients have FSGS. Approximately 70% of children with MCD are under 5 years of age, while FSGS is typically not diagnosed until after the age of 6 [11]. This disparity in age was evident in our patient population, with the SRNS patients being on average 5 years older than the SSNS group (p=0.001). However, several previous studies from our laboratory have demonstrated that urine NGAL concentrations are not affected by age or gender in the pediatric population [14, 15, 30], lending support to our conclusion that the elevated urine NGAL levels seen in the SRNS subjects were not due to their relatively older age. Furthermore, FSGS is a histopathological diagnosis that can represent multiple underlying pathologies, therefore it is not surprising that specific protein levels might be expressed with some variability. The strength of the significant differences (p<0.001 or better) in our study, however, leads us to believe the results are indeed powerful. One possibility that we cannot ignore is that our results might indicate that urine NGAL concentrations are reflecting a difference between FSGS and MCD. Unfortunately, we cannot conclude this with certainty, because although 13/15 of our SRNS patients had biopsy-proven FSGS, we typically do not biopsy patients who respond to steroid treatment due to the patient’s age and the invasiveness of the procedure. So while our SSNS group likely includes a majority of patients with MCD, we cannot say this with scientific certainty.
Our goal is to use this pilot data to garner interest for a multi-center prospective study, where we can capture a large number of idiopathic NS patients and obtain NGAL levels at baseline before treatments are administered, and follow the progress of the disease and NGAL levels longitudinally. We could use this study model to determine whether higher initial NGAL, levels predict worsening renal function as was found by Bolignano et al. in other forms of CKD [17]. An additional benefit of tracking the NGAL levels of these patients over time would be to determine if NGAL could be used to detect early response to therapy. Bin-markers that can be used as surrogate endpoints are valuable in clinical trials and can allow for more rapid drug development. Data gleaned from a longitudinal study would also allow us to determine if urine NGAL levels could be utilized to predict therapeutic response. While the presence of a urine biomarker is not likely to take the ultimate place of a biopsy for definitive diagnosis, in conjunction with other clinical findings it can offer the physician valuable information to more effectively plan personalized treatment strategies in the care of patients with NS.
References
- 1.Cattran DC, Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis. 1998;32:72–79. doi: 10.1053/ajkd.1998.v32.pm9669427. [DOI] [PubMed] [Google Scholar]
- 2.Hari P, Bagga A, Jindal N, Srivastava RN. Treatment of focal glomerulosclerosis with pulse steroids and oral cyclophosphamide. Pediatr Nephrol. 2001;16:901–905. doi: 10.1007/s004670100680. [DOI] [PubMed] [Google Scholar]
- 3.Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, Gibson K, Thomas DB. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344–349. doi: 10.1007/s00467-005-2097-0. [DOI] [PubMed] [Google Scholar]
- 4.Roberti I, Vyas S. Long-term outcome of children with steroid-resistant nephrotic syndrome treated with tacrolimus. Pediatr Nephrol. 2010;25:1117–1124. doi: 10.1007/s00467-010-1471-8. [DOI] [PubMed] [Google Scholar]
- 5.Gipson DS, Gibson K, Gipson PE, Watkins S, Moxey-Mims M. Therapeutic approach to FSGS in children. Pediatr Nephrol. 2007;22:28–36. doi: 10.1007/s00467-006-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulati S, Sharma AP, Sharma RK, Gupta A. Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis. 1999;34:646–650. doi: 10.1016/S0272-6386(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 7.Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, Verani R. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children1. Kidney Int. 1999;55:1885–1890. doi: 10.1046/j.1523-1755.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. 1999;13:13–18. doi: 10.1007/s004670050555. [DOI] [PubMed] [Google Scholar]
- 9.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Pediatr Transplant. 2007;11:366–373. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 10.Korbet SM. Clinical picture and outcome of primary focal segmental glomerulosclerosis. Nephrol Dial Transplant. 1999;14:68–73. doi: 10.1093/ndt/14.suppl_3.68. [DOI] [PubMed] [Google Scholar]
- 11.Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- 12.Gulati S, Sharma AP, Sharma RK, Gupta A, Gupta RK. Do current recommendations for kidney biopsy in nephrotic syndrome need modifications? Pediatr Nephrol. 2002;17:404–408. doi: 10.1007/s00467-002-0840-3. [DOI] [PubMed] [Google Scholar]
- 13.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 14.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 15.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 20.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2009;21:189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, D'Amico G, Goldsmith D, Devarajan P, Bellomo R. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36:452–461. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, 3rd, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22:2089–2095. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 23.Wasilewska A, Zoch-Zwierz W, Taranta-Janusz K, Michaluk-Skutnik J. Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of cyclosporine nephrotoxicity? Pediatr Nephrol. 2010;25:889–897. doi: 10.1007/s00467-009-1397-1. [DOI] [PubMed] [Google Scholar]
- 24.Woroniecki RP, Orlova TN, Mendelev N, Shatat IF, Hailpem SM, Kaskel FJ, Goligorsky MS, O'Riordan E. Urinary proteome of steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome of childhood. Am J Nephrol. 2006;26:258–267. doi: 10.1159/000093814. [DOI] [PubMed] [Google Scholar]
- 25.Woroniecki RP, Shatat IF, Supe K, Du Z, Kaskel FJ. Urinary cytokines and steroid responsiveness in idiopathic nephrotic syndrome of childhood. Am J Nephrol. 2008;28:83–90. doi: 10.1159/000109396. [DOI] [PubMed] [Google Scholar]
- 26.Nishida M, Kawakatsu H, Okumura Y, Hamaoka K. Serum and urinary neutrophil gelatinase-associated lipocalin levels in children with chronic renal diseases. Pediatr Int. 2010;52:563–568. doi: 10.1111/j.1442-200X.2010.03067.x. [DOI] [PubMed] [Google Scholar]
- 27.Bolignano D, Coppolino G, Campo S, Aloisi C, Nicocia G, Frisina N, Buemi M. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant. 2008;23:414–416. doi: 10.1093/ndt/gfm541. [DOI] [PubMed] [Google Scholar]
- 28.Bolignano D, Lacquaniti A, Coppolino G, Campo S, Arena A, Buemi M. Neutrophil gelatinase-associated lipocalin reflects the severity of renal impairment in subjects affected by chronic kidney disease. Kidney Blood Press Res. 2008;31:255–258. doi: 10.1159/000143726. [DOI] [PubMed] [Google Scholar]
- 29.Bolignano D, Coppolino G, Lacquaniti A, Nicocia G, Buemi M. Pathological and prognostic value of urinary neutrophil gelatinase-associated lipocalin in macroproteinuric patients with worsening renal function. Kidney Blood Press Res. 2008;31:274–279. doi: 10.1159/000151665. [DOI] [PubMed] [Google Scholar]
- 30.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–1115. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]